Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The development of the colonoscope followed that of upper panendoscopes. Rigid proctoscopes were developed in the late 1800s, and fiberoptic techniques were adapted to visualize the sigmoid and descending colon in the 1960s. In the 1970s, the colonoscope was lengthened with four-way tip deflection, allowing for modern day colonoscopic techniques.
The first mass-produced video instruments were introduced in the 1980s and 1990s. Pediatric colonoscopes with a range of outer diameters are currently commercially available. These small-caliber endoscopes have found widespread application in treatment of both children and adults and are now being used with increased frequency in adults who are undergoing unsedated endoscopic procedures and in those with decreased luminal size due to their underlying disease process such as stricture or malignancy.
The personnel, facilities, sedation, and monitoring requirements and antibiotic prophylaxis regimens for colonoscopy in pediatric patients are discussed in Chapter 60 . Colonoscopy is technically more challenging than esophagogastroduodenoscopy (EGD), especially in pediatric patients. Individuals performing colonoscopy in children should be specifically trained to do so and have met the standard of current training guidelines.
Colonoscopes are similar in construction to the upper endoscopes, with different diameters, instrument lengths, range of tip bending, flexibility characteristics, and fields of view. In the last several years, colonoscopes of variable stiffness have been developed to decrease loop formation and assist with loop reduction. Although they are available for use in pediatric patients, variable stiffening of the colonoscope should not be used to replace good endoscopic technique, with the primary emphasis on minimal loop formation and rapid loop reduction.
The most important issues related to pediatric endoscopy equipment are the diameter and length of the insertion tube, the degree of tip angulation, and the depth of field. Colonoscopy in infants and young children has traditionally been performed with instruments designed for upper endoscopy because of their smaller diameter. Newer ultrathin pediatric colonoscopes are available—with an outer diameter in the range of 9.7 mm, comparable to standard upper endoscopes, and with length (1330 mm for standard, 1680 mm for long), channel diameter, and flexibility characteristics of colonoscopes. Polypectomy snares can be passed via an upper endoscope or small colonoscope with a 2.8-mm channel. Colonoscopy in normally developed children older than 4 to 5 years of age can usually be performed with a standard pediatric colonoscope. These colonoscopes, including those with variable stiffness capabilities, have a distal end with an outer diameter in the range of 11.3 to 11.6 mm with a 3.2- to 3.8-mm channel, compared with an outer diameter of 12.8 mm for adult colonoscopes. (Endoscope information courtesy of Olympus America Inc., Center Valley, Pennsylvania, and Pentax Precision Instrument Corporation, Orangeburg, New York.)
The indications for colonoscopy vary with the age of the patient. The need for colonoscopy in neonates and infants is usually suggested by physical signs reported by parents or other observers, which include hematochezia, melena, hypotension, or anemia. With toddlers and older children, the history is of greater importance in identifying gastrointestinal disorders ( Box 61.1 ). The sensitivity of gastrointestinal endoscopy in establishing a diagnosis varies with the indication for the procedure.
Gastrointestinal hemorrhage
Chronic diarrhea
Suspected inflammatory bowel disease
Cancer surveillance
Inflammatory bowel disease
Polyposis syndromes
Therapeutic intervention
Polyp removal
Foreign body removal
Decompression of toxic megacolon
Endoscopic hemostasis (cautery, clip, injection/ablation)
Percutaneous cecostomy
Stricture dilation
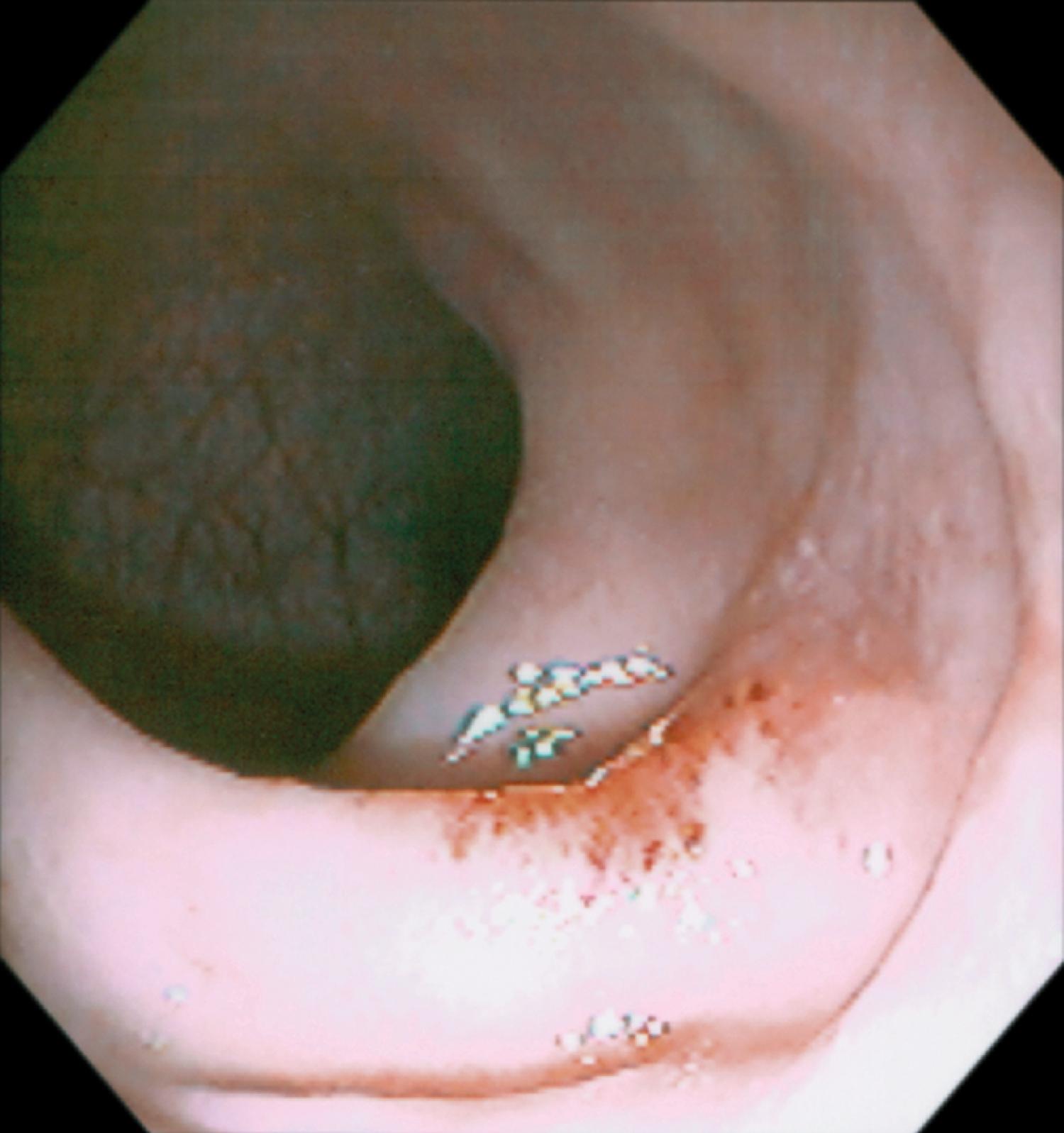
The major indications for colonoscopy in infants and children include rectal bleeding, unexplained diarrhea, and abdominal pain with abnormal growth, weight loss, or other constitutional symptoms. Colonoscopy is also performed to investigate abnormalities suspected on barium enema, small-bowel follow-through, computed tomography (CT), or magnetic resonance imaging (MRI). Colonoscopy is typically the first line of investigation for patients with suspected mucosal disease, and barium enema is rarely performed for this indication. If inflammatory bowel disease (IBD) is suspected, colonoscopy can provide visual evidence of the nature and extent of disease, and evaluation of biopsies obtained at the time of the procedure may define the nature of the underlying inflammation. Terminal ileal intubation and biopsy are especially important in the diagnosis of IBD and in the evaluation of known IBD. Infectious colitis may be difficult to distinguish visually from IBD of recent onset, both visually and histologically, especially in patients younger than 10 years of age ( Fig. 61.1A and B ). , Crohn disease involving only the colon may also present with a focal active colitis pattern of injury on biopsy similar to that seen with infection. Some patients may require follow-up endoscopic examination with biopsy to look for evidence of chronicity before establishing a firm diagnosis of IBD. Colonoscopy is also indicated in the postoperative setting in patients with IBD to evaluate a stoma to look for evidence of Crohn disease, to examine “out of circuit” bowel, and to evaluate the pouch for pouchitis or dysplasia in patients following ileal pouch anal anastomosis.

Visualization and biopsy of the terminal ileum may be helpful in the diagnosis of infectious ileitis. In some infections, such as ileocecal tuberculosis, colonic schistosomiasis, and amebiasis, stool cultures are usually negative, but colonoscopy with biopsies may identify the etiologic organism on biopsy specimens or cytologic brushing.
Lymphonodular hyperplasia of the colon, although not an indication for colonoscopy, may be a cause of painless rectal bleeding identified at endoscopy. Thinning of the surface epithelium over the protruding lymphatic tissue with subsequent trauma from the passage of fecal material is thought to lead to ulceration and hematochezia. Prominent lymphatic nodules can be found throughout the large and small bowel. They appear as smooth, round, 2- to 4-mm nodules with normal overlying mucosa. Occasionally larger nodules may have central umbilication or overlying erosion. This nodular lymph node tissue is thought to represent a self-limiting response to antigenic stimulation and does not require any additional evaluation or therapy.
