Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
High altitude or space environments present a number of extreme physiologic challenges that must be overcome in order to survive.
Given sufficient time, humans can adapt to both hypobaric hypoxia and microgravity.
Lack of adaptation can lead to environment-specific illnesses, such as acute mountain sickness, high-altitude pulmonary edema, decompression illness, or the acute worsening of comorbid conditions.
These conditions can rapidly become fatal if not treated appropriately (e.g., with either descent to lower altitudes or returning to the Earth’s surface).
Providing critical care or anesthesia in such environments is further complicated by their extreme levels of remoteness.
Exploratory missions to such environments depend on the development and vetting of robust and simple health care protocols.
An estimated 140 million individuals live at altitudes over 2500 m, whereas sojourns to altitude are undertaken by large numbers of individuals each year for leisure, work, and religious reasons. It can be expected that a significant proportion of these individuals will require medical care, either directly related to altitude or for any other reason. As such, there is a clear need for medical practitioners to appreciate the specific environment, physiology, and pathologic conditions at altitude.
At high altitude, a number of environmental changes may be observed. These include decreased temperature, increased ultraviolet exposure and, particularly in mountainous environments, remoteness combined with challenging access and egress, and challenging weather patterns. Overall, the area of primary focus to the critical care and anesthetic practitioner is hypobaric hypoxia and the resultant physiologic changes associated with altitude exposure.
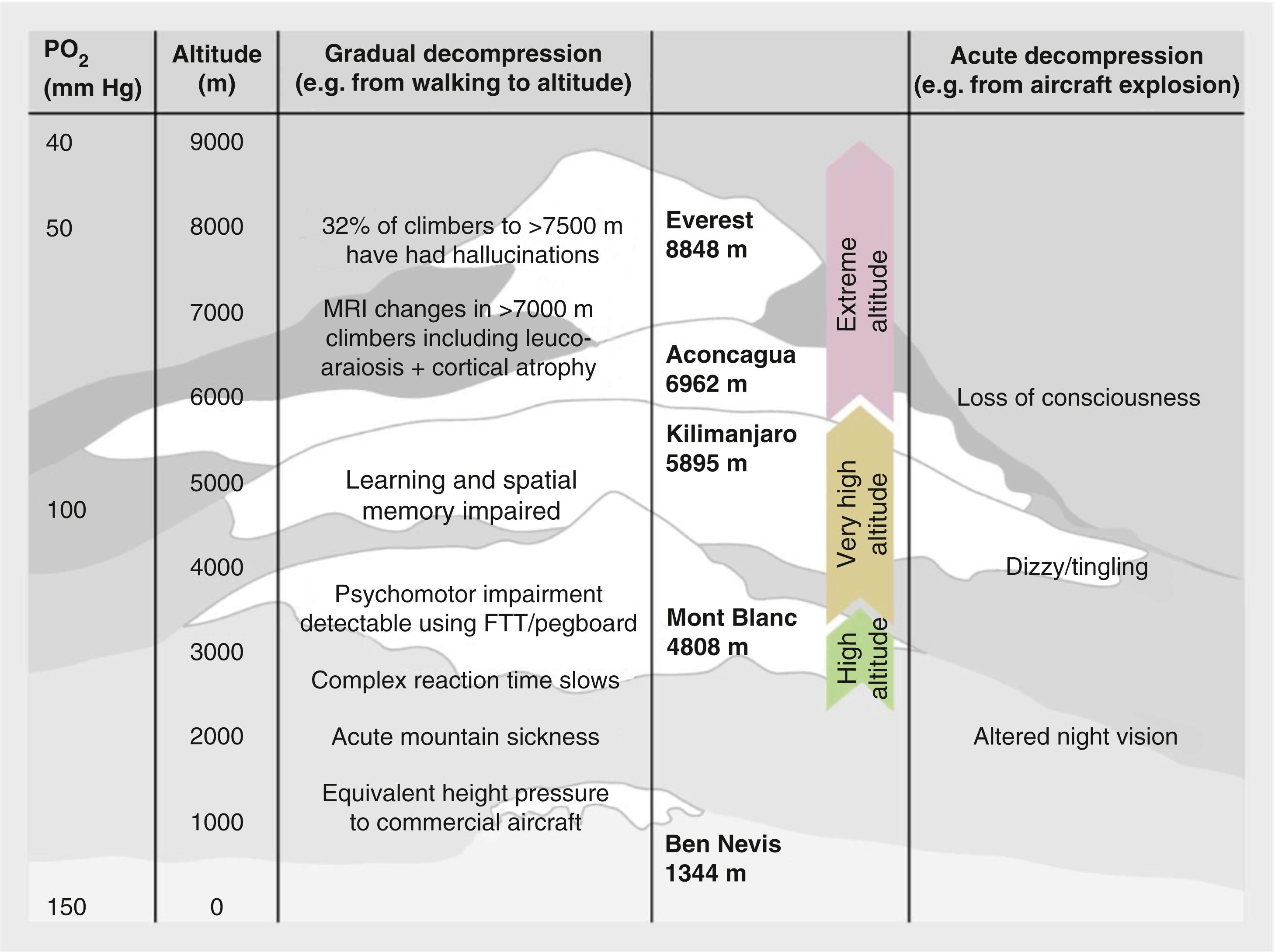
As altitude (i.e., vertical height above sea level) increases, the barometric pressure (P B ) decreases in a nonlinear manner ( Fig. 74.1 ), resulting in a P B at the summit of Mount Everest (8848 m) approximately one third that of sea level. The relative concentrations of gases, including oxygen, remain static but because of the falling P B, the partial pressure of each gas decreases. The resultant hypoxia is of great clinical significance and results in many physiologic changes. These physiologic changes vary with the time course of exposure and a number of long-term adaptations in high-altitude populations have been observed. This chapter considers the high-altitude environment directly, and the consequences of it on clinical practice; however, it should be noted that many consider the hypoxia encountered at altitude to be of translational value to hypoxia at sea level, for example, in critically unwell patients.
Initial cardiovascular changes occur rapidly. Following the initial ascent, however, more gradual changes may be observed over a period of weeks ( Fig. 74.2 ). On exposure to hypoxia at altitude, peripheral arterial chemoreceptors are stimulated, triggering increased sympathetic activation. The resultant increased heart rate (HR) leads to a rise in cardiac output. A concurrent systemic vasodilation is seen on ascent to altitude. This is antagonized by increased sympathetic tone and the overall effect is one of increased blood pressure (BP), although in the early hours of exposure vasodilation may predominate. After several days, cardiac output returns to baseline but with a lower stroke volume (SV) and higher HR, while BP continues to rise for several weeks.
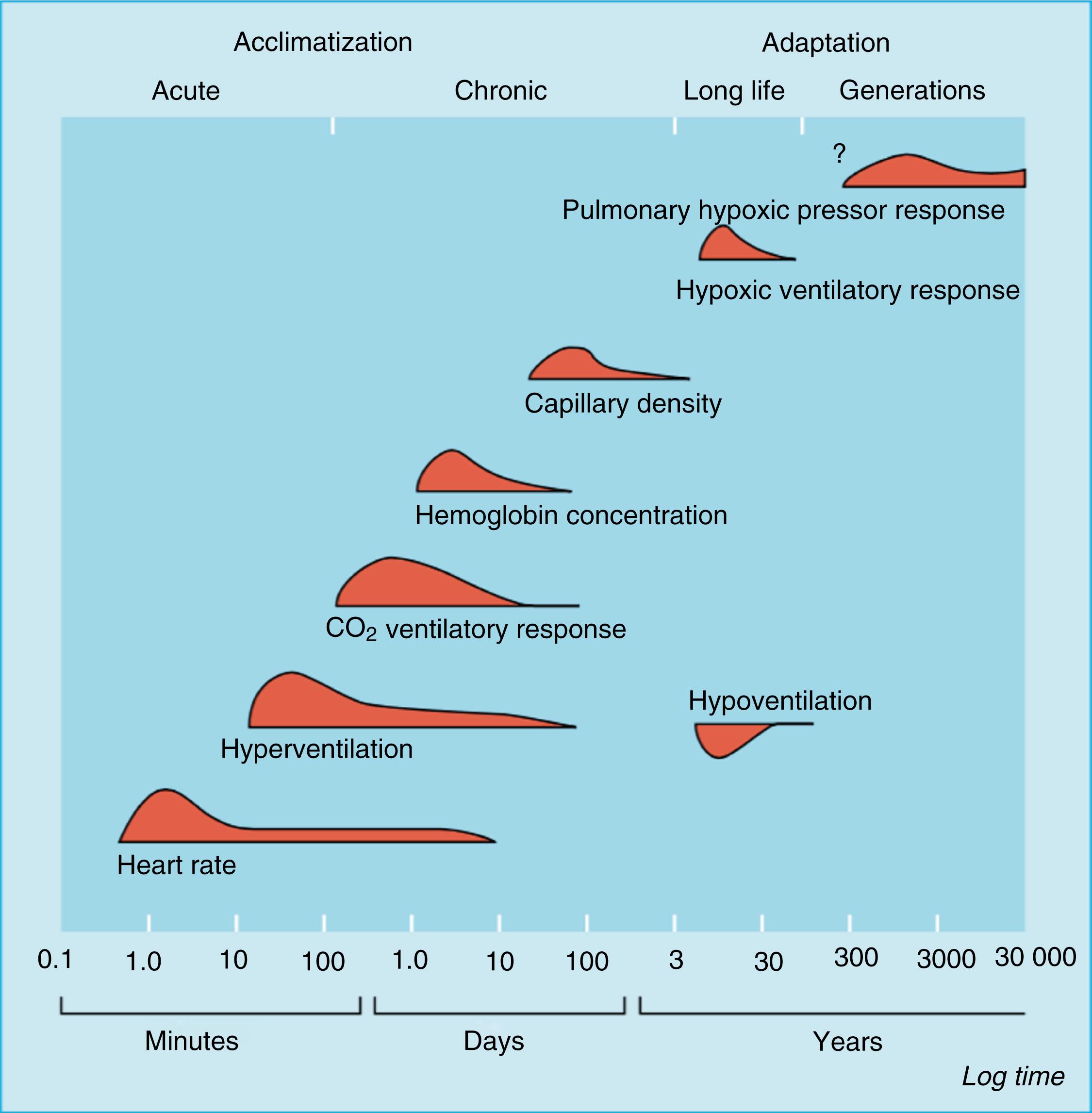
This pattern of increased HR and reduced SV persists across exercise states at altitude. In both athletes and nonathletes, maximal oxygen consumption (VO 2 max) decreases with increasing altitude, estimated at approximately 1% per 100 m above 1500 m.
Hematologically, a rise in hemoglobin (Hb) concentration is seen in response to altitude exposure. Over the initial days, this is related to a fall in plasma volume of approximately 20%, followed by a rise in red cell production secondary to increased levels of erythropoietin (EPO; which begins to rise within hours of ascent). This change in Hb concentration increases arterial oxygen content (CaO 2 ) and theoretically improves oxygen delivery (DO 2 ) to tissues.
However, this may be compromised because there is a marked increase in hematocrit and plasma viscosity. Viscosity has been observed to increase by 38% in healthy volunteers on ascent to 5800 m. Changes in microcirculation, important for the final phase of DO 2 , have been observed in vivo at altitude with a significant reduction in microcirculatory flow seen in healthy volunteers during altitude exposure, although this does not seem to be directly related to the increase in hematocrit and viscosity. Subsequent studies have shown that this flow is significantly greater in the highly altitude-tolerant Sherpa population, raising the possibility that such microcirculatory adaptation may play an important role in optimizing DO 2 and adaptation to high altitude.
The falling P B at altitude results in a decreased atmospheric partial pressure of oxygen (PO 2 ). This in turn results in reduced alveolar (PAO 2 ) and arterial (PaO 2 ) partial pressures of oxygen. However, the reduction in PAO 2 is accentuated because the saturated vapor pressure of water (P SVP Water ) is unchanged (6.3 kPa) at altitude. Examining the alveolar gas equation, we can see that a proportionally higher saturated water vapor at body temperature (
) will result in a decreased PAO 2 .
This decrease in PAO 2 leads to the hypoxic ventilatory response (HVR), which is the most important aspect of acclimatization to high altitude. Studies have found that successful climbers demonstrate an improved HVR. The decrease in PaO 2 leads to hypoxic stimulation of peripheral arterial chemoreceptors, which in normoxia are normally suppressed by central chemoreceptors responding to changes in carbon dioxide (CO 2 ). The peripheral arterial chemoreceptors trigger rapid increases in minute volume (V E ) over seconds to minutes. A resulting decrease in PaCO 2 also helps increase the PAO 2 , as explained by the alveolar gas equation, which demonstrates that any fall in PaCO 2 will result in an increased PAO 2.
The resultant hypocapnia following HVR leads to a leftward shift in the oxygen dissociation curve (ODC). This shift improves oxygen uptake at the lungs, but the increase in affinity may have consequences for DO 2 at end organs. A leftward shift and increased oxygen affinity of Hb are key components of the adaptive response in a number of animals who live in hypoxic conditions. However, with altitude exposure in humans, this leftward shift is opposed by an increase in 2,3-diphosphoglycerate, over several days, causing a rightward shift. The final position of the curve varies depending on altitude exposure and level of acclimatization, but in sojourners it appears to equilibrate at almost sea level values, whereas certain groups of high-altitude natives are able to sustain a leftward shift through hyperventilation.
As PaCO 2 decreases, a respiratory alkalosis develops in both arterial blood and cerebral spinal fluid (CSF). This alkalosis initially antagonizes HVR; however, after several hours and continuing for over 2 weeks, the body begins to correct the alkalosis through bicarbonate (HCO 3 − ) excretion, as well as the increased concentrations of protein anions (such as phosphate and albumin). These processes allow the continued increase in V E (and thus improved acclimatization) to progress throughout this period.
The pulmonary circulation is also modulated at altitude. In response to the reduced PAO 2 , hypoxic pulmonary vasoconstriction (HPV) occurs within minutes. The mechanism is a contraction of pulmonary smooth muscle cells. This process is independent of any external regulation and has been demonstrated in the laboratory setting in pulmonary smooth muscle cells completely isolated from all other tissues. The exact mechanism of oxygen sensing is a source of ongoing research, but it is known that the smooth muscle contraction itself, as in other tissues, relies upon a rise in intracellular Ca 2+ . Although the contraction occurs in isolation, it is modulated by both the endothelium and systemic factors such as sympathetic activation. The process is biphasic in nature, with an initial contraction reaching its maximal effect between 2 and 15 minutes. A secondary phase occurs between 30 and 60 minutes, causing further vasoconstriction in sustained hypoxia. This secondary phase appears to be dependent on the presence of endothelial cells.
In contrast to HPV in localized hypoxia (as seen in many pathologies found at sea level, such as pneumonia, atelectasis) where this response is beneficial for correcting ventilation-perfusion (V/Q) mismatch, the global alveolar hypoxia seen at high-altitude HPV can be significantly deleterious. Widespread vasoconstriction increases pulmonary vascular resistance 50% to 300%, with a consequent precipitous pulmonary arterial pressure (PAP) increase that may be responsible for altitude-specific pathologies such as high-altitude pulmonary edema (HAPE) (see later in this chapter). This rise in PAP is further exacerbated by physical exercise with chamber studies reporting a PAP of 54 mm Hg on maximal exercise at a P B equivalent to 7620 m.
Renal responses play a key role in altitude acclimatization and have long been observed. The hypoxic diuretic response (HDR) was first observed in 1944. It is characterized by both natriuresis and diuresis and is seen in humans and many other mammals.
The earliest phase of the HDR occurs within hours of ascent and is in fact a diuresis alone, with a static fractional sodium clearance. Hypoxia and hypocapnia appear to be the driving factors (although the exact mechanism remains uncertain) and natriuresis soon follows. During the HDR, a total water loss of approximately 3 L is possible during initial exposure and a decrease of approximately 40% plasma volume.
A number of studies suggest that HDR undergoes humoral regulation. The renin-angiotensin-aldosterone system is one of the major systems regulating fluid balance at sea level. Renin and aldosterone promote retention of both water and sodium. However, although renin activity and aldosterone levels are observed to decrease in response to altitude, this response has not been consistently shown to be directly related to the level of natriuresis observed, suggesting there may be a mediation driven by chemoreceptor activation. It is also of note that although the aldosterone exercise response (leading to an increase in plasma aldosterone) remains present at altitude, it appears to be reduced. Indeed, further elements of the hormonal response also appear inconsistent. An increase in serum osmolality is reported with altitude ascent and although this may be expected to produce a concomitant increase in arginine vasopressin (AVP), such a response is often not observed, suggesting a change in AVP regulation at altitude in favor of a diuresis.
The other side of the coin, from a fluid homeostasis perspective, is atrial natriuretic peptide (ANP). It is produced by cardiomyocytes in response to atrial distension and plays an important role in fluid balance; an increase in ANP will induce a natriuresis. It has been observed to increase in response to both pure hypoxia and altitude ascent and may play an important role in the initial diuresis, although any direct causal relationship between ANP levels and the observed diuresis/natriuresis have been questioned.
Although controversy remains around the exact relationship between any hormonal changes and the observed diuresis/natriuresis, a number of studies have reported an association between a picture of increased fluid retention, or hormonal changes promoting fluid retention, and altitude pathologies such as acute mountain sickness (AMS) and HAPE. This suggests that, regardless of the underlying regulation, diuresis remains an important part of successful acclimatization.
In addition to fluid balance, the renal system is also important in mitigating the pH changes generated by the HVR, as discussed previously. Urinary bicarbonate excretion increases over a period of hours to more than 2 weeks, in a process that appears to be unrelated to the natriuresis previously discussed. This reduces blood pH, and may play a role in facilitating the ongoing increase in V E and, therefore, acclimatization to altitude.
Additional endocrine changes may also be observed following altitude exposure. Cortisol, a stress hormone secreted by the adrenal glands, appears to increase at altitude, although exceptions are found in the literature. It may be that this variation is related to duration of exposure, exercise state, and other stress factors, such as coexisting pathology.
As previously discussed, sympathetic activation forms an essential part of the response to acute altitude exposure, which drives many other physiologic changes. As expected, levels of norepinephrine and epinephrine increase, as does nerve fiber activity. However, after prolonged exposure and despite persistently elevated catecholamine levels, maximal HR and response to chronotropic drugs (e.g., isoprenaline) are both reduced, which suggests that a degree of desensitization is also occurring.
Several central nervous system (CNS) changes are found at high altitude. Many of the most frequently observed were eloquently described by John West, one of the leading figures in respiratory and high-altitude physiology. On ascending to altitude, unacclimatized lowlanders typically complain that they take longer to get to sleep, wake frequently, often have unpleasant dreams and do not feel refreshed in the morning. The resultant difficulties are not confined to the night because, as a result of poor sleep, people often feel somnolent and fatigued during the following day, their productivity is reduced and they are more liable to make errors.
This quote encapsulates two separate changes observed at altitude: sleep disturbance and cognitive changes.
Sleep disturbance is a frequently occurring symptom, with incidences of up to 65% reported on ascent. Indeed, a network analysis of symptom scores in altitude sojourners identified the largest single cluster of symptoms to be one primarily characterized by sleep disturbance. Subjectively, individuals report reduced general sleep quality, difficulty in falling asleep, frequent wakening, as well as temperature-related discomfort.
At high altitude, the sleep architecture is altered. The duration of light sleep, and non-rapid eye movement (NREM) sleep stages I and II, is increased. Deep sleep and NREM stages III and IV are reduced dramatically, whereas rapid eye movement (REM) sleep duration appears to be more variable in duration. Frequent awakenings are also observed, which appear to be closely linked to another phenomenon seen during sleep at altitude: periodic breathing.
Periodic breathing, as described by John Cheyne and William Stokes in the 19th century and named eponymously for them, has long been observed at altitude. Reports date back as early as 1857 before it was first described fully by Angelo Mosso in 1894. Characterized by periods of crescendo-decrescendo hyperpnea followed by apnea at altitude, periodic breathing is seen most commonly during NREM stages I and II and appears to be largely absent during REM sleep. Periodic breathing may be responsible for the frequent awakenings seen at altitude that often occur at the transition from apnea to hyperpnea.
This breathing pattern is caused largely by hypocapnia, which is itself a result of HVR. Although this hypocapnia during wakefulness does not adversely affect patterns of breathing during sleep, both the rate and depth of respiration can decline, culminating in apnea. During this apnea, the partial pressure of carbon dioxide (PCO 2 ) increases as PO 2 falls, ultimately leading to a return of ventilation with hyperpnea. This theory is supported by the observation that those who demonstrated the most brisk HVR also experienced the highest rates of apnea, along with the efficacy of supplemental oxygen to improve sleep quality. Periodic breathing tends to increase with ascent and may even occur during REM sleep at extremes of altitude. With acclimatization, periodic breathing can be seen to decrease.
The previous quote from John West also alludes to cognitive changes, which are frequently observed at altitude. Studies have demonstrated deficits across a wide range of domains under hypoxic conditions including arithmetic, memory, language, and motor skills. In addition to direct cognitive impairment, there are adverse changes in mood state and even new onset anxiety disorders secondary to high altitude. The exact etiology of these cognitive changes has not been fully elucidated, although fatigue does appear to be correlated with a number of cognitive domains, and as such, sleep disturbance may be contributory.
On ascent to altitude, anorexia is frequently observed but not fully understood. Leptin, a hormone with elevated levels that are associated with satiety, has been hypothesized as playing an important role but the literature is conflicting; elevated, reduced, and unchanged levels have all been reported. Cholecystokinin, another satiety hormone, has been less widely studied but has been found to be elevated, particularly in those with marked anorexia at altitude.
In concert with anorexia, weight loss of both fat and muscle occurs in response to prolonged exposure. This may be related primarily to reduced intake, although a modest increase in basal metabolic rate is observed, and may be partly attributable to sympathetic drive.
Several distinct pathologies are observed in association with both acute and chronic altitude exposure. Although each is considered separately here, some share pathologic features. In addition, the hypoxic environment can also affect individuals with other ongoing health considerations, such as comorbidity or pregnancy.
AMS is the most common acute altitude pathology. It is a clinical syndrome of nonspecific symptoms, occurring on ascent to altitude (>2500 m). The onset is usually seen between 4 and 12 hours after ascent.
The prevalence of AMS is closely linked to altitude attained—approximately 25% at moderate altitude (<3000 m) and approximately 50% above 4000 m. Other factors exerting a strong influence are rate of ascent, degree of acclimatization, and individual susceptibility. It is relevant to note that physical fitness appears to confer no risk reduction. Physical exertion at altitude may increase risk, although studies remain conflicted.
The pathophysiology of AMS is not yet fully understood. A number of theories have been suggested and current opinion appears to favor CNS dysfunction as the primary cause. Imaging studies in moderate-severe AMS have suggested some degree of cerebral edema, and increased intracranial pressure (ICP) has also been observed. In one remarkable study by Brian Cummins in 1985, three individuals, including Cummins himself, were fitted with invasive telemetric ICP monitoring. On ascent, two subjects remained well but one developed symptoms of AMS, including headache. This individual was also the only subject to demonstrate a raised ICP. The symptoms were seen on minimal exertion; turning his head increased ICP from 14 to 24 mm Hg, and a press up led to an ICP of 51 mm Hg. This study has never been, and is unlikely to be, replicated but suggests elevated ICP may be pathologically significant and potentially related to reduced intracranial compliance. Cerebral blood flow (CBF) is known to increase transiently on ascent and, although wide interindividual variations have been observed, it may play a role. A unifying hypothesis has been proposed whereby hypoxia and hypoxemia result in increased CBF, which alongside changes in blood-brain barrier permeability, leads to brain swelling. Finally, in those with reduced intracranial compliance, this leads to AMS. This hypothesis, however, remains speculative, with some believing venous outflow may be more significant than intracranial compliance.
The diagnosis of AMS is clinical. The symptoms are variable in presentation but may include headache, nausea, anorexia, dizziness, sleep disturbance, and fatigue. Headache is the most frequently observed indication and remains a mandatory symptom for diagnosis in a number of research tools. Several scoring systems of AMS exist for research purposes. A number of these have been applied to clinical practice, although they are not validated formally for this use. The most widely used, the Lake Louise Score, is ubiquitous in the literature and consists of five simple, self-reported symptom-related questions. This scoring system was revised in 2018 with the removal of sleep disturbance after studies suggested that it is likely to be a separate phenomenon unrelated to AMS ( Table 74.1 ). Laboratory testing and clinical examination in AMS are both unremarkable. Oxygen saturation (SpO 2 ) monitoring has been suggested as a useful tool but although SpO 2 is seen to fall with ascent, there appears to be no independent relationship with AMS.
| 2018 Lake Louise Acute Mountain Sickness Score | ||
|---|---|---|
| Symptom | Description | Score |
| Headache | ||
| None at all | 0 | |
| A mild headache | 1 | |
| Moderate headache | 2 | |
| Severe headache, incapacitating | 3 | |
| Gastrointestinal symptoms | ||
| Good appetite | 0 | |
| Poor appetite or nausea | 1 | |
| Moderate nausea or vomiting | 2 | |
| Severe nausea and vomiting, incapacitating | 3 | |
| Fatigue and/or weakness | ||
| Not tired or weak | 0 | |
| Mild fatigue/weakness | 1 | |
| Moderate fatigue/weakness | 2 | |
| Severe fatigue/weakness, incapacitating | 3 | |
| Dizziness/light-headedness | ||
| No dizziness/light-headedness | 0 | |
| Mild dizziness/light-headedness | 1 | |
| Moderate dizziness/light-headedness | 2 | |
| Severe dizziness/light-headedness, incapacitating | 3 | |
| AMS Clinical Functional Score | ||
| Overall, if you had AMS symptoms, how did they affect your activities? | ||
| Not at all | 0 | |
| Symptoms present, but did not force any change in activity or itinerary | 1 | |
| My symptoms forced me to stop the ascent or to go down on my own power | 2 | |
| Had to be evacuated to a lower altitude | 3 | |
Optimal clinical management of AMS begins with prevention. The most significant measure should be a slow ascent, with a limit of 300 m gain in sleeping altitude per day at altitudes above 3000 m generally accepted as best practice, although 600 m is proposed as an alternative. The sleeping altitude is generally accepted to be the most important consideration and the mantra of “climb high, sleep low” can be found widely in the literature. Other nonpharmacologic measures suggested, with some evidence, include: preacclimatization, avoidance of exercise, adequate hydration, and oxygen supplementation. Pharmacologic prophylaxis has been widely studied with acetazolamide the most recommended at an adult dose of 125 mg twice daily. Higher doses of acetazolamide remain efficacious; however, they may be associated with more deleterious effects. Dexamethasone (2 mg every 6 hours or 4 mg every 12 hours) has also been shown to be beneficial, but remains a second-line chemoprophylaxis. Individual risk is highly variable based on both personal (e.g., previous history of AMS or High-Altitude Cerebral Edema [HACE]) and environmental (e.g., rate of ascent, highest altitude attained) factors. Prescribing of prophylactic medications and indeed any recommendations regarding individuals should consider this personalized risk.
When treating AMS, because of the nonspecific nature of the symptoms, consideration must be given to other causes. There may be more severe altitude pathologies such as HACE, or common conditions related to the nature of much travel to altitude such as dehydration, exhaustion, hypothermia, hypoglycemia, or infection. In cases where AMS is diagnosed, then the single most efficacious treatment is descent. This may present other dangers, given the terrain often found in the high altitude environment; however, in severe cases, descent until resolution of symptoms (which typically occurs after a descent of as little as 300 m) remains the gold standard treatment. Measures aimed at correcting hypobaric hypoxia, such as supplemental oxygen and hyperbaric chambers, can offer possible alternatives to descent but in a remote high-altitude setting these treatments present marked logistical challenges, may only offer temporary benefit, and also carry their own risks. Dexamethasone has been shown to be an effective treatment, but it does not aid acclimatization and further ascent is not recommended until the patient is asymptomatic without dexamethasone.
HACE is a severe, life-threatening pathology. It is rare, with a prevalence of 0.28% to 1%. Because of its rarity, there is limited systematic evidence regarding risk factors. However, (not yet conclusively proven) HACE is now considered to be a severe form of AMS and may share the same pathophysiology and risk factors. It is important to note though that while AMS may rapidly progress to HACE if left untreated, HACE may also present suddenly without any preceding symptoms of AMS.
Both animal studies and postmortem examinations have demonstrated gross edema in HACE sufferers, with magnetic resonance imaging (MRI) studies suggesting this is likely vasogenic in nature. In contrast to AMS, in HACE there is evidence of microhemorrhage, primarily within the corpus callosum. The presence of microhemorrhage may indicate venous obstruction as a key pathologic process, which may differentiate AMS from HACE.
HACE may be clinically differentiated from AMS by the presence of ataxia, confusion, psychiatric changes, or altered consciousness that may progress rapidly to coma and death. Investigations offer limited benefit on acute presentation; however, lumbar puncture may reveal elevated ICP, whereas MRI and computed tomography may reveal changes associated with edema.
Prevention of HACE should be guided by the same principles as AMS, with slow ascent, preacclimatization, and pharmacologic strategies as previously described. The diagnosis should consider other possible causes for the observed presentation, but it is important to remember the mantra that any illness at altitude should be treated as altitude-related until proven otherwise. When HACE is diagnosed, its severity should not be underestimated and prompt management is essential. Immediate actions include supplemental oxygen (aiming for an SpO 2 > 90%), administration of dexamethasone (initial dose of 8 mg by mouth, or intravenous or intramuscular injections; and immediately followed by a dose of 4 mg every 6 hours) and, where logistically possible, descent. If descent is not possible, and the airway is adequately protected, then a hyperbaric chamber is an acceptable temporary alternative.
HAPE is a form of noncardiogenic pulmonary edema that occurs in unacclimatized individuals on ascent to altitude (>2500 m). The condition was first described in South American lowlanders on ascent to altitude in 1955, and subsequently in 1960 by two separate American physicians.
The condition generally occurs within 2 to 5 days of ascent and is more common with greater altitude. It remains relatively uncommon under 3000 m and after more than 1 week at altitude. As with many altitude pathologies, the rate of ascent, altitude, and individual susceptibility are the most significant risk factors for the development of HAPE. There is evidence of other predisposing factors including: male sex, preexisting respiratory infection, and cold temperatures. Cardiorespiratory diseases, including anatomical abnormalities affecting pulmonary blood flow, have also been shown to increase risk of HAPE and it should be noted that altitude dwellers may suffer from “re-entry” HAPE following a sojourn to lower altitudes. The prevalence of HAPE varies greatly depending on the aforementioned risk factors. For example, in the general population prevalence of HAPE is estimated at less than 0.2% when climbing to 4500 m in 4 days. However, for a 1- to 2-day ascent to the same altitude, the prevalence rises dramatically to 6%. In those with a known susceptibility to HAPE, this could reach as high as 60%.
The pathophysiology of HAPE is closely related to HPV and the increase in PAP. Pulmonary hypertension is seen on ascent to altitude prior to the development of HAPE. Individuals who are susceptible to HAPE tend to have a brisk HPV response, and often a relatively blunted HVR which further drives HPV. This accentuated rise in PAP of approximately 60 mm Hg in untreated HAPE on ascent may also be partly related to decreased nitric oxide (NO) bioavailability. The subsequent edema appears to be directly related to this increase in pressure, with bronchoalveolar lavage in early HAPE showing little inflammatory change, while pulmonary capillary pressure exceeds values shown in animal models to cause edema.
Early clinical presentation is most commonly exertional dyspnea, often associated with a dry cough. This results in reduced exercise performance. Dyspnea is normally progressive, leading to dyspnea at rest, while the cough may become productive of pink frothy sputum—with hemoptysis of frank blood being rare. Orthopnea is additionally seen as edema progresses. Symptoms of AMS may coexist, but around 50% of sufferers display none. Examination findings typically include tachycardia and tachypnea. Cyanosis may be present and, although not universal, crepitations are invariably audible on auscultation of the lungs. SpO 2 and blood gas analysis show more profound hypoxia than in healthy controls, while radiographic findings demonstrate patchy edema, generally starting peripherally.
As with AMS, if appropriate steps are taken, the incidence of HAPE can be significantly reduced. Gradual ascent remains the single most effective means of reducing HAPE occurrence. Individuals with a history of HAPE should take additional care and in such cases pharmacologic prophylaxis may be warranted. Nifedipine, a calcium channel blocker, has been shown through randomized control trial and clinical experience to be effective in high-risk individuals, delivering significantly lower PAP and improved prophylaxis of clinical HAPE over a placebo. Other medications, such as salmeterol and tadalafil, have shown promise in clinical trials but clinical experience remains limited. Further research is required, although salmeterol is considered as an adjuvant to nifedipine in high-risk cases. Dexamethasone, which is used widely in AMS/HACE, is not as regularly utilized in HAPE. There is some data supporting its use, but again further study is advised to confirm.
As with AMS/HACE, descent remains the most effective treatment for HAPE. Improvements may be seen after only minimal descent, although descent of 1000 m or until symptoms resolve is advocated. The degree of exertion on descent should be minimized as much as possible to reduce further rise in PAP. Supplemental oxygen and hyperbaric chambers are further measures to consider when descent is not possible. Nifedipine continues to have a role in treatment, with a single unblinded trial showing some clinical improvement and extensive clinical experience supporting its role. Phosphodiesterase inhibitors and dexamethasone may both confer some benefit and case reports of their use exist. It is important to point out that unlike other forms of pulmonary edema, the use of diuretics is not advocated in HAPE, particularly as patients may have concurrent hypovolemia. In the hospital setting it may be possible to manage HAPE without descent using supplemental oxygen and close observation. The use of continuous positive airway pressure has been advocated but remains unreported.
Chronic mountain sickness (CMS), also known as Monge disease, is a syndrome affecting lifelong altitude dwellers and native populations. First described by Carlos Monge in 1928 in Peru, it is characterized by excessive erythrocytosis (EE), which may lead to pulmonary hypertension, cor pulmonale, and congestive cardiac failure.
CMS prevalence varies widely among high-altitude populations around the world. Tibetans have a low prevalence with 1.2% reported, while with the Han Chinese in the same region the rate is 5.6%. The rates in South America are also generally higher with a prevalence of 4.5% in the general population, and increasing up to 33.7% in miners 60 to 69 years of age. These ethnic variations may be explained by significant differences in adaptation to high altitude. Tibetan natives have been dwelling at altitude for significantly longer than Andean natives and have adapted very differently. Andeans show lower HVR and stronger HPV than Tibetans and even in health show a preponderance for higