Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Each day, 1200 to 1500 mL of ileal effluent enter the colon, 200 to 400 mL of which are excreted as stool. The colon mixes its contents to facilitate transmural exchange of water, electrolytes, and short-chain fatty acids and, in doing so, stores stool for extended periods. The mixing process involves rhythmic to-and-fro motions, together with short stepwise movements of contents, resulting in an overall net aboral flow rate that averages 1 cm/hr. When dehydration threatens survival, such as with water deprivation or severe diarrhea, the ability of the colon to reabsorb fluids is of major physiologic significance; appropriate motility patterns are important in achieving this function. The colon has the capacity to increase its fluid absorption 5-fold when required, but this ability is greatly impaired when transit is accelerated. Under normal circumstances, viscous contents are occasionally propelled aborally at a rapid rate, and if circumstances are appropriate, stool is evacuated under voluntary control. Thus, the colon is capable of showing a diverse range of motor patterns suited for particular physiologic functions. The generic term motility describes the range of motor patterns and the mechanisms that control them.
Common sensorimotor bowel symptoms (e.g., constipation, diarrhea, bloating, abdominal pain, rectal urgency) can arise from disturbances of ileocolonic delivery, colonic propulsion, or stool expulsion. Clearly these symptoms and dysmotility must be linked, although our current understanding of such linkages is limited, largely because of technical difficulties involved in studying the human colon. Because of differences between species, care is required in extrapolating data from animal studies to humans. For many years, intraluminal motility recordings in humans were obtained mainly from the rectum and sigmoid, but it is now clear that the motor activity of these distal regions is not representative of the colon as a whole. The contents of the colon become increasingly viscous distally, and this alteration complicates the relationship between propulsion and the contractile activity of the smooth muscle. Colonic movements are much less frequent and transit is considerably slower than in other regions of the GI tract. The highly propulsive stereotypical motor patterns associated with stool expulsion generally occur only once or twice daily. Hence, study of the motor patterns in the human cannot be achieved using contrast radiology. Prolonged recording techniques must be used to capture such infrequent motor patterns.
Simultaneous recording of colonic intraluminal pressure across multiple sites can only be achieved by manometry. Recent advances in high-resolution manometry have made it possible to record detailed pressure profiles throughout most of the colon. Measurement of colonic wall tone using a barostat provides information on nonocclusive colonic wall movements, but imparts no information about the spatiotemporal patterning of motility. Smooth muscle electromyography provides insight into the patterning of muscle activity but generally requires access to the muscular wall of the colon, which is problematic in humans for ethical reasons. Scintigraphy recorded over prolonged periods, with suitably high frame rates, can resolve discrete movements of the contents within the colon, but it is time-consuming and suboptimal for measuring actual wall motion. Within the last few years, MRI has been used to detail wall motion and flow of content in discrete sections of the colon over short periods of time (approximately 20 seconds). The recent success of videocapsule imaging of the GI tract, along with the desire to avoid radiologic studies, has driven a move to ingestible telemetric (wireless) devices to assess GI motility and transit; 2 such devices are the Wireless Motility Capsule (SmartPill, Given Imaging, Yokneam, Israel) and the 3D-Transit system (Motilis Medica, SA, Lausanne, Switzerland). The former can provide total colonic transit times, while the latter can be tracked in real time and thus provide detailed movements of the capsule within specific regions of the colon. In-vitro study of the cellular basis of motility using isolated specimens of colon faces fewer technical and ethical limitations, but data obtained at the cellular level, often under rather nonphysiologic conditions, can be difficult to extrapolate to the more complex integrated responses of the entire organ in vivo. Although we recognize the intrinsic limitations of all these measurement techniques, in combination they have allowed us to piece together a number of concepts that have provided important insights into the inter-relationships of muscle activity, wall motion, intraluminal pressure, and flow.
The human colon is just over 1 meter long and divided anatomically into the cecum; the ascending, transverse, descending, and sigmoid colon; and the rectum, which lies between the rectosigmoid junction and the anal canal. The outer longitudinal smooth muscle layer forms 3 thick, cord-like structures called the teniae coli, which are spaced evenly around the circumference of the colon. Between teniae, the longitudinal smooth muscle is much thinner, allowing the wall to bulge noticeably.