In children with suspected polyps, colonoscopy is both diagnostic and therapeutic. In diseases associated with an increased rate of malignancy, such as ulcerative colitis, Crohn disease, or familial adenomatous polyposis and other polyposis syndromes, colonoscopic surveillance for dysplastic changes is usually performed at regular intervals. Colonoscopy has been reported for the diagnosis and evaluation of smooth muscle tumors in children with acquired immune deficiency syndrome. These lesions may appear as a submucosal nodule with central umbilication. Colonoscopy may rarely identify colonic neoplasia in pediatric patients. These lesions may be primary or metastatic and may present with significant gastrointestinal bleeding. Endoscopic biopsy is useful in establishing the diagnosis and potentially the extent of intraluminal disease, but endoscopic resection should be avoided because of the possibility of submucosal or deeper extension and the associated risk of significant hemorrhage or colonic perforation. Colonoscopy is usually not indicated in children complaining of chronic abdominal pain or constipation with unremarkable physical examinations and laboratory studies. However, changes of mucosal prolapse in patients with chronic constipation may on occasion be noted at endoscopy ( Fig. 61.2 ). Colonoscopy or more typically flexible sigmoidoscopy is used in the evaluation of suspected acute graft-versus-host disease (GvHD) following hematopoietic stem cell transplantation. ,
Ileal examination with endoscopic biopsy typically via a stoma is the primary method used to identify graft rejection following small bowel or multivisceral transplantation. Endoscopic biopsies from the allograft are routinely obtained starting in the immediate posttransplantation period, and regularly thereafter, these are immediately performed for symptoms including increased stomal output, unexplained fever, or gastrointestinal bleeding to assess for infection, rejection, or posttransplantation lymphoproliferative disorder (PTLD). Small bowel transplantation is discussed in more detail in Chapter 36 .
Indications for therapeutic colonoscopy include polypectomy, therapy for gastrointestinal bleeding including bleeding following polypectomy, vascular ablation, retrieval of a foreign body, placement of a percutaneous cecostomy (PEC), and colonic decompression in toxic megacolon. Colonoscopy with therapeutic intervention for vascular lesions can eliminate the need for surgery in some cases. The types of lesion encountered include cavernous hemangiomas and small telangiectasias. Syndromes associated with vascular lesions in the gastrointestinal tract include Osler-Weber-Rendu, CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia), dyschondroplasia (Maffucci syndrome), blue rubber bleb nevus syndrome, diffuse neonatal hemangiomatosis, Turner syndrome, and pseudoxanthoma elasticum.
Colonic strictures can also be dilated through the endoscope channel by using the controlled radial expansion (CRE) balloon dilators in conjunction with other endoscopic instruments. Intussusception has been identified and reduced during colonoscopy, but air-contrast reduction remains the procedure of choice. Colonoscopy has been used as an alternative to surgery to direct colonic Gastrografin administration in patients with cystic fibrosis and distal intestinal obstruction syndrome refractory to medical therapy. Only practitioners with adequate experience should undertake technically advanced therapeutic endoscopic procedures.
Absolute contraindications to gastrointestinal endoscopy include suspected perforation of the intestine and peritonitis in a toxic patient. There are several relative contraindications, including patients who are severely neutropenic or have bleeding disorders and children with a recent history of bowel surgery. In addition, patients with connective tissue disease, especially Ehlers-Danlos syndrome type 4 and Marfan syndrome, are at increased risk of perforation during endoscopy. , Toxic dilation of the bowel carries an increased risk of perforation during colonoscopy, although the procedure may relieve the distension. Other relative contraindications include partial or complete bowel obstruction and aneurysm of the abdominal and iliac aorta. In all endoscopic procedures, the clinician and endoscopist must determine whether the potential information gained or therapeutic intervention outweighs the risk of the procedure.
In addition to a period of preprocedure fasting, colonoscopy can be accomplished successfully only if the colon is free of fecal debris. Large amounts of stool compromise the ability to negotiate the curves of the large bowel and prohibit complete visualization of the mucosa. Stool adherent to the lens often requires additional maneuvering and added air-water insufflation to clear the field of vision. Liquid stool can be aspirated through the suction channel but increases the examination time and excess stool may preclude complete colonoscopy. Quality indicators of colonoscopy in adults including percent mucosa visualized, polyp detection rate, and scope insertion and withdrawal time can be correlated to the quality of the bowel preparation. In adults, there are a number of bowel preparation rating scales that can be used to study the clinical effectiveness of different bowel preparations. These include the validated Boston Bowel Preparation Scale (BBPS), which assigns a score of 0 to 3 to each of the three colon segments (right, left, and transverse colon) and a total score ranging from 0 (no prep) to 9 (complete mucosal visualization). , Other relevant scales include the Ottawa and Aronchick Scales. To date, comparable pediatric bowel preparation scores have not yet been developed but will aid in both the comparison of bowel preparation regimens and in the development of quality indicators in pediatric colonoscopy.
Many bowel preparations have been used successfully to cleanse the colon. The method depends on the age and cooperation of the child and the individual experience of the examiner. In infants who are totally breast- or bottle-fed, adequate preparation can usually be obtained with the use of small-volume enemas and substituting clear liquids for breast- or bottle-feeding for 12 to 24 hours. In older children and adolescents, the best preparation is usually obtained with the use of colonic lavage solutions that contain a nonabsorbable solution of polyethylene glycol and electrolytes (PEG-ELS), which causes an osmotic diarrhea. The risk of dehydration is minimal because of the limited transmural flux of sodium and water. To be effective, a large volume of solution must be ingested over a relatively short period. In adolescents of adult size, the usual regimen is 240 mL every 10 minutes until the fecal effluent is clear. This often requires 3 or 4 L of solution. Smaller volumes are recommended for younger children ( Table 61.1 ). Occasionally, nasogastric administration may be necessary. The rate of nasogastric administration is 20 to 30 mL/min, or 1.2 to 1.8 L/h in an older child or adolescent. Appropriate volume reductions can be made for infants and smaller children.
| Weight of Child (kg) | Volume (mL) Each 10 min Until Passage of Clear Fecal Effluent | Maximum Volume (mL) |
|---|---|---|
| <10 | 80 | 1100 |
| 10–20 | 100 | 1600 |
| 20–30 | 140/ | 2200 |
| 30–40 | 180 | 2900 |
| 40–50 | 200 | 3200 |
| >50 | 240 | 4,000 |
Oral cleansing solutions are effective in more than 95% of the patients who drink adequate amounts of the fluid. An occasional patient may experience presumed allergic reactions manifested as dermatitis, urticaria, and rhinorrhea. Combination regimens have been evaluated in adults using bisacodyl in addition to PEG-ELS. Addition of bisacodyl may allow for a reduction in the volume of PEG-ELS required or allow for the use of split-dose regimens. , , Split-dose regimens of PEG-ELS that incorporate a portion of the preparation being given the evening prior to colonoscopy and the remainder given early in the morning of the procedure have been determined to be more effective and associated with increased patient tolerability in adults compared with a standard 4-L preparation administered the evening before colonoscopy. Studies show split-dosing preparations result in improved bowel preparation and resultantly increased sessile-serrated polyp detection rates. , PEG-3350 without electrolytes (e.g., Miralax, Merck & Co., Inc., Whitehouse Station, NJ, USA) in doses as much as 10 times higher than those recommended for standard treatment of constipation up to 255 g is emerging as the bowel preparation of choice in many pediatric centers. Recent evaluations of 1-day PEG 3350 along with sports drink bowel preparation regimen in children show that it is a safe, effective, and moderately tolerable bowel preparation regimen for colonoscopy in children. , Because of their hyperosmotic nature compared with PEG-ELS, which is iso-osmotic, minor electrolyte changes may be noted with these regimens even when mixed with sports drinks. Initially, this regimen involved ingestion of the PEG without ELS solution over several days. Use of a single-day or 2-day preparation of this type of solution has been reported, frequently in combination with administration of oral bisacodyl or enemas. , , Although likely to improve compliance because of increased palatability, these regimens appear to be less effective in terms of bowel preparation quality while demonstrating no significant reduction in adverse effects or enhanced polyp detection compared with standard PEG-ELS–based solutions. , Therefore, until regimens with comparable efficacy are identified, a variety of regimens will likely be used in pediatric and adolescent patients.
If the child cannot comply with a large-volume lavage preparation, most endoscopists use an alternative regimen of clear liquids for 48 hours, accompanied by magnesium citrate or milk of magnesia one or two nights before the procedure, with or without saline enemas administered the night before and the morning of the procedure to clear any residual stool ( Table 61.2 ).
