Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Colonic strictures are encountered with relative frequency in gastroenterology and colorectal surgery practices. The causes of these strictures are quite varied but the most common etiologies in adults include malignancy, diverticular disease, ischemic injury, inflammatory bowel disease (Crohn's disease), surgical anastomoses, and radiation injury. Less commonly, they can be seen in association with nonsteroidal antiinflammatory drug (NSAID)-induced injury, pancreatitis, endometriosis, and as a complication of infections such as amebiasis. A number of technical advances have made endoscopic management of these strictures possible. Endoscopic balloon dilation has been utilized since the 1980s, whereas endoscopic placement of self-expanding metal stents (SEMS) has become increasingly utilized since the early 1990s. Prior to any diagnostic or therapeutic endoscopy, patients with suspected colonic stricture or obstruction must be assessed with a careful physical examination and noninvasive radiographic imaging to exclude a surgical abdomen and the presence of luminal perforation.
Understanding the underlying etiology of a colonic stricture is critical to managing it appropriately. The diagnostic and therapeutic approach varies widely depending on whether a stricture is related to benign disease versus a malignancy. Inflammatory, ischemic, or anastomotic colon strictures can often be managed with endoscopic balloon dilation, but a malignant colonic stricture typically will not respond to conventional dilation therapy and will require either placement of a SEMS or surgical alternatives, such as diversion or resection.
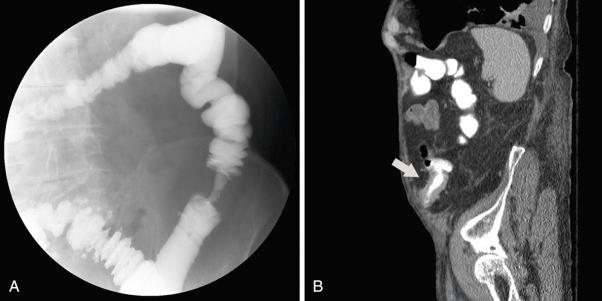
In addition to identifying the etiology of a colonic stricture, it is also critical to define the location and morphology of the stricture. Fluoroscopic imaging (typically a barium enema or Gastrografin [Bracco Diagnostics Inc., Monroe Township, NJ] enema if a leak is suspected; Fig. 40.1A ) or cross-sectional imaging (typically computed tomography scan [CT scan] with intravenous contrast in addition to oral or rectal contrast; see Fig. 40.1B ) are important in confirming and delineating a colonic stricture. These radiographic imaging modalities are important to determine the number and location of colonic strictures, the degree and length of stricturing, and whether a perforation has already occurred. Additionally, cross-sectional imaging (typically a CT scan) enables assessment of the surrounding soft tissue, lymph nodes, and more distant organs, which can be critical in cases of mass lesions, as well as in inflammatory bowel disease, which can be further complicated by fistulas or abscesses. Diagnostic endoscopy is also important in determining the etiology of a stricture as it can enable sampling for pathological diagnosis.
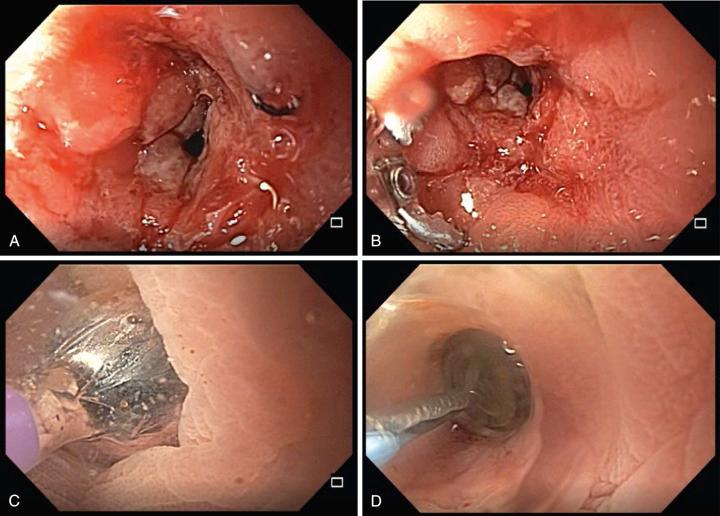
Dilating balloons are commercially available from various manufacturers. Balloon lengths range from 5.5 to 8 cm, and the dilating widths range from 6 to 20 mm, with various atmospheres of pressure being required to achieve a different balloon size depending on the balloon and manufacturer. Dilating balloon catheters come in gastroscope and colonoscope/enteroscope lengths and they can be purchased either with or without the ability to accommodate a guidewire. Most diagnostic gastroscopes (with a 2.8-mm accessory channel), and both pediatric and adult colonoscopes will accommodate these “through-the-scope” (TTS) catheters.
The commercially available dilating balloons can appear somewhat opaque when dilated in an insufflated lumen (particularly if they are filled with contrast for visualization under fluoroscopy). Replacing the air/CO 2 interface with water while pulling the dilated balloon snugly against the endoscope lens makes the balloon more transparent, allowing some visualization of the stricture lumen during dilation ( Fig. 40.2 , ). When the lumen proximal to the stricture is endoscopically visible, fluoroscopy is not required. However, when visualization is poor due to tight, long, or angulated strictures, fluoroscopic guidance facilitates passage of a guidewire and then the dilating balloon catheter. Fluoroscopy is also valuable as contrast can be injected after dilation to ensure that contrast remains intraluminal and that there is no extravasation of contrast to suggest a bowel perforation.
For strictures that are tight, long (> 4 cm), or otherwise complex, a “ball-tipped” catheter can be used to inject contrast, navigate the stricture (given its atraumatic tip), and pass a guidewire. Additionally, dilation of strictures with significant inflammation, evidence of fistula, or an associated abscess should be avoided. Whereas most endoscopists likely have experience with TTS balloon dilation, there are no well-studied standard protocols for how to perform balloon dilation. In general, communication between the endoscopist and the assistant who is inflating the dilating balloon is critical, particularly as resistance to expansion of the balloon allows real-time assessment of the adequacy and risk of the dilation. Once a balloon size is reached that results in significant resistance to moving the balloon, most endoscopists adhere to the “rule of 3s,” whereby dilation to three additional sizes provides treatment effect but also limits the risk of perforation (e.g., sequential dilation from 8 to 9 mm and then to 10 mm, etc.).
Examination of a stricture can be done through the balloon or by deflation of the balloon in between sequential dilations. Evidence of a significant mucosal rent, or particularly of visible muscle fibers, should be a signal to cease further dilation. For a tight stricture, repeated dilations could be done in 2 to 4 weeks, with a reasonable eventual goal luminal diameter of 15 to 20 mm. If a stricture fails to respond or remain patent over two to three serial dilations, an alternative approach, such as surgical resection, should be strongly considered.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here