Irregularly spaced circumferential constrictions pinch the colon into a series of pockets called haustra that give the colon a sacculated appearance for much of its length. Some haustra are relatively fixed structures and can be readily seen during colonoscopy. Localized contractions of the circular muscle result in functional haustrations that move, disappear, and re-form during the mixing and propulsion of colonic contents. Myogenic activity alone, however, does not seem sufficient to explain haustration, and neural input likely contributes to their formation, especially during active propulsion of content.
The 3 teniae represent narrow bands of longitudinal muscle which fuse at the rectosigmoid junction to form a continuous outer longitudinal smooth muscle layer, which then continues down to the distal margin of the anal canal, insinuating itself between the internal and external anal sphincters. Throughout the length of the colon, the circular smooth muscle layer consists of thick bundles of cells separated by connective tissue septa. The internal anal sphincter consists of a thickening of the circular muscle layer over the last 2 to 4 cm of the anal canal.
Gross anatomy of the colon and anorectum are discussed in Chapters 98 and 129 , respectively.
Smooth muscle cells in the human colon, as in other muscular organs, are spindle-shaped, nucleolated cells with tapered ends. The surface area of the smooth muscle cell membrane is increased greatly by numerous caveolae, or small pits. Individual smooth muscle cells are connected mechanically to neighboring cells by intermediate junctions and electrically by gap junctions that allow ions and small molecules—those with molecular weights up to about 1000 kilodaltons (kd)—to diffuse between the cells, thereby ensuring that the cells are functionally coupled to one another. Smooth muscle cells therefore do not contract as individual cells but together in a large coordinated assembly called a syncytium .
Like smooth muscle throughout the GI tract, colonic smooth muscle shows spontaneous oscillatory mechanical and electric activity even when all neural activity is blocked. Three types of rhythmic myogenic activity have been identified in isolated preparations of human colon. These include rhythmic motor patterns at 3 to 6 cycles per minute (cpm), 10 to 12 cpm, and a slower pattern at 0.3 to 0.6 cpm. The corresponding myoelectric activity of the 3 to 6 cpm activity is the slow waves generated by a major pacemaker region located at the submucosal border of the circular muscle. This region produces larger-amplitude, slower myogenic oscillations in membrane potential (slow waves), which spread decrementally through the thickness of the circular smooth muscle by means of gap junctions. When slow waves reach a threshold for contractions, phasic pressure waves are often recorded by manometry. Slow waves occur throughout the human colon at a frequency of 2 to 4/min and propagate over short distances up or down the colon. Complex interactions occur as waves coming from different initiation sites collide, leading to mixing of contents with slow overall propulsion.
The corresponding myoelectric activity of the 10 to 12 cpm small rhythmic contractions are the myenteric potential oscillations (MPOs). MPOs are small-amplitude rapid oscillations with a frequency of 10 to 20/min that originate in the plane of the myenteric plexus. These small oscillations spread via gap junctions into both the longitudinal and circular smooth muscle layers, where they summate with slow waves and often reach the threshold potential to generate smooth muscle action potentials. The currents produced by pacemaker cells at the submucosal and myenteric borders decay as they spread through the thickness of the circular muscle layer. Cells in the middle of the circular smooth muscle layer therefore display complex spontaneous electrical activity consisting of a mixture of MPOs and slow waves, with superimposed smooth muscle action potentials. The myoelectric activity that corresponds to the slow myogenic contractions (0.3 to 0.6 cpm) has not yet been positively identified, but it is likely to involve the interstitial cells of Cajal (ICC) network near the myenteric border (ICC MY ).
These myogenic patterns do not function in isolation. Enteric neural activity generates motor output that is superimposed on the myogenic activity, adding considerably more variety to colonic motor patterns. Enteric neuronal output can simply augment the phasic myogenic contractions, bringing them to threshold level to drive simple rhythmic activity, or, alternatively, enteric neural circuits can generate powerful patterned contractions of much longer duration than those produced by slow waves. These contractions can propagate for long distances along the colon and include patterns such as high amplitude propagating contractions (see later, “Propagating Motor Patterns”) , the manometric equivalent of the mass movements described in radiologic observations. These are understood to primarily be the result of activation of polarized enteric neural pathways, and thus represent a form of “neurogenic peristalsis.”
The functions of the colon circular smooth muscle are quite well understood; in contrast, the role the longitudinal muscle layer plays in colonic motility, mixing, and propulsion is a matter of some controversy. The longitudinal muscle probably acts in synergy with the circular muscle, preventing excessive lengthening when the circular muscle contracts. It may also contribute to propulsion by pulling the colon over its contents so that circular muscle contractions gain more purchase.
Since 1991, ICC have been shown to play at least 2 important roles in the control of GI motility: control of myogenic activity and mediating or amplifying the effects of motor neurons on the smooth muscle apparatus. ICC are non-neuronal in origin and are derived from common progenitors of smooth muscle cells. Mutant mice and rats deficient in ICC have profoundly disturbed intestinal motility, an observation that provides insight into the roles of ICC in the human GI tract.
In the human colon, 3 types of ICC are recognized, usually by their immunoreactivity for c-Kit (CD117). They are named according to their locations: ICC in the plane of the myenteric plexus (ICC MY ), ICC near the submucosal plexus (ICC SM ), and intramuscular ICC located between the circular and the longitudinal muscle layers (ICC IM ). ICC MY and ICC SM form extensive networks along the colon and are electrically coupled to one another and to the smooth muscle layers by gap junctions ( Figs. 100.1 and 100.2 ). ICC MY are probably the pacemakers for the small, rapid (12 to 20/min) MPOs of longitudinal and circular smooth muscle layers. ICC SM are the pacemakers for the large-amplitude slow waves (2 to 4/min) that originate in the plane of the submucosal plexus; these slow waves have a powerful influence on the patterning of circular muscle contraction.
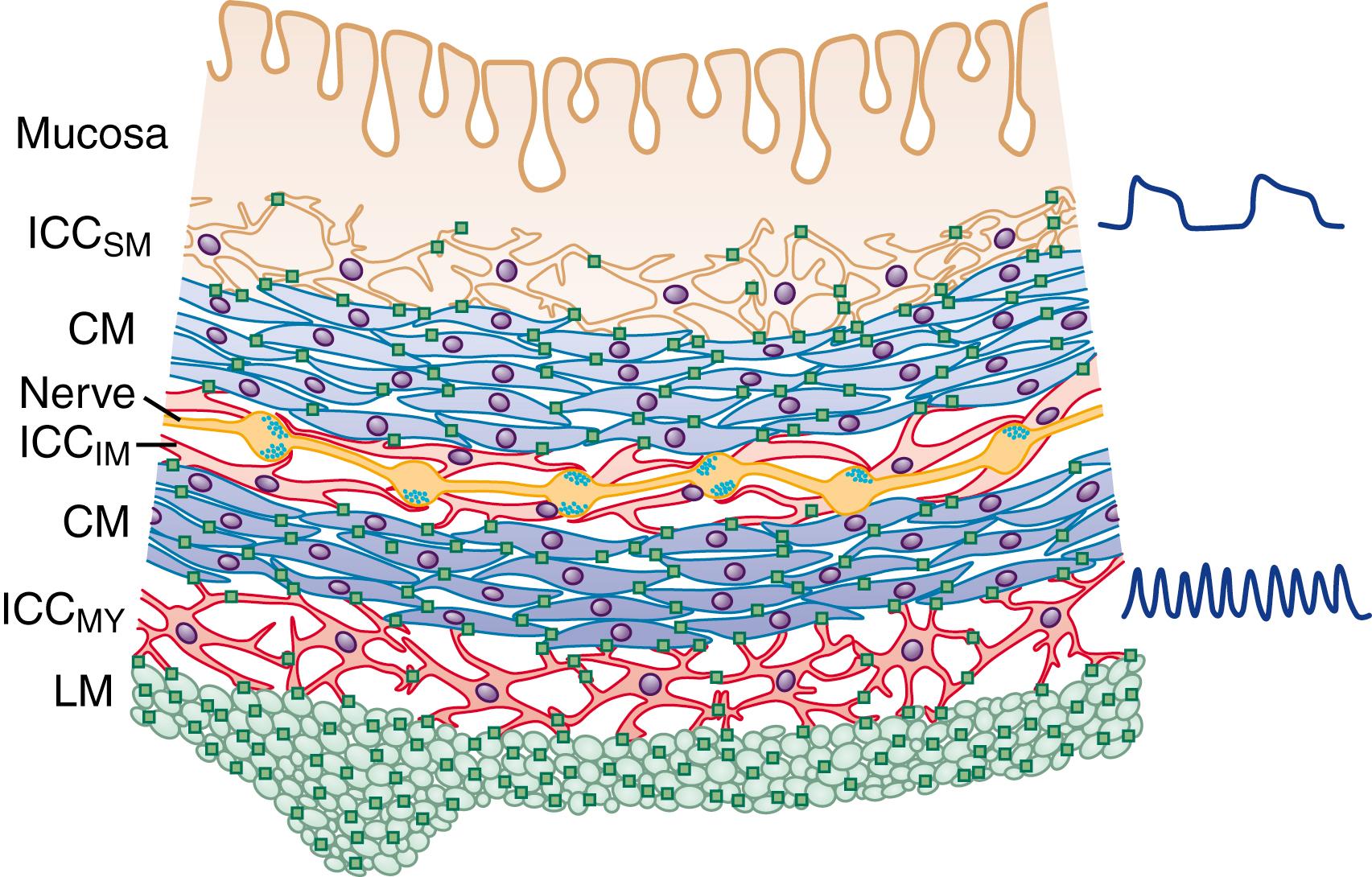
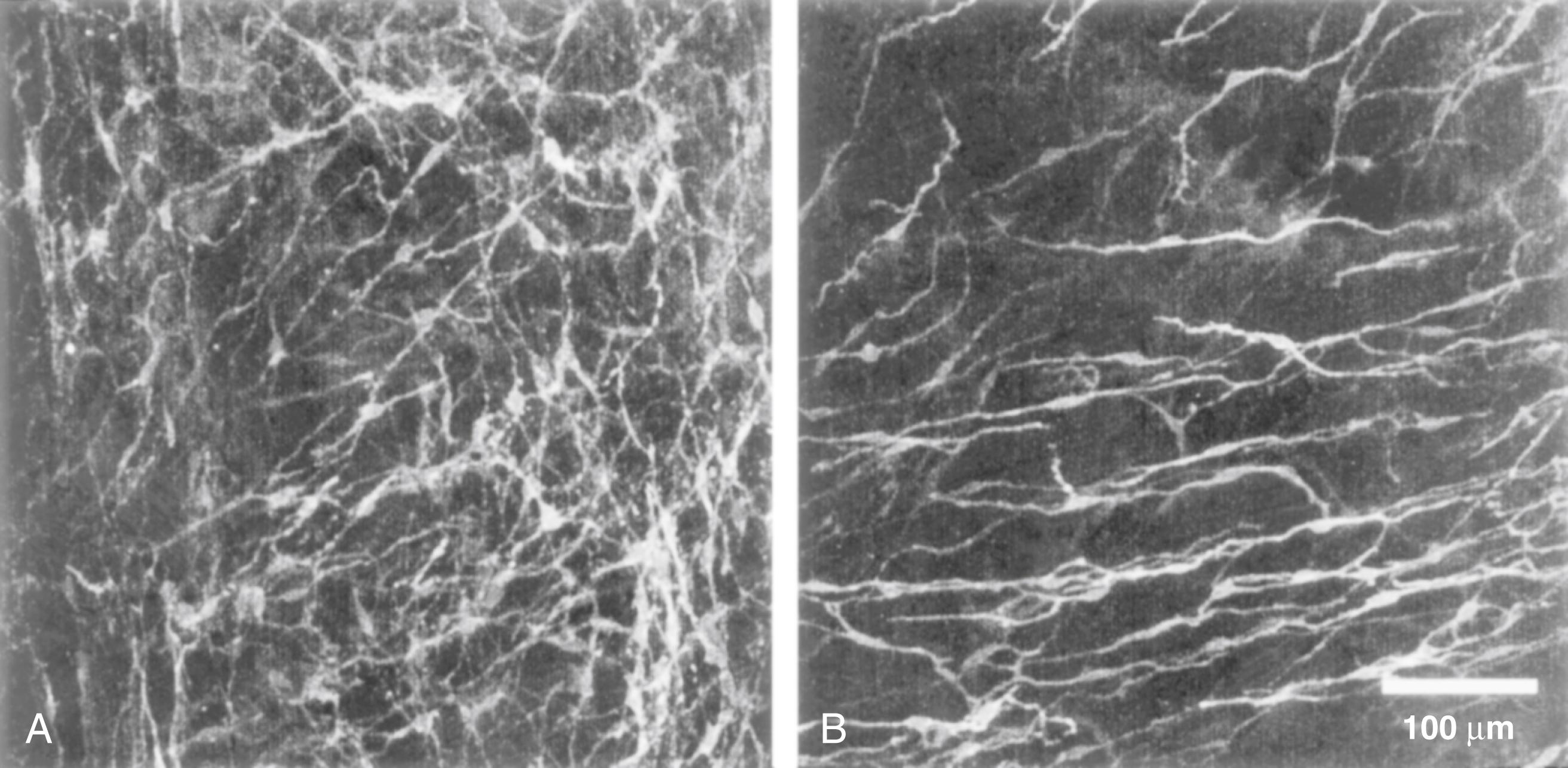
Although the exact ionic basis of rhythmicity in ICC MY and ICC SM that gives rise to MPOs and slow waves is not entirely clear, oscillations in membrane potential are an intrinsic property of both ICC MY and ICC SM . ICC IM are a major target of neurotransmitters released from the axons of excitatory and inhibitory enteric motor neurons. Acetylcholine and nitric oxide (ACh and NO), and probably several other motor neuron transmitters, evoke changes in the membrane potential of ICC IM , which then spread through the smooth muscle by means of gap junctions. ICC IM may also be involved in amplifying the slow waves as they spread through the muscle layers. Thus, these cells appear to be key players in integrating non-neuronal pacemaker activity and neuronal inputs to smooth muscle.
Recently, another cell type that likely contributes to colonic motility control has been identified in the human colon. These “fibroblast-like cells” are characterized by immunoreactivity for platelet-derived growth factor receptor alpha but not for the prototypical marker of ICCs, c-Kit. They form plexuses within the longitudinal and smooth muscle layers and are closely intertwined with networks of ICC. Like ICC IM , they are also closely apposed to axons of enteric motor neurons within the muscle layers. In animal models, these fibroblast-like cells are involved in inhibitory neurotransmission to the smooth muscle and appear to mediate the purinergic component due to ATP. The close association of smooth muscle, ICCs, and fibroblast cells has led to the suggestion that these should be considered to form a SIP (smooth muscle/ICC/platelet-derived growth factor receptor alpha) syncytium.
The discovery that cellular mechanisms long considered to be the properties of smooth muscle cells are actually mediated by ICC may have important clinical implications. In the distal bowel, reduced numbers of ICC or reduction in the total volume of ICC have been associated with anorectal malformations, colonic manifestations of Chagas disease, normal aging, and possibly some cases of slow-transit constipation. Some reports have suggested that the density of ICC may be reduced in the aganglionic segments of colon in Hirschsprung disease and that this deficit might contribute to diminished propulsive activity; this finding, however, has not been consistent between studies.
Smooth muscle in the colon does not just contain 3 types of cells. In addition to smooth muscle cells, ICCs, and fibroblast-like cells, there are also axons of neurons and supporting glial cells, an extensive vasculature, true fibroblasts, and a range of immune cells including a substantial population of resident macrophages. These macrophages play a role in the paralytic ileus that often follows surgery on the bowel, through the release of chemoattractants and prostaglandins which activate extrinsic sensory nerve endings (see Chapter 124 ).
The membrane of colonic smooth muscle cells contains a variety of ion channels, including multiple potassium, calcium, chloride, and nonselective cation channels. Although the exact physiologic roles of many of these ion channels are unknown, the high-threshold voltage-operated calcium channels ( l -type calcium channels) play a crucial role in colonic muscle contractility. These channels open when the membrane potential of smooth muscle cells is depolarized beyond a voltage threshold, and they are responsible for the rapid upstroke of smooth muscle action potentials. The influx of calcium through l -type calcium channels during action potentials is a major trigger for activation of the contractile apparatus. It is therefore not surprising that pharmacologic blockade of l -type calcium channels by dihydropyridine drugs like nifedipine can substantially reduce the contractility of colonic smooth muscle. Release of calcium from intracellular stores, which is triggered by excitatory neurotransmitters, may also play a role in muscle contraction.
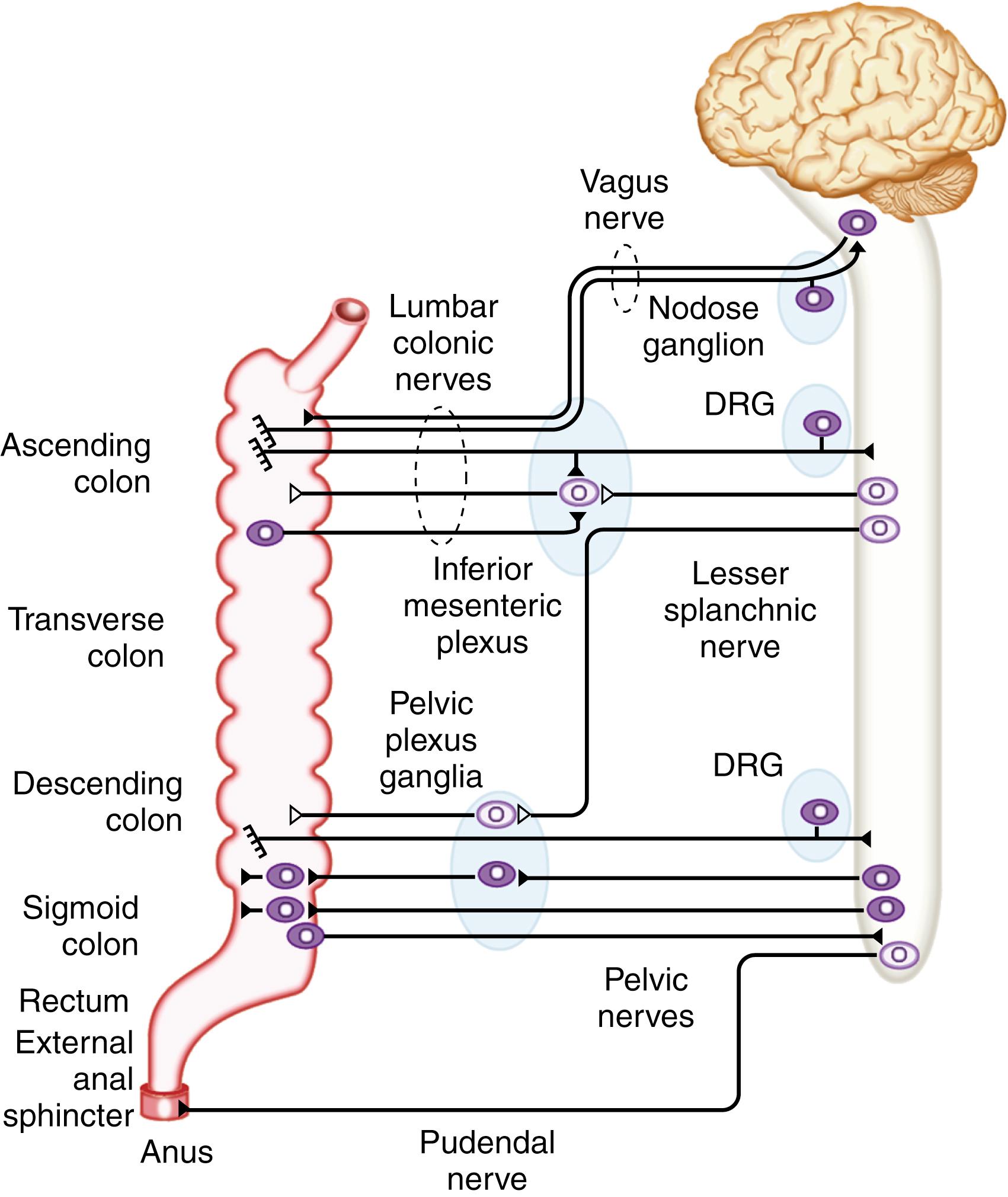
Direct neuronal control of colonic motility is mediated mostly by the ENS. Although the ENS is capable of autonomously driving a diverse repertoire of motor patterns, its functions are often modulated by sympathetic, parasympathetic, and extrinsic afferent pathways ( Fig. 100.3 ). In terms of numbers of nerve cells, the ENS is by far the largest component of the autonomic nervous system, with considerably more neurons than those of the parasympathetic and sympathetic divisions combined. The nerve cell bodies of the ENS are located in plexuses of myenteric ganglia (Auerbach plexus) that lie between the longitudinal and the circular muscle layers of the muscularis externa, or in the submucosal ganglia that lie between the muscle and mucosa ( Fig. 100.4 ).
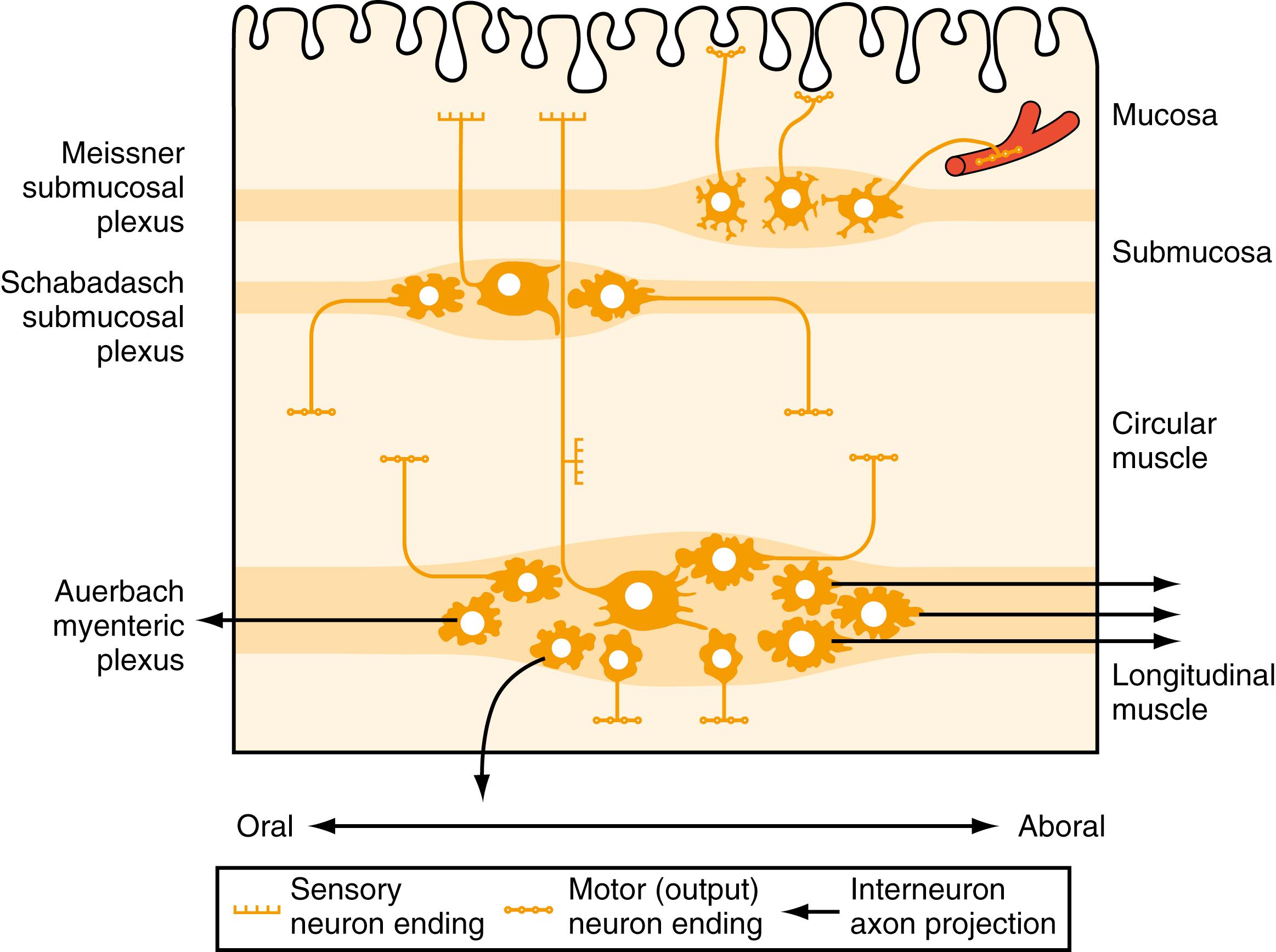
The submucosal plexus comprises at least 2 networks: Meissner plexus , which lies closer to the mucosa, and Schabadasch plexus , which lies adjacent to the circular muscle; some authors have identified an additional intermediate plexus. Internodal strands that contain hundreds of axons run within and between the different plexuses. Finer nerve trunks innervate the various target tissues of the intestinal wall, including the longitudinal muscle layer, circular muscle, muscularis mucosae, mucosal crypts, and mucosal epithelium. Within the ganglia of each plexus, different functional classes of enteric nerve cell bodies are intermingled, and differences in the proportions of cell types between the plexuses have been observed. It has become clear that an exquisite degree of organization is characteristic of the ENS, each class of nerve cell making highly specific and precise projections to its particular target.
The ENS uses many transmitters in addition to the major transmitters (ACh and NO), including tachykinins, purines, numerous other modulatory peptides, and some amines. Many other substances released from neural and non-neural cells also modulate neuronal and muscular excitability, including gaseous mediators (NO, carbon monoxide, and hydrogen sulfide) and, in inflammation, prostanoids, cytokines, purines, bradykinin, H + ions, and neurotrophins.
Much of the motor and secretory activity of the intestine can be conceptualized as a series of reflexes evoked by mechanical or chemical stimuli. These reflexes involve activation of enteric primary afferent neurons (PANs), integration by interneurons, and execution of appropriate responses by motor neurons. The first neurons in these reflex circuits are PANs (sometimes called “sensory” neurons, although they do not give rise to conscious sensation). These neurons are located in both myenteric and submucosal plexuses and characteristically have several long axonal processes. Some PANs fire action potentials in response to stretch or tension in the bowel wall; others are activated by chemical or mechanical stimuli of the mucosa. These mucosal stimuli probably work at least in part by activating specialized enteroendocrine cells (e.g., serotonin-containing enterochromaffin cells) in the mucosal epithelium. The PANs then release synaptic transmitters, such as ACh or tachykinins or other peptides, to excite other classes of enteric neurons in nearby ganglia. Enteric PANs also make excitatory synaptic contacts onto other neurons of their own class, so that they fire as coordinated assemblies.
Enteric motor neurons typically have smaller cell bodies than afferent neurons, with a few short dendrites and a single long axon. Separate populations of motor neurons innervate the circular and longitudinal muscle layers. Excitatory motor neurons synthesize ACh, which they release from their varicose axons in the smooth muscle layers; some also release the tachykinin peptides substance P and neurokinin A, which also excite smooth muscle. Typically, axons of excitatory motor neurons project either directly to the smooth muscle close to their cell bodies or orad for up to 10 mm. Once in the smooth muscle layers, the axons turn and run parallel to the smooth muscle fibers for several millimeters; they branch extensively and form many small varicosities, or transmitter release sites, closely associated with ICC IM and fibroblast-like cells.
Inhibitory motor neurons are typically slightly larger than excitatory motor neurons and also have short dendrites and a single axon, but unlike excitatory motor neurons, they project aborally to the smooth muscle layer for distances of 1 to 15 mm in the human colon. Once the axon reaches the smooth muscle, it branches extensively to form multiple varicose release sites. Inhibitory motor neurons release a cocktail of transmitters that inhibit smooth muscle cells, including NO, ATP (or a related compound like NADH), and peptides like vasoactive intestinal polypeptide and pituitary adenyl cyclase-activating peptide. The varicose transmitter release sites of inhibitory motor neurons are also associated with ICC IM , as are the release sites of excitatory motor neurons. Interstitial cells probably mediate a large component of the electrical effects on smooth muscle of neurotransmitters released by enteric motor neurons. Inhibitory motor neurons are usually tonically active, modulating the ongoing contractile activity of the colonic circular smooth muscle. Inhibitory motor neurons are particularly important in relaxing sphincteric muscles in the ileocecal junction and the internal anal sphincter. Typical polarity of excitatory and inhibitory motor neurons to human colonic smooth muscle is illustrated in Fig. 100.5 .
When a region of colon is stimulated, such as by a bolus that distends it, enteric PANs are activated. These neurons then activate excitatory and inhibitory motor neurons that, because of their polarized projections, cause contraction of the muscle orad to the bolus and relaxation aborally. These effects tend to propel the contents aborally. From the new position of the bolus, another set of polarized reflexes is triggered, and peristaltic propulsion results. The ascending excitatory reflex and the descending inhibitory reflex are sometimes called the “law of the intestine.” These reflexes spread farther than is predicted by the projections of the excitatory and inhibitory motor neurons, because interneurons are also involved in these neuronal pathways. Ascending cholinergic interneurons in the human colon have axons that project up to 40 mm orad and extend the spread of ascending excitatory reflex pathways. In addition, several classes of descending interneurons are present in the human colon, with axons that project 70 mm or further aborally. Some of these interneurons are involved in spreading descending inhibition along the colon, but others are likely to be involved in the propagation of migratory contractions. It is also likely that some interneurons are themselves stretch sensitive, thereby functioning as PANs. In addition to the sensory neurons, interneurons, and motor neurons, viscerofugal nerve cells project to the sympathetic prevertebral ganglia, vasomotor neurons innervate blood vessels, and secretomotor neurons stimulate secretion from the colonic epithelium.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here