| Product | Dose | Duration | Maximum | Additional Comments | Side Effects (Noninclusive) | References |
|---|---|---|---|---|---|---|
| PEG (Miralax) | 1.5 g/kg daily ÷ bid | 2 days | 51 g–136 g/day | ±Normal saline enema ±Bisacodyl Clears day before. Mixing beverage: patient choice |
Nausea, abdominal pain, vomiting, mild electrolyte changes (K, CO 2 , BUN, Cr) | , |
| PEG (Miralax) | 238 g in 1.9 L of Gatorade | 1 day | 238 g (age 8–18 years) | Clears day before | Nausea, abdominal pain, vomiting, mild electrolyte changes (K, CO 2 , BUN, Cr) | |
| Magnesium citrate | 1 oz per year of age | Evening before procedure | 10 oz | ±Saline enema | ||
| Magnesium citrate with X-prep | Age 2–5 years: 4 oz magnesium citrate and 2.5 oz X-prep | 1 dose on day before colonoscopy | ||||
| Age > 5 years: 6 oz magnesium citrate and 2.5 oz X-prep | ||||||
| Pico-Salax (picosulfate, magnesium oxide, and citric acid) (Canadian availability) | Age 1–5: ¼ sachet (mixed and dissolved in 150 mL water) 6 p.m. day prior to procedure, followed by ¼sachet (mixed and dissolved in 150 mL water) on the morning of procedure Age 6 to 12: ½sachet (mixed and dissolved in 150 mL water) 6 p.m. day prior to procedure, followed by ½ sachet (mixed and dissolved in 150 mL water) on the morning of procedure |
Evening before and day of procedure | Liberal clear liquids required day before; avoid in patients with risk of dehydration, electrolyte imbalance, kidney disease, or bowel obstruction | Dehydration and electrolyte imbalance | , | |
| Picosulfate, magnesium oxide, and citric acid: Prepopik | Age 18 and older 150 mL (5 oz) in early evening prior to colonoscopy (4–6 p.m.) followed by a second 150 mL (5 oz) 6 h later (10 p.m.–12 a.m.) in the evening prior to colonoscopy |
Evening before procedure | 10 oz total | Liberal clear liquids required after administration (40 oz after first dose and 24 oz required after second dose) and on day before procedure; avoid in patients with risk of dehydration, electrolyte imbalance, kidney disease, or bowel obstruction | Dehydration and electrolyte imbalance, including magnesium | |
| Bisacodyl (Dulcolax) | Age 2–5 years: 5 mg | Each day for 2 days before colonoscopy | Saline enema on morning of colonoscopy. May be associated with inadequate mucosal visualization | |||
| Age 5–12 years: 10 mg | ||||||
| Age ≥12 years: 15 mg |
Oral sodium phosphate is an alternative regimen, available in both liquid and tablet form. The cathartic action of oral sodium phosphate is due to its osmotic properties. Oral sodium phosphate solutions are generally safe and effective in most adult patients without significant comorbid conditions. , Aphthous ulceration of the colon has been observed following sodium phosphate bowel preparation. However, the cathartic effects of oral sodium phosphate may result in significant hypovolemia. , There are a number of case reports of serious adverse effects, related primarily to inappropriate dosing or noncompliance with the prescribed regimen of medication dilution or administration of additional fluids. , , In addition, acute phosphate nephropathy followed by chronic renal insufficiency in patients with normal precolonoscopy renal function has been reported following oral sodium phosphate preparation. Extra caution must be used in patients who are vulnerable to minor shifts in intravascular volume or transient increases in serum phosphate levels. , , This regimen is contraindicated in patients with renal disease, megacolon, bowel obstruction, ascites, or congestive heart disease or in patients who are on medications that affect renal function. Because of reported significant adverse effects with oral sodium phosphate regimens, significant caution must be exercised with utilization of these products for any indication in patients of any age, and the most current U.S. Food and Drug Administration (FDA) and manufacturer’s recommendations should be followed. Although there are no data regarding specific safety risks of oral sodium phosphate in children, black box warnings posted in 2008 from the FDA regarding its association with acute phosphate nephropathy have led to removal of this product as an over-the-counter (OTC) precolonoscopy bowel preparation, and some manufacturers recommend against the use in any child or adolescent younger than 18 years of age. , An additional warning was issued by the FDA in January 2014 of possible harm from exceeding the recommended OTC dose of sodium phosphate products to treat constipation, and therefore use of these products should be avoided.
More recently, a dual-action nonphosphate natural orange-flavored low-volume preparation containing sodium picosulfate and magnesium citrate (P/MC) was studied in a large randomized multicenter trial in adults. The picosulfate inhibits absorption of water and ELS while enhancing motility, whereas the magnesium works as a hyperosmolar laxative. Based on the Aronchick and Ottawa Scales of bowel cleansing, successful overall bowel cleansing was similar in adult patients receiving P/MC and patients receiving 2 L of PEG 3350 and oral bisacodyl tablets. Patient-reported acceptability and tolerability was significantly greater for the P/MC regimen. Sodium picosulfate with citric acid and magnesium oxide has been used in children for bowel preparation outside of the United States and is available for pediatric use in Canada. In one study, this regimen, which is typically administered in two divided doses in conjunction with a clear liquid diet for 24 hours, was comparable in efficacy and tolerability to PEG-ELS. Recently, a randomized study in Sweden compared the quality of bowel prep using PEG or sodium picosulphate (NaPi co ) in pediatric patients, with no significant difference in bowel cleansing quality detected according to the Ottawa Score, but with higher rates of acceptability and tolerability found in the NaPi co group. Thus NaPi co may be a recommended option for children ages 10 and older. Although not yet approved for use in pediatric patients in the United States, modification of this type of regimen may be an acceptable alternative to current bowel preparations in patients without renal insufficiency, bowel obstruction, cardiovascular issues, or in patients at risk for dehydration, if these results are validated in additional pediatric series.
A variety of other regimens with variable efficacy have been reported in children, including use of a combination of bisacodyl tablets, enema administration, and a clear liquid diet. , , A recent clinical report reviewed several pediatric bowel preparation regimens, including the mechanism of action, efficacy, and ease of use, and reported on their use among North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) members. Findings showed that overall, PEG 3350–based bowel preparation protocols are the most popular pediatric cleanout regimen.
To investigate quality measures specific to children undergoing colonoscopy, a prospective review was performed using a central registry known as Pediatric Endoscopy Database System–Clinical Outcomes Research Initiative (PEDS-CORI). Examination of key quality indicators including bowel preparation, ileal intubation rate, and procedure time was performed. This study showed significant variations and inconsistent documentation of bowel preparation among pediatric colonoscopists highlighting the need for developing quality measures specific to pediatric colonoscopy. A recent study evaluating colonoscopy quality outcomes in pediatric patients showed that in patients with failed cecal or terminal ileal intubation, a poor bowel preparation was the cause in 23% and 14% of patients, respectively. In addition, the study found that the presence of a training pediatric gastroenterology fellow during colonoscopy resulted in scant variability among terminal ileum intubation rates with 90% intubation success with a fellow present versus 94% without, although fellow presence did significantly increase the procedure duration.
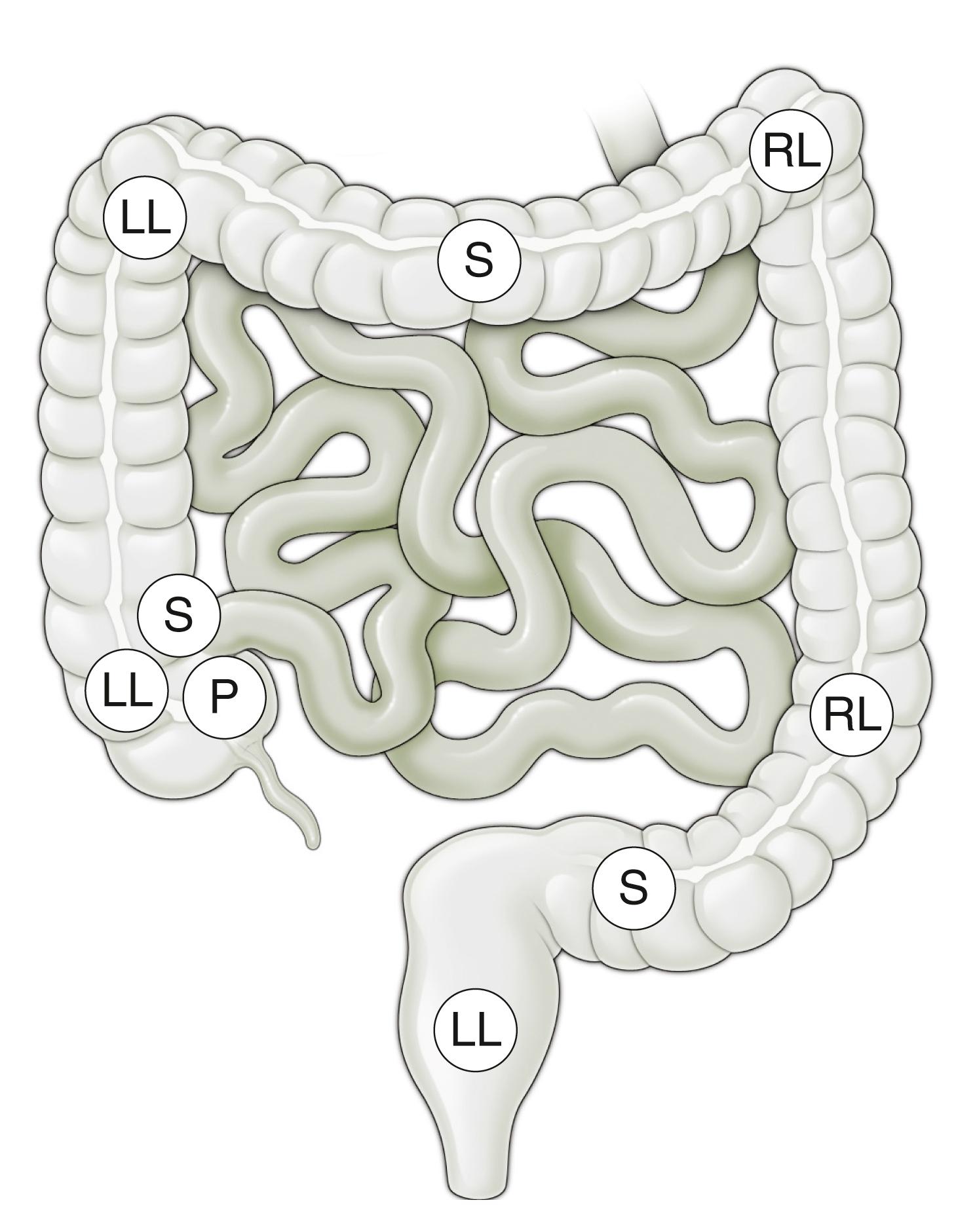
The small intestine and transverse and sigmoid colons are attached to mesentery and shift positions freely ( Fig. 61.3 ). Their excursion during a procedure is limited by the length of bowel and the length of their attachment. Disease processes, masses, prior surgical procedures, or adhesions may influence their configuration or limit their mobility. The ascending colon and descending colon are relatively fixed in the retroperitoneum. The rectum is also relatively fixed as it passes through the pelvic connective tissue, with only a short segment free within the peritoneal cavity.
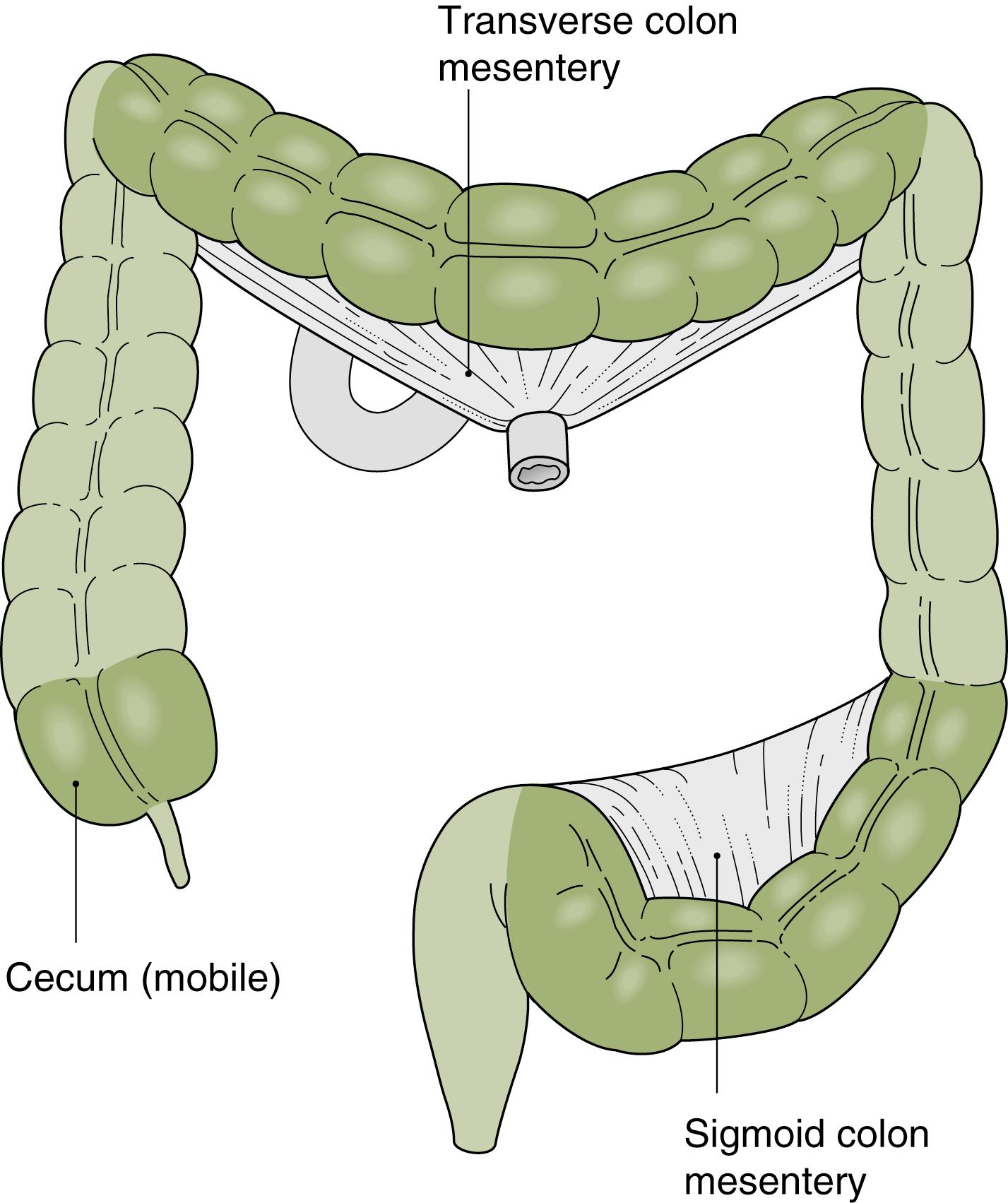
From the endoscopist’s point of view, the anus is first intubated, and then the rectum courses posteriorly to the coccyx and follows the gentle angle of the sacrum to the peritoneal reflection. Three “valves of Houston,” two on the right and the middle one on the left, are encountered. These are crescentic, or semilunar, folds and are due to tension by the longitudinal muscle fibers of the teniae coli. The colonic assumes a more circular pattern in the sigmoid and descending colon and a characteristic triangular pattern in the transverse colon.
The sigmoid colon is attached to a mesentery and is relatively mobile. The descending colon is usually straight and ends at the splenic flexure, which forms an acute angulation with the transverse colon, turning abruptly to the right and anteriorly. The hepatic flexure forms the distal margin of the transverse colon and forms another abrupt but inferior and posterior angle with the ascending colon. The transverse colon has a variable configuration and is suspended on a mesentery between the two fixed flexures. The ascending colon is fixed in the retroperitoneum and is relatively straight, ending in the mobile cecum. At the pole of the cecum, the tenia fuse to cause a marked cecal haustration, often forming a “crow’s foot” or “Mercedes Benz” sign. The appendiceal orifice is usually a crescentic opening slightly to the left of the midline ( Fig. 61.4 ). Occasionally, it may appear as a tubular diverticulum. The postappendectomy opening appears identical unless the appendiceal stump has been inverted.
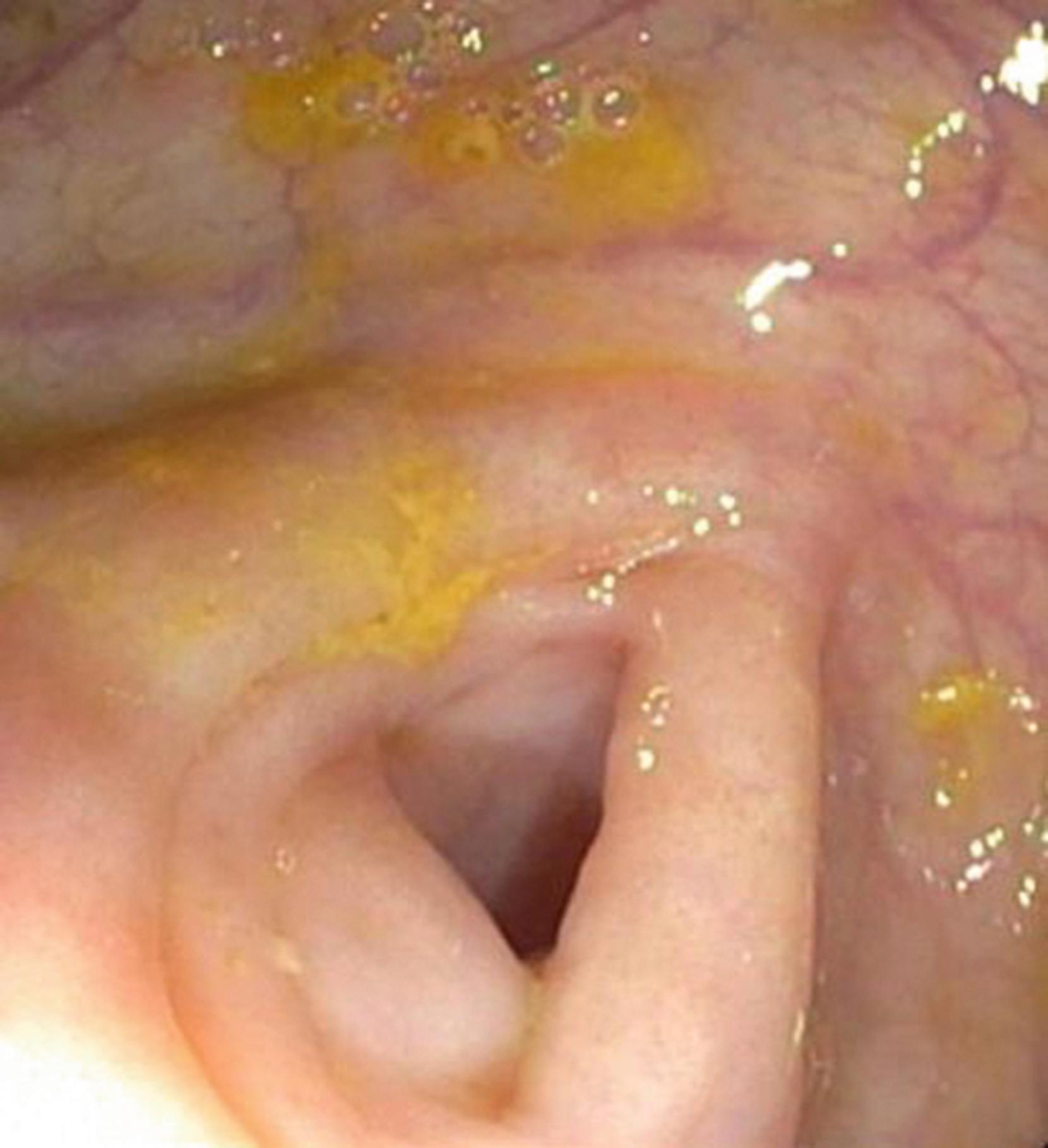
The ileocecal valve lies proximal to the cecal pole on the prominent ileocecal fold. It usually cannot be directly visualized but can be identified by a slight irregularity of the fold, with the ileum passing obliquely downward from its cecal opening. The ileum is characterized by the circumferential valvular conniventes and a predominance of lymphoid tissue, especially in younger children ( Fig. 61.5 ).
The endoscope is held in the left hand for right-handed individuals. The thumb is used to turn the large dial; the index finger and sometimes the middle finger are used to control the suction and air and water valves; and the remaining fingers hold the control handle. The insertion tube is held in the right hand. Some endoscopists advocate holding the insertion tube in the fingers like a pencil, rather than in the hand like a tennis racquet. The lateral wheel, which controls right and left tip deflection, is usually manipulated with the right hand by endoscopists with smaller hands but may be controlled by either the left hand or the right hand in endoscopists with larger hands. Endoscopists with large hands may also adopt the “left hand scope grip” whereby the insertion tube is placed between the fourth and fifth fingers of the left hand for portions of the colonoscopy. The advantage of this technique is that it allows simultaneous movement of the up/down dial with the left hand and right/left control with the right hand, which may be useful for detailed therapeutic work and passage of complex colonic turns.
The instruments used via the biopsy channel include needles for injection, biopsy forceps, cytology brushes, foreign body retrieval instruments, polypectomy snares, probes for cautery, and syringes for irrigation, and these are usually inserted with the right hand. Before initiating a procedure, the suction and air channels should be checked to ensure proper functioning.
Colonoscopy is traditionally performed in the left lateral decubitus position with the knees bent. Patients who are receiving general anesthesia may have the procedure performed in the supine frog leg position. A lubricant is usually applied to the tip of the instrument before insertion into the anus. In patients with active perianal disease, lubrication with viscous lidocaine may relieve some discomfort. It is usually necessary to reapply lubricant during the procedure because the lubricant tends to dry as the endoscope is serially advanced and withdrawn. The endoscopist may mistake the increased resistance secondary to poor lubrication as resistance coming from the more proximal colon.
The anus is inspected visually, examining for fissures, fistulas, or skin tags, and the instrument is inserted into the anus and advanced into the rectum. The anal canal is 2 to 3 cm long (in the older child and adolescent) and is angled toward the umbilicus. The instrument is inserted until there is an abrupt decrease in resistance, indicating that the tip has passed from the anal canal into the rectal ampulla. Following insertion, the scope tip usually comes to rest near the anterior wall of the rectum, because the junction of the rectum and anal canal forms an angle of approximately 90 degrees. If the lumen is not in view, a small puff of air or withdrawal of the endoscope a few centimeters will usually locate the lumen. Negotiating the colon is similar to the examination of the duodenum. Rather than simply “pushing,” the instrument should be subtly advanced using the dials for tip deflection and the techniques of air insufflation and suction, rotation or torquing of the insertion tube, external pressure applied to the abdomen, and changing the position of the patient. The elastic nature of the colon allows it to become long and tortuous when it is stretched by the colonoscope. Alternatively, it can be telescoped onto the instrument with little stretching by experienced endoscopists. The fundamentals of colonoscopy are the following. ,
Advance the endoscope under direct observation
Use as little air as possible while maintaining adequate visualization
Avoid forming loops; when loops are formed, reduce them as quickly as possible
Pull back and telescope the bowel onto the colonoscope whenever possible
The most prominent landmarks in the rectum are the fixed “valves of Houston.” These folds are accentuated haustral markings, and the lumen will veer toward the side of the fold. The endoscope is advanced with a series of short movements, jiggling motions, or torquing technique, depending on the skill and preference of the examiner. The junction of the rectum and sigmoid usually presents the first major problem and opportunity to form a loop. If the endoscopist simply advances the instrument, the force of the colonoscope is transmitted to the wall of the colon, with progressive distension of the bowel and its mesentery; the patient usually experiences pain, and the examiner feels increasing resistance to forward motion. This is often accompanied by the failure of the tip to advance despite insertion of the colonoscope. Paradoxic movement may occur when the tip recedes as the instrument is advanced and a larger loop is formed.
Early colonoscopes with limited tip deflection and fields of view required a “slide by” technique because the lumen could not always be kept in view. The instrument was advanced while the endoscopist had only a red field of view as the mucosa moved past the tip of the instrument. Advancement was halted when the mucosa was no longer slipping past the instrument tip or the mucosa took on a whitish hue, suggesting that compression of the bowel wall by the colonoscope was decreasing perfusion to the mucosa.
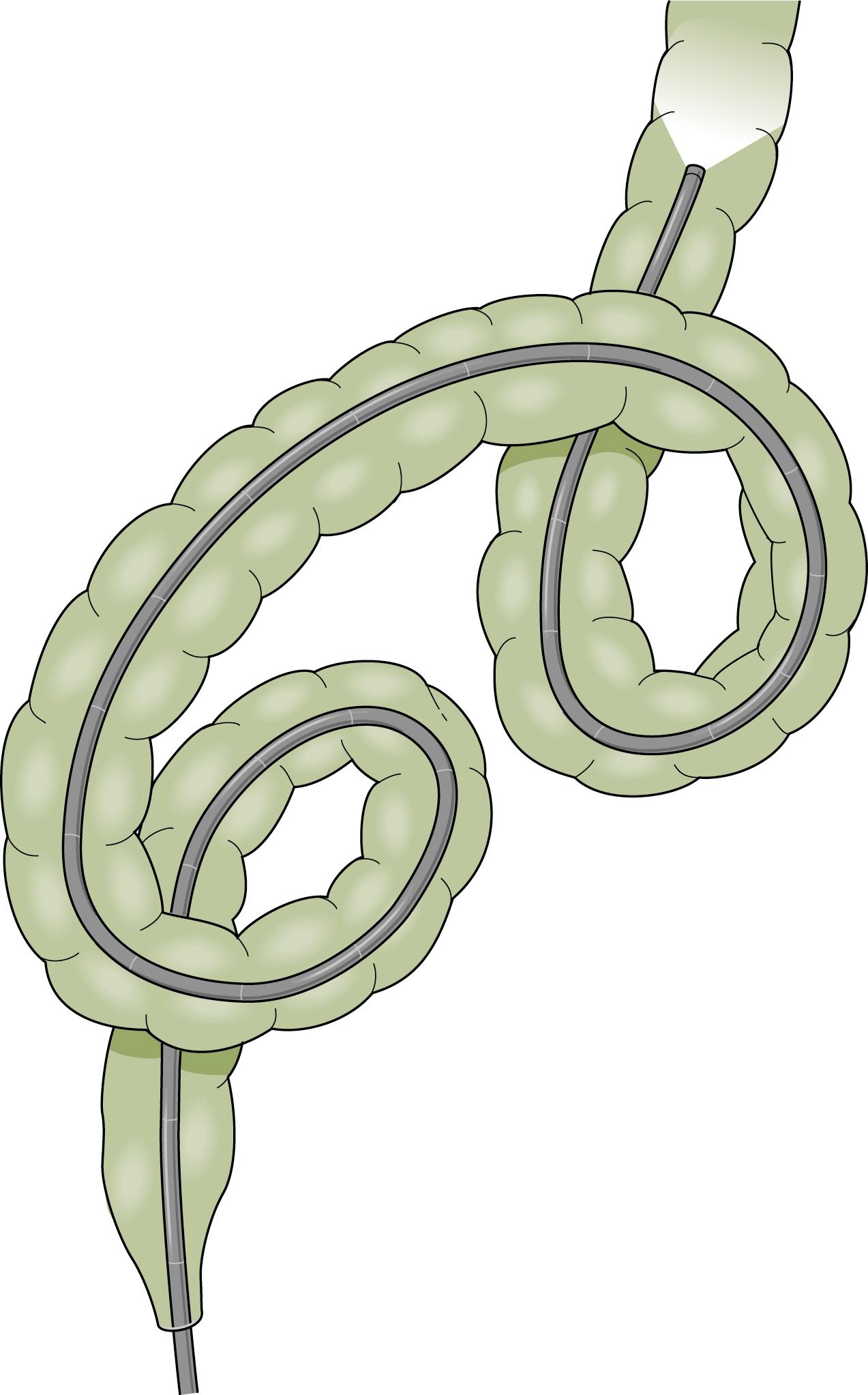
The instruments currently available reduce some of the technical difficulties; the instrument should be advanced under direct visualization and not by the “slide by” technique. The skillful negotiation of the rectosigmoid junction is the key to successful colonoscopy. The experienced endoscopist will anticipate many of the potential problems. Several loops may be formed in the sigmoid colon. Names have been given to various configurations as they were seen under the fluoroscope with the patient positioned in the anterior-posterior position ( Fig. 61.6 ). The α loop resembles the Greek letter α and may occur in either a normal or reversed configuration. The most common loop is the “N” loop, which is shaped like the letter N. Each of these loops has a similar appearance when viewed from the lateral position. The sigmoid colon is usually directed upward and then forms a caudad-directed convex loop in the anterior-posterior plane and a second cephalad convex loop with an oblique orientation to the anterior-posterior plane ( Fig. 61.7 ). Less common loops such as the double α loop can occur in a forward or backward orientation ( Fig. 61.8 ). Regardless of the anatomy in the individual patient, the endoscopist is unaware of the anatomy unless fluoroscopy or another form of scope position tracking, such as use of a three-dimensional (3D) imaging probe discussed later in this chapter, is used during the procedure. Because it is rarely needed to complete the procedure and exposes the patient, physician, and equipment to radiation, the experienced endoscopist will anticipate the potential problems rather than use fluoroscopy.

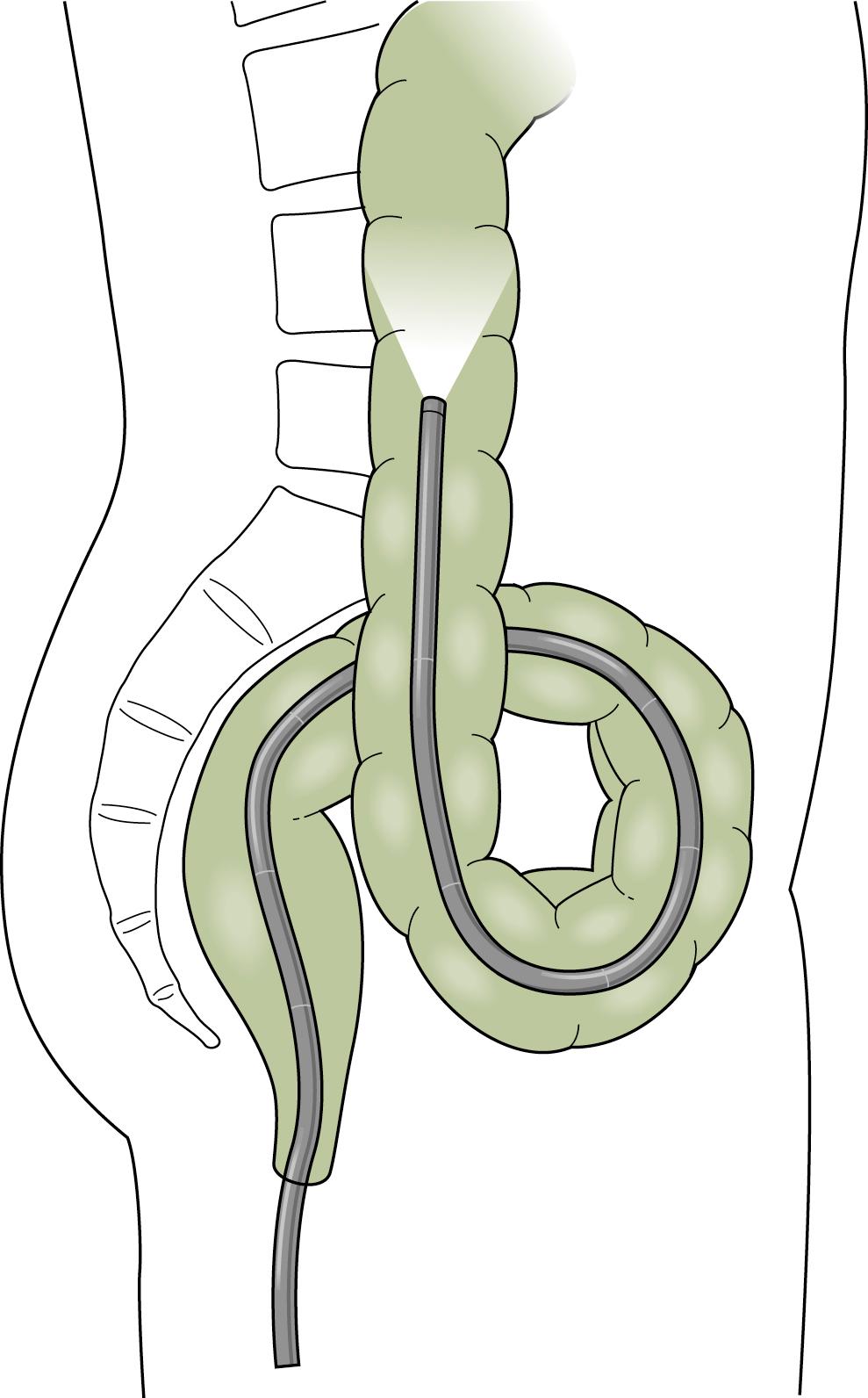
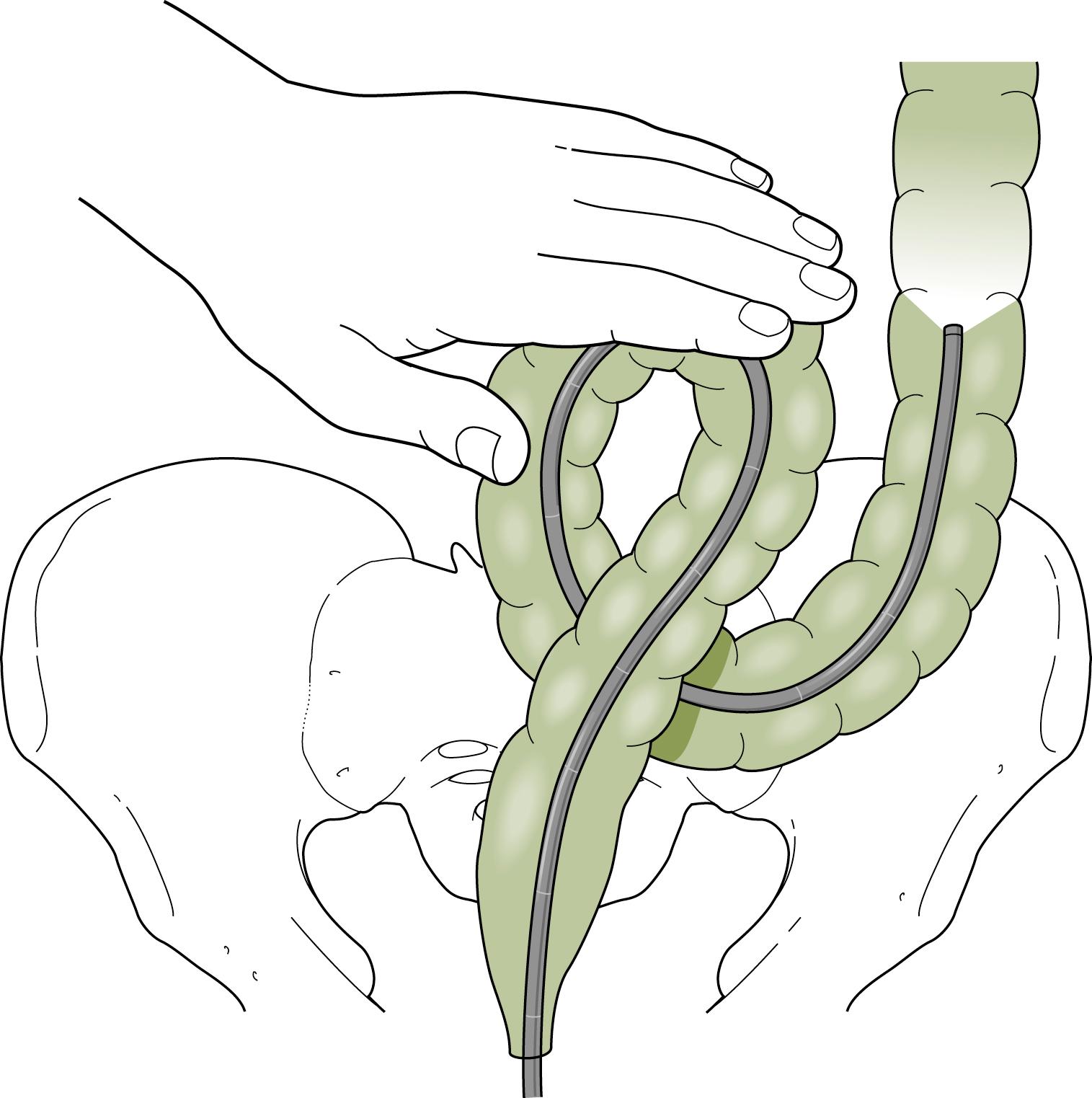
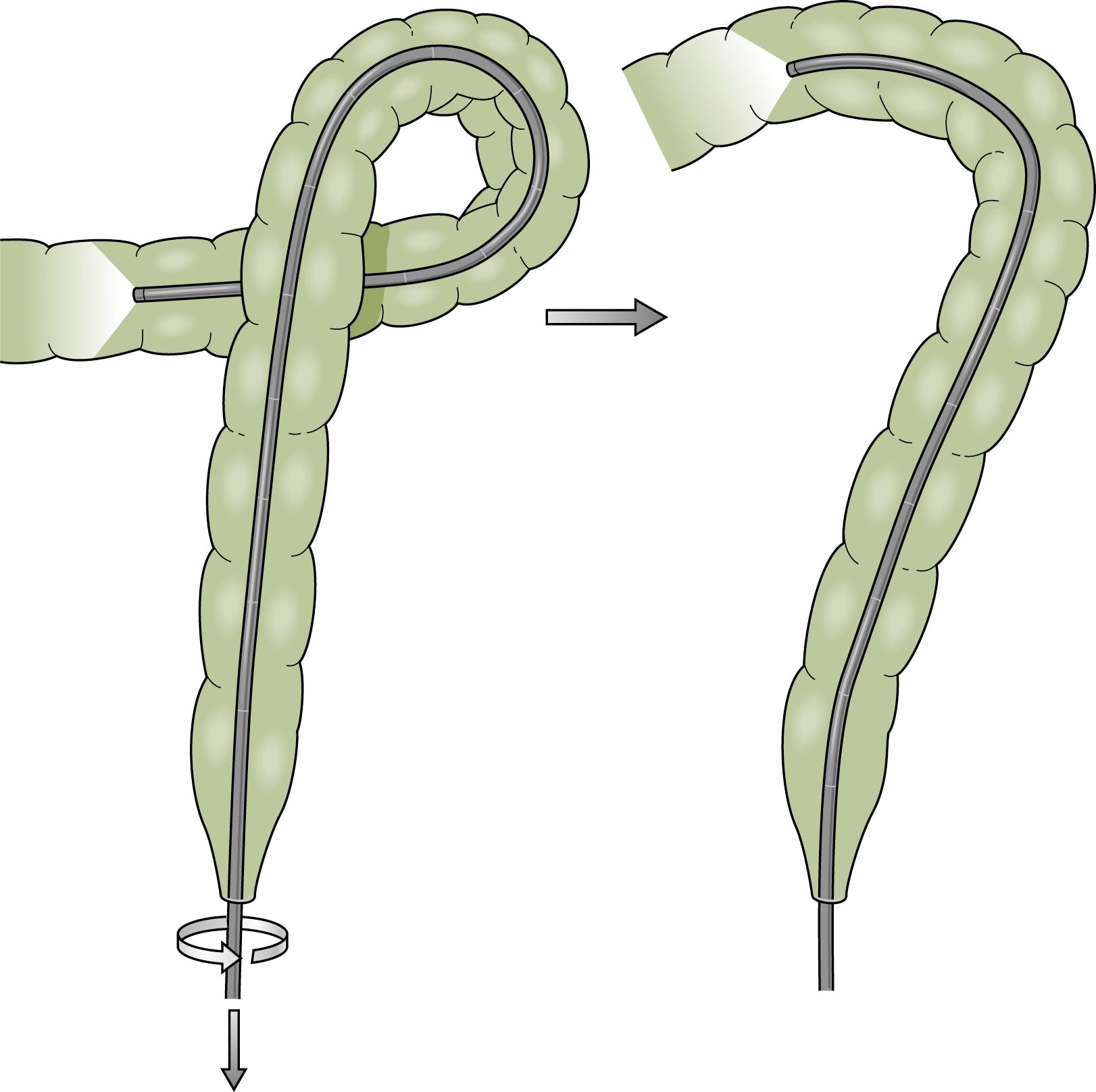
Advancing the endoscope through the sigmoid colon should be a smooth and coordinated procedure. This often involves torquing the instrument along its shaft with the right hand and using the left hand to control the tip deflection. Repeated backward and forward motions are usually needed to telescope the colon onto the instrument. As the tip of the instrument reaches a bend, the dials or a combination of dials and torque are used to rotate the tip around the bend into the lumen. Withdrawing the instrument will often straighten the loop and allow advancement to the next bend. To reduce an α loop, the scope is withdrawn so the tip lies just distal to the rectosigmoid junction and then is rotated 180 degrees ( Fig. 61.9 ). Failure of “one to one” movement, increasing patient discomfort, and increased resistance to insertion of the endoscope are usually signs of loop formation. Repeated attempts at loop removal are often necessary to traverse the sigmoid colon. In an occasional patient, it may be necessary to push through the loop and then reduce it after the instrument reaches the more proximal colon. Hand pressure by an assistant on the anterior abdominal wall may be beneficial during this maneuver ( Fig. 61.10 ). This should be done with caution only by an experienced endoscopist. A change in patient position may in some instances help the colonoscope tip advance, but some providers use this primarily when other measures are unsuccessful Fig. 61.11 .
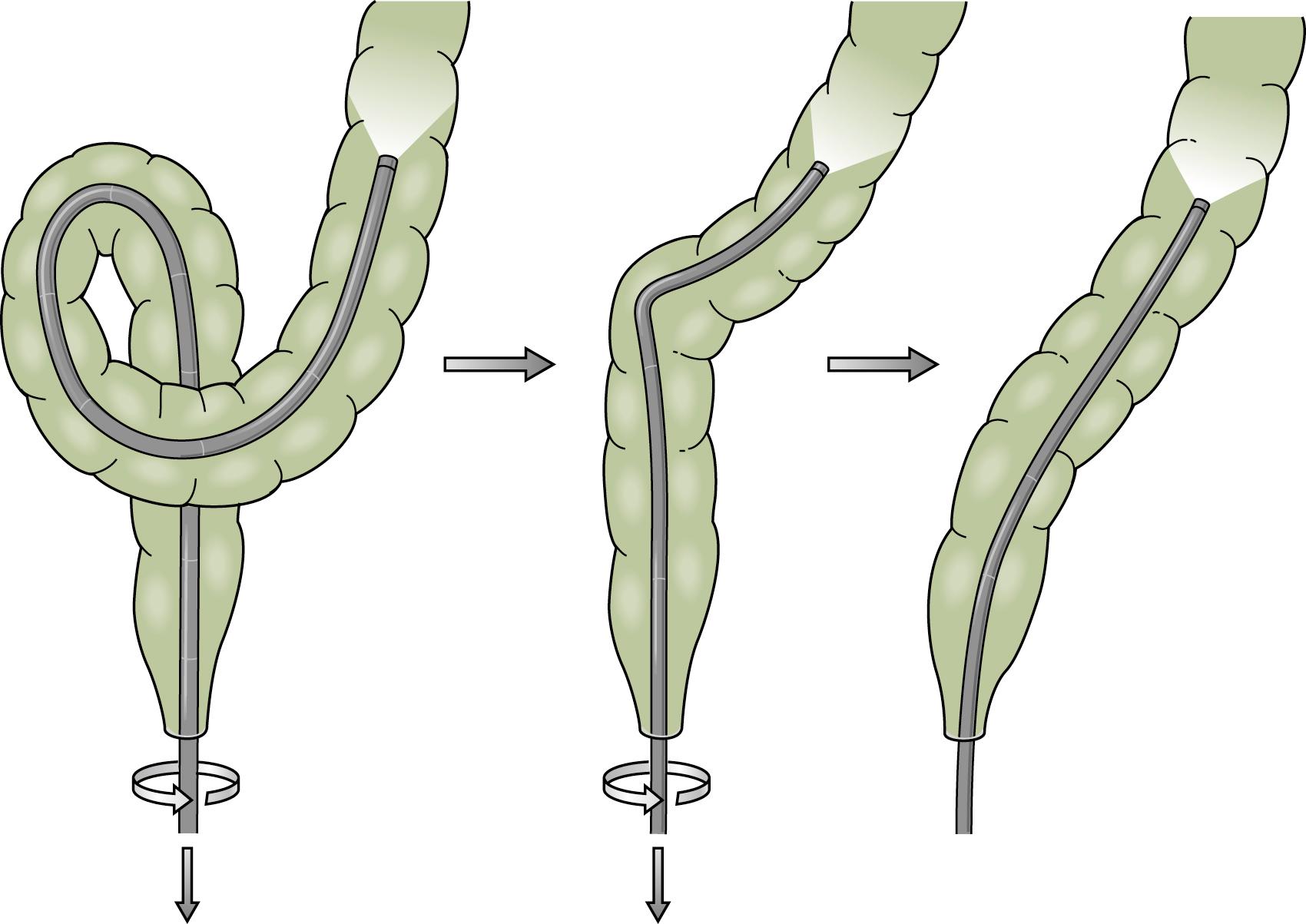
α Sigmoid loops, with the endoscope advanced into the distal descending colon, can be reduced by rotation (usually clockwise) and withdrawal. N loops can usually be removed with simple withdrawal. Occasionally an N loop has to be converted to an α loop for reduction to be successful, which can be accomplished by withdrawal and counterclockwise rotation. The conversion usually allows the endoscope to be advanced to the level of the descending colon, as most of the forward force on the N loop is directed at the apex of the loop and the forces in the α loop are more radially distributed. This permits advancement of the instrument despite the presence of the α loop. When the instrument reaches the descending colon, the loop can be reduced. Attempted examination of the proximal colon is usually limited if there is a residual sigmoid loop. Presence of two simultaneous loops preclude complete colonoscopic examination. At least one and preferably both loops should be reduced if possible, to achieve complete examination and ileal intubation.
There is no anatomic demarcation between the sigmoid and descending colon, but there is a tendency for the descending colon to be slightly smaller in diameter and to contain residual fecal debris because of its dependent position. Often a long tunnel view may be obtained, and if the sigmoid loops have been reduced, the endoscope can usually be advanced to the level of the splenic flexure without difficulty. A straight sigmoid colon is essential for successful maneuvering around the splenic flexure. Expert endoscopists have counseled to “master the left colon” as the key to successful colonoscopy with “time spent in the left colon saving twice as much time beyond the splenic flexure.” Paradoxic motion is often noted as the endoscope is advanced into the transverse colon. The configuration and forces resemble those of the N loop in the sigmoid colon. To reduce this tendency, the patient is often rotated from the left lateral to the supine position. Clockwise rotation of the endoscope may also be helpful. The relationship of the transverse colon and splenic flexure also varies with respiration. Deep sustained inspiration in the cooperative child may decrease the acute angle of the splenic flexure and allow easier passage of the endoscope. If the endoscopist is unsuccessful, the instrument should be withdrawn to ensure reduction of a sigmoid loop that may not have been reduced or one that may have reformed during maneuvers to negotiate the flexure ( Fig. 61.12 ). It is recommended that the splenic flexure should be a “stop point” for colonoscopy, when reached loop removal and straightening of the scope should be performed to relieve any tension in the scope. Multiple attempts by trial and error may be necessary to advance the instrument into the transverse colon.
The transverse colon is characterized by triangular haustral folds ( Fig. 61.13 ). The endoscope can usually be advanced without difficulty to the hepatic flexure, which is easily identified by the bluish discoloration of the outer wall caused by its close approximation to the liver. Occasionally a U-shaped loop is formed; this requires reduction before attempted negotiation of the hepatic flexure (see Fig. 61.6 ). If the transverse colon is redundant, a γ loop may form. Reduction of this loop is difficult because rotational forces are poorly transmitted and the loop is usually large. Reduction is less difficult if the instrument can be advanced into the ascending colon and then withdrawn to reduce the loop.
The fixed position of the splenic flexure acts as a fulcrum during attempts to negotiate the hepatic flexure. Suctioning in the region of the hepatic flexure usually draws the mucosa to the tip of the endoscope, and subsequently the tip can be maneuvered around the flexure into the ascending colon. If the flexure cannot be negotiated, withdrawal, manual rotation of the insertion tube, suctioning, manual compression of the abdominal wall, and/or sustained deep inspiration may be attempted to facilitate passage. If these measures fail, it may still be possible to advance the endoscope, but the patient usually experiences some discomfort. Rotation of the patient from the left lateral position to a supine or right lateral decubitus position may also facilitate scope advancement from the hepatic flexure into the ascending colon. Reduction of sigmoid looping is key to successful negotiation of the hepatic flexure.
To advance the endoscope into the ascending colon, the tip of the instrument is usually turned into the lumen with the dials. Additional procedures such as withdrawal and rotation may be necessary if paradoxic movement is encountered. Deep inspiration may be effective in “pushing” the instrument into the ascending colon. After the tip is aligned with the lumen, suctioning will also collapse the cecum toward the tip of the instrument. If there have been any difficulties with cleansing of the colon, they are most likely to become apparent in the ascending colon. Multiple washings of the mucosa and lens may be necessary to allow full examination of the mucosa. The cecum can be recognized by the typical anatomic landmarks of the triangular folds and the appendiceal orifice. The tip of the endoscope can often be identified in the right lower quadrant as it transilluminates the anterior abdominal wall. The ileocecal valve can be identified on the lateral surface of the prominent ileal fold as a slight irregularity of the valve contour. If loops have not been formed or have been successfully reduced, the opening of the ileocecal valve is usually in the 7 o’clock position (left lower quadrant) of the field ( Fig. 61.14 ). Occasionally, the valve can be directly intubated, but usually it must be approached indirectly. The tip of the endoscope is brought to a position parallel to the valve, and the dials are used to deflect the tip 90 degrees toward the valve ( Fig. 61.15 ). The maneuver is designed to catch the proximal lip of the valve with the tip of the endoscope. If the maneuver is successful, the lumen of the ileum will open, and adjustment of the tip will advance the instrument several centimeters. Several attempts may be needed to achieve success. It may be helpful to rotate the patient and suction the air from the cecum to draw the valve opening closer to the endoscope.
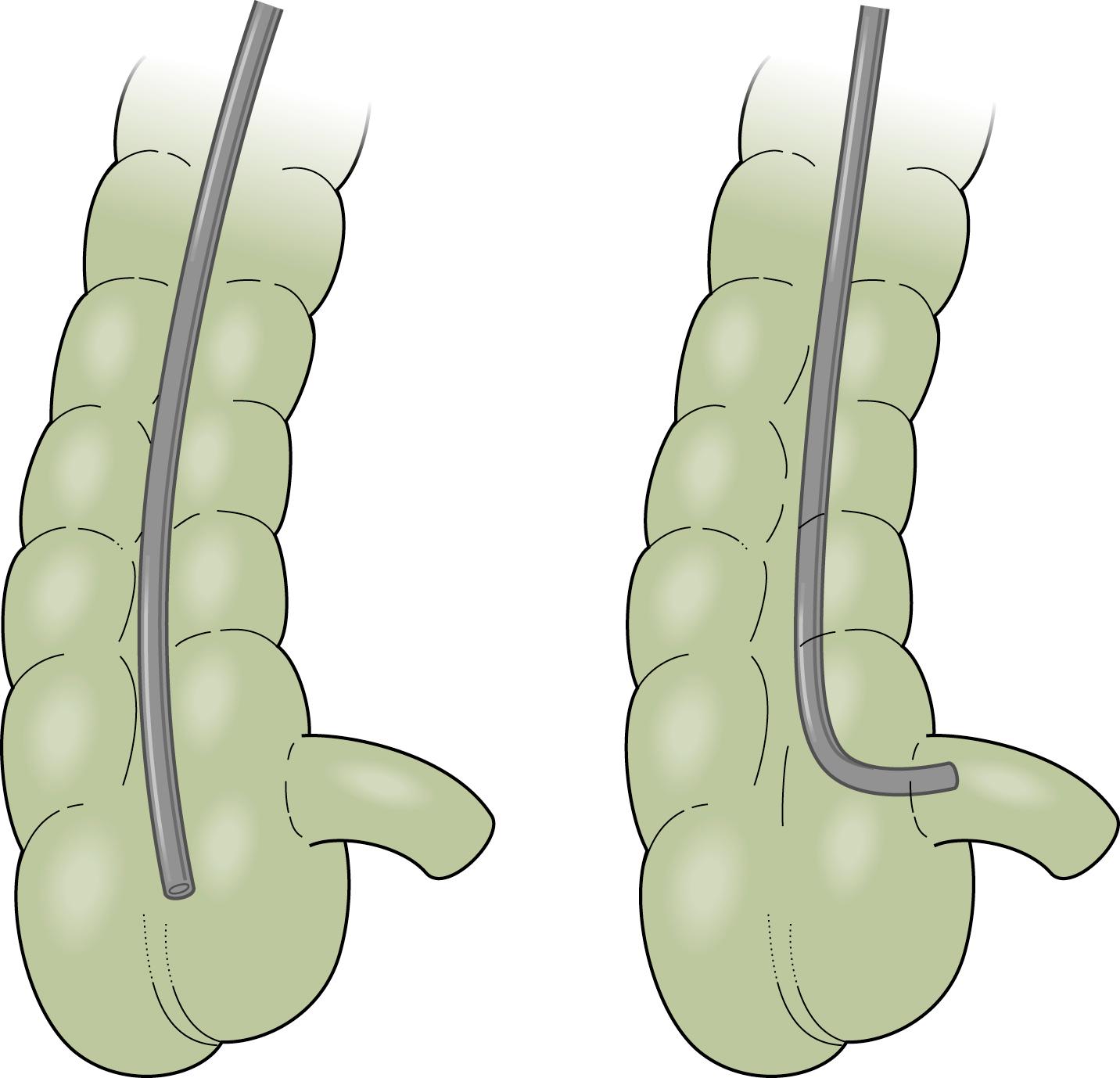
Following terminal ileal intubation, having the assistant gently hold the proximal end of the insertion tube while the endoscopist gently inserts the biopsy forceps may aid in keeping the scope within the ileum in cases of difficult intubation, as there is a tendency of the scope to come back to the cecum as the forceps are inserted as they straighten out the colonoscope.
The mucosa of the ileum is easily identified by the presence of valvula conniventes and lymphoid aggregates, which appear as 2- to 4-mm submucosal mounds of tissue with normal overlying mucosa. The presence of lymphoid aggregates in adults has been associated with immunoglobulin A (IgA) deficiency but is normal in children and even adolescents; the IgA level tends to decrease with advancing age. If the valve cannot be cannulated and pathologic evaluation is needed, the forceps can sometimes be gently advanced through the valve and biopsy material obtained.
Examination of the mucosa and therapeutic procedures such as polypectomy are conducted principally as the instrument is withdrawn. All areas must be routinely examined, paying special attention to the areas behind folds. This is important for mucosal evaluation for infectious organisms, such as enterobius vermicularis (pinworm), often identified within the cecum, appendix, and adjacent bowel ( Fig. 61.16 ). Observation of areas around flexures may not be adequate if the scope slips back rapidly, and the instrument may have to be advanced back through areas not well visualized. Observation of the distal rectum requires a retroflexed maneuver for complete examination. This is done by turning the dials to their maximum deflection in the midrectum. The endoscope is then slowly rotated to obtain an unobstructed view. The scope must be straightened before removal from the anus.
Colostomies and ileostomies can usually be examined as long as the openings are large enough to admit the tip of the instrument. In some cases an upper endoscope or the new ultrathin pediatric colonoscope is a more appropriate size for stomal examination. The length of bowel that can be examined is usually dependent on whether adhesions have formed. Advancement of the instrument around flexures in the bowel again involves repeated advancement, withdrawal, and torque of the insertion tube. Ileoscopy of the allograft after transplantation is typically performed using an instrument designed for upper endoscopy including “neonatal” smaller diameter endoscopes but usually with minimal or no sedation unless an EGD is also being performed. In the immediate postoperative period, the endoscope is advanced only 5 to 10 cm with minimal air insufflation to avoid anastomotic trauma. Subsequently, the endoscope can be advanced further with both random and directed endoscopic biopsies. Indications for small bowel ileoscopy after transplantation include routine surveillance, fever, bacteremia, increased stoma output, diarrhea, and bleeding. In addition to detection of rejection, this technique is helpful for detection of infection, such as Epstein-Barr virus and cytomegalovirus, and PTLD. , ,
There are a number of excellent references on colonoscopy tips in adults. , , Some of the caveats also potentially applicable to pediatric patients, which will be described later in the chapter, include maximizing sensory feedback from the insertion tube, problem solving in an algorithmic fashion, which includes changing solutions quickly, and consideration of use of CO 2 insufflation rather than air to reduce pain. ,
Routine flexible proctosigmoidoscopy is useful in some pediatric patients. In compliant, usually older children with a chronic illness such as ulcerative colitis, routine office examination to assess the extent and severity of disease when the history is in doubt or before initiating topical therapy for localized disease is useful. Examinations can be performed routinely with the same instruments that are used for full colonoscopy. When it is necessary to sedate pediatric patients for their comfort, it is usually appropriate to be prepared to perform a full colonoscopic examination. If pathology is encountered that is compatible with the patient’s symptoms and therapeutic options do not rest on establishing the extent of the disease, the procedure can be terminated without attempting to examine the entire colon. Flexible sigmoidoscopy with biopsy can be performed in the infant with rectal bleeding with suspected cow’s milk or soy protein allergy. Use of a small-diameter upper endoscope in the range of 5 to 6 mm and the brief nature of the examination often allow the procedure to be performed without sedation.
Anoscopy and rigid proctosigmoidoscopy are infrequently used procedures in the pediatric patient. Most pediatric gastroenterologists and their patients prefer flexible instruments when examination is required. However, the anoscope remains a useful office instrument for better visualization of the anal canal and identification of sources of bleeding. In addition, pediatric general and colorectal surgeons will occasionally use this type of instrument.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here