Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Almost any type of fracture can occur during athletic activities. This is because of the many different types of sports and the corresponding situations in which athletes are found. All athletes undertake various fracture risks with participation. Many of these specific fractures are covered in detail in other chapters. This chapter focuses on certain specific fractures and their management, particularly on stress fractures in various bones in the extremities and spine. Also discussed are some of the more commonly seen acute fractures in sports, such as clavicular and rib fractures. Returning an injured athlete to practice and competition, whether a recreational or an organized sport, is an issue specific to athletes. Return to participation guidelines are addressed and guidance for practicing sports medicine clinicians is offered further on.
Stress fractures are commonly seen in athletes and in the military, particularly in trainees. They are seen in as many as 20% of military trainees and in up to 20% of individuals presenting to a sports medicine clinic. History of a prior bone stress injury is the strongest predictor of a new injury. Athletes are susceptible to developing stress fractures because they are often exposed to repetitive high-intensity, load-bearing activities in both training and competition. This overuse leads to microdamage to the involved bone that is not repaired by normal bone homeostasis. These microcracks accumulate and lead to fatigue failure and eventually a visible fracture line can be seen on imaging. Stress injuries occur on a continuum from an asymptomatic stress reaction visible only on magnetic resonance imaging (MRI) as edema, to a symptomatic fracture line visible on imaging, to a chronic nonunion. Unfortunately physical exam including the use of a tuning fork and ultrasound are of little use for diagnosing a stress fractures. Both bone scan and MRI are commonly used for imaging diagnosis, although MRI is more useful; it involves comparatively less radiation and testing time, fewer false positives, and better correlation with return to participation. A recently proposed classification system ( Table 10.1 ) has been found to have high intra- and interobserver reliability and was also found to be easily remembered.
| Grade | Pain | Radiographic Findings (CT, MRI, Bone Scan, or Radiography) | Description |
|---|---|---|---|
| I | No | Imaging evidence of stress fracture, no fracture line | Asymptomatic stress reaction |
| II | Yes | Imaging evidence of stress fracture, no fracture line | Symptomatic stress reaction |
| III | Yes | Nondisplaced fracture line | Nondisplaced fracture |
| IV | Yes | Displaced fracture (≥ 2 mm) | Displaced fracture |
| V | Yes | Nonunion | Nonunion |
Certain bone locations are more susceptible to stress injuries. Slower bone remodeling occurs in cortical bone; therefore cortical regions are more likely to sustain stress fractures. On the other hand, cancellous bone has a higher turnover and is susceptible to altered homeostasis from poor bone quality (i.e., osteoporosis). Typically cancellous bone stress injuries occur in athletes with low bone mineral density (BMD). Furthermore, the location of the fracture within the bone can be predictive of healing potential. Injuries on the compression side of a bone are likely to do well with nonoperative management. Those that occur on the tension side of a bone are inherently unstable and may require surgery to heal. Some specific bone locations, such as the fifth metatarsal and navicular, are also predisposed to stress injury due to poor vascularity. These watershed areas are likely less able to respond to mechanical stress with an appropriate and robust healing response.
Female sex is the most important predictor of stress fracture in endurance athletes. Long-distance running, in particular, poses the highest risk; it can predispose to stress fractures by itself. However, associated nutrition and menstrual irregularities are likely major contributors to injury. Lower levels of estrogen in the amenorrheic female athlete may be due to lower levels of gonadotropins. A relative lack of estrogen disrupts the normal bone turnover response to repetitive stress.
The female athlete triad involves low energy availability, menstrual dysfunction, and low BMD. Early intervention is important to prevent the development of eating disorders, amenorrhea, and osteoporosis. Cardiovascular health as well as endocrine, gastrointestinal, renal, and neuropsychiatric health can also be affected. There is evidence that athletic performance may worsen in athletes with triad symptoms. Athletes can be stratified as at low, moderate, and high risk based on specific criteria involving the presence of a dietary restriction, low body mass index (BMI), delayed onset of menarche, other menstrual abnormalities, low Z score on Dexa scan, and the presence of a bone stress injury. Female athletes who fall into the moderate- and high-risk categories are two and four times more likely to develop a bone stress injury.
Biomechanical factors may also be associated with stress fracture risk. Interestingly, ground reactive forces do not seem to be different between uninjured and injured athletes. Increased lower body muscle mass may help to absorb forces during running. Athletes with smaller calf girth and less lower limb muscle mass have a higher risk of injury. Lower extremity limb alignment may matter as well. A quadriceps angle greater than 15 degrees is related to a higher risk of stress fractures. Also, leg-length discrepancies are related to injury risk in runners. There may be a training adaptation period during which an athlete or military recruit is at increased risk of stress fracture. After this period, the stress injury risk may decrease.
There is some association between athlete fatigue and changes in loading patterns in the lower extremity. This may place a fatigued athlete at higher risk of fracture. Changes in training regimen, such as sudden increases in running mileage, are also risk factors for injury. Running in a fatigued state may lead to greater lower extremity mechanical forces.
Nutrition and diet influence the development of stress injuries. Higher dairy and calcium intake results in fewer stress fractures. Also, a higher intake of calcium may have a protective effect when taken in doses of 1500 to 2000 mg/d. Both vitamin D intake and vitamin D levels are lower in athletes who develop stress fractures and are inversely correlated with the risk of developing a stress fracture in both the adolescent and adult population.
Some recent data support a genetic predisposition to the development of stress injuries. The RANK pathway has been implicated in distance athletes. The androgen receptor gene CAG allele distribution is different among military recruits with bone stress injuries versus those without injury. The CALCR and VDR genes may also confer a risk for stress fracture. Other gene sequences have been implicated as well. To date, however, no clear cause-and-effect relationship has been determined.
The following sections focus on the specific stress fractures most commonly seen in athletes. Risk factors for injury, recommended diagnostic workup, treatment strategies, and in some cases prevention are discussed.
A femoral neck stress fracture can be devastating to an athlete and the long-term health of his or her hip. Care should be taken to avoid missing a rare presentation in children. Fractures cause anterior groin pain of insidious onset that is worse with weight bearing. Fractures can be bilateral even with unilateral groin pain. A missed stress fracture can lead to displacement and the development of femoral head avascular necrosis (AVN). These fractures are described in long-distance runners and military recruits, specifically those with lower levels of fitness at the start of training. Internal forces in the femur are concentrated along the femoral neck with running, and there may be a genetic predisposition to the development of femoral neck stress fractures. Additionally there is an association of femoroacetabular impingement, specifically acetabular overcoverage or retroversion, with the development of femoral neck stress fractures.
Athletes with groin pain should undergo a standard hip radiographic series. If no fracture is visualized on radiographs, then an MRI can serve to evaluate the femoral neck in more detail ( Fig. 10.1 ). Most fractures are first diagnosed on MRI, and an abbreviated MRI sequence is effective. A bone scan positive on all three phases also indicates a stress fracture, and a single photon emission computed tomography (SPECT) scan has improved diagnostic accuracy. However, lack of anatomic detail limits its usefulness for treatment planning. Fractures can be classified based on whether they are nondisplaced or displaced, complete or incomplete, tension side or compression side. These characteristics have implications for recommended treatment.
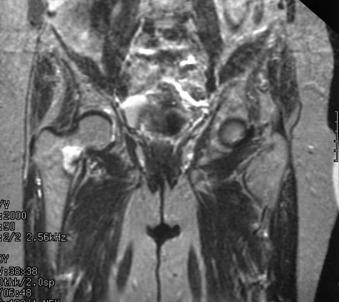
Displaced fractures require urgent treatment, as they are typically seen in younger patients in their 20s and 30s. Open reduction and internal fixation is required, with a goal of restoring function and decreasing the chance of developing AVN and secondary osteoarthritis. Treatment of nondisplaced fractures depends on fracture location and the extent of involvement of the femoral neck. Fractures that are incomplete and involve only the compression side (medial aspect) of the femoral neck can be treated nonoperatively with an extended period of non–weight bearing ( Fig. 10.2 ). Time to return to running for compression-sided injuries increases with the severity of injury as seen on MRI. This ranged from 7.5 weeks for low-grade injuries seen only as edema without a visible fracture line to 17.5 weeks when a fracture line is visible.
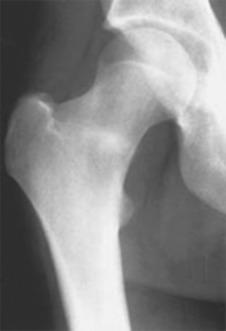
Fractures that involve the tension side (lateral aspect) of the femoral neck are typically treated surgically ( Fig. 10.3 ). Percutaneous internal fixation is achieved with cannulated screws to prevent varus displacement. Of note, there is no correlation between the femoral neck shaft angle and the outcome of femoral neck stress fracture treatment. Postoperatively these patients are also treated with an extended period of non–weight bearing. Both nonoperatively and operatively treated patients require an extended period of time for rehabilitation and return to running as well as other high-impact athletic activities. It is important to gradually increase loading during the rehabilitation process so as to be sure that the femoral neck is healed and able to handle the required stresses.
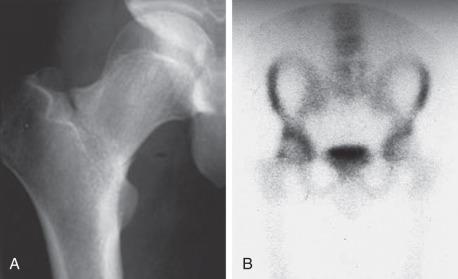
Tibia stress fractures are the most common stress injuries in endurance athletes. Athletes place significantly more load on the tibia while running than while walking. It is unclear if mechanical factors alone can account for tibia stress fractures; overall bone health along with other factors likely play an important role. Running speed has been correlated with risk of tibial stress fracture. Although such fractures are not as devastating as some other lower extremity stress injuries, they can lead to prolonged time off from running and impact activities.
Running athletes, including military trainees, who experience focal tibial pain with impact exercise should be evaluated for a tibial stress fracture. Patients who have such fractures are tender focally over the location of the fracture. Provocative maneuvers such as hopping on the injured leg and using the leg as a fulcrum may cause pain. A stress fracture can be distinguished from medial tibial stress syndrome (MTSS) by the focal nature of the fracture compared with the classic diffuse area of tenderness in MTSS. Radiographs are often negative, although the presence of the “dreaded black line” is a poor prognostic indicator for fracture healing with nonoperative treatment. In most cases this represents a chronic fracture nonunion. Stress injuries not visible on radiographs can be imaged with MRI. Bone scan is used less commonly for reasons listed previously.
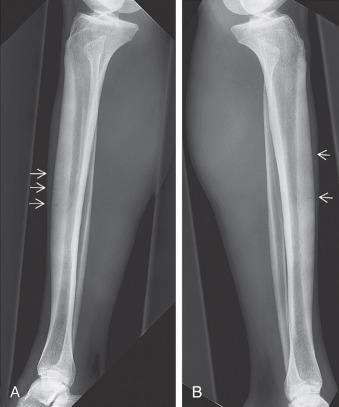
Tibial stress fractures are most commonly located posteromedially on the compression side of the proximal tibia. Fractures can also occur on the tension side, and these are seen along the anterior cortex of the midshaft of the tibia. Those fractures occurring on the compression side of the tibia can be treated with a period of non–weight bearing and immobilization ( Fig. 10.4 ). Fractures that occur on the tension side of the tibia and those that are visible on radiographs typically require operative intervention to heal ( Fig. 10.5 ). Traditionally these fractures were treated with an intramedullary nail. The intramedullary device promotes fracture healing by creating a load-sharing environment with the native tibia. Additionally, reaming is thought to stimulate bone marrow and provide a source of internal bone graft within the canal of the tibia to assist with healing. These fractures can also be treated with anterior tibial cortex plating with or without local bone grafting. The advantages of plating include placing the implant on the tension side of the fracture, allowing access for direct fracture débridement and bone grafting, as well as not violating the knee joint. Such patients may experience less anterior knee pain, which is as high as 80% in some studies involving the use of an intramedullary nail to treat these injuries. Drawbacks include plate prominence over a location of the tibia with minimal soft tissue coverage as well as the potential to devitalize the tibial cortex surrounding the fracture.
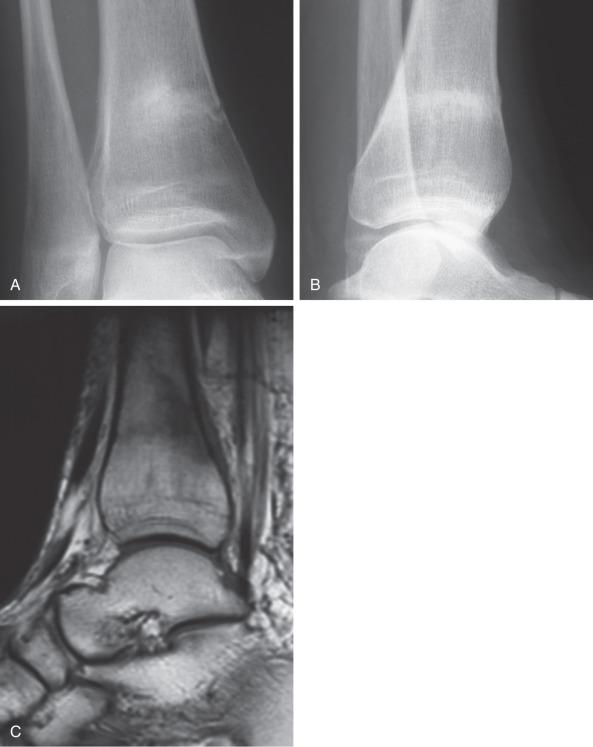
The proximal aspect of the fifth metatarsal is also a common site for a stress fracture. Athletes of any running or marching sport are susceptible. Abnormal forefoot loading may contribute to these injuries, especially with minimalist shoe wear, which increase forefoot loading. Athletes with a cavovarus hindfoot place additional stresses on the lateral border of the foot, predisposing them to injury. Additionally, the proximal metatarsal is a vascular supply watershed area, which perhaps leads to a poor regenerative response to repetitive injury. Also, toe grip weakness is correlated with an increased incidence of fifth metatarsal stress fractures.
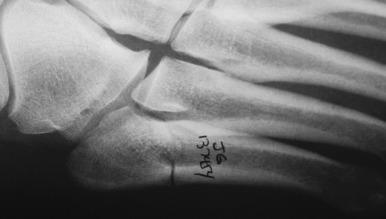
These injuries should be suspected in athletes with pain along the lateral border of the foot. The pain is often insidious and chronic, although the injury may also present with pain after a twisting type of injury. Stress fractures of the proximal fifth metatarsal should be distinguished from Jones fractures, which involve the fourth and fifth metatarsal articulation, as well as smaller, proximal avulsion type injuries. This distinction can be made on plain radiographs, where stress injuries are visible more distal at the proximal junction of the metatarsal shaft and metaphysis. These injuries can be seen as a lateral cortical thickening representing attempted healing in response to repetitive stress. They may also present with a visible fracture line that most often is incomplete and involves the lateral cortex. MRI and bone scan can be used for diagnosing injuries not seen on radiographs. Ultrasound has been proposed as a cheaper imaging option, although its sensitivity is not as high as that of MRI.
When a fracture is visible on radiographs, most patients are treated with surgical stabilization ( Fig. 10.6 ). Distinguishing a true Jones fracture from a diaphyseal fracture may not be important. Nonoperative treatment has higher nonunion rates and longer time to sports and physical labor. Typically surgical repair involves placing an intramedullary screw with sufficient screw threads past the fracture ( Fig. 10.7 ). Intramedullary cortical thickening may make canal drilling difficult. Additionally, it is important not to penetrate the cortex distally with the drill or screw, thus creating a stress riser susceptible to fracture. There is debate over screw diameter, since larger screws are less susceptible to fatigue fracture. For most patients a screw 5 mm to 50 mm in diameter, based on computed tomographic (CT) studies, is appropriate. Some surgeons prefer a solid screw as opposed to a cannulated one to also help resist fatigue failure. Most commonly, exposing the fracture site to débride the fracture and place bone graft is not necessary for a first-time attempt at fixation, although it may be considered on a case-by-case basis and might be more likely in revision cases. Patients with incomplete fractures on radiographs and a plantar fracture gap less than 1 mm healed faster than those with a gap greater than 1 mm. Patients with a cavovarus hindfoot might require a valgus-producing calcaneal osteotomy to unload the lateral aspect of the foot and create a more favorable mechanical environment. This is especially important in athletes who sustain recurrent injuries.
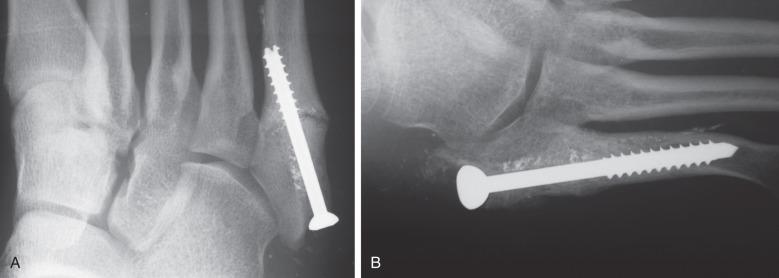
Postoperatively an assessment should be made of the factors, in addition to patient-specific health characteristics, that may have contributed to the injury. These may include running style, training volume, running shoe quality and wear, as well as foot shape. Additionally, athletes with low vitamin D levels (<30 ng/mL) have a higher incidence of fifth metatarsal stress fracture and should therefore be treated with oral supplementation. Adjusting each of these factors is important in order to minimize the chance of recurrence. Custom orthotics should be considered to ensure that stresses to the athlete's feet are properly distributed. Recurrence of injury after operative fixation is more likely in athletes who return to sports earlier, prior to complete healing. This is also true for those with a higher BMI and a wider fourth-to-fifth intermetatarsal angle and curved fifth metatarsal head.
Stress fractures involving the first to fourth metatarsals are also common, especially in running athletes and dancers. The second metatarsal is subjected to the highest bending stresses and is therefore the most commonly involved of the metatarsals. This is followed by the third metatarsal.
Fractures can occur anywhere along the metatarsal and maximal stress occurs 3 to 4 cm distal from the base of the bone. Fractures that are more distal heal more predictably. In most cases, with a period of immobilization and limited weight bearing, treatment is successful. Once the foot pain has subsided, it is important to progress activities in a gradual process to allow for mechanical adaptation of the foot to increased loads. It is also important to address any factors involving the patient's systemic homeostasis as well as foot biomechanics and loading patterns. Achilles stretching, cross training, limiting mileage, regular shoe replacement, and custom orthotics are all treatment strategies that should be considered.
Forth metatarsal fractures can have delayed or incomplete healing with nonoperative treatment, especially in patients with metatarsus adductus. Open reduction and plate fixation is an option in these injuries to hasten return to athletics.
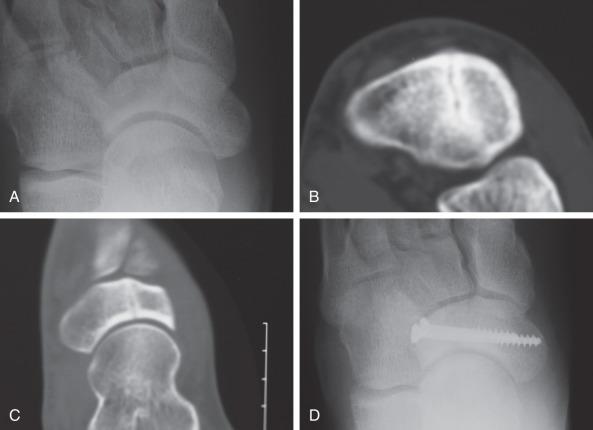
Navicular stress fractures, while uncommon, are challenging to diagnose and treat. Delay in diagnosis is the norm. They are seen in sprinting and jumping sports such as basketball and, unlike most stress fractures, are more common in male athletes. The central third of the bone is an area of high shear stress. The navicular is the keystone to the medial longitudinal arch and the link between the midfoot and hindfoot. It serves as an important structural support for both the longitudinal and transverse arches. With weight bearing, forces are generated medially across the navicular from the first metatarsal through the medial cuneiform and the talus. Forces through the second metatarsal and middle cuneiform are not resisted in the same way, thereby generating shear forces. Additionally, the central third of the navicular is thought to be a relatively avascular zone, which limits its ability to resist changes in forces and subsequently its healing potential. Recent data note that an avascular zone in the central navicular is present only 12% of the time. The majority of the time the navicular is well perfused from the dorsalis pedis and branches of the posterior tibial artery.
Patients present with insidious dorsal foot pain, and diagnosis of a navicular stress injury can often be delayed. Tenderness is described over the navicular. Pain can be exacerbated by axially loading a plantarflexed foot such as standing on tiptoe or hopping on a single leg. Radiographs may become positive only on a delayed basis owing to lack of involvement of the plantar surface. Therefore MRI is more commonly used for fracture detection.
Once the fracture has been diagnosed, treatment requires an extended period of immobilization. When fractures are diagnosed in early stages, they can be treated successfully with casting for a minimum of 6 weeks. Non–weight bearing during this period is also important; allowing weight bearing too soon can lead to nonunion. On average, these fractures result in about 4 months loss of athletic participation. Pain with therapeutic ultrasound can be used to follow navicular healing and correlates with MRI findings.
Athletes with fracture lines visible on radiographs or those hoping to return to participation sooner may be candidates for operative fixation. Operative treatment allows return to athletics at a mean of 16 weeks, compared with 22 weeks for conservative treatment. In most cases two partially threaded screws placed perpendicular to the fracture line from lateral to medial suffice to generate compression and healing ( Fig. 10.8 ). Postoperative limited weight bearing for 1 to 2 months is important. Repeat imaging to confirm fracture healing can be considered prior to release to return to sports participation. Outcomes of navicular stress fractures are not always optimal regardless of treatment. There is a high rate of persistent pain and disability and those patients are more likely to develop a nonunion. With operative treatment there is a high rate of secondary osteoarthritis and need for reoperation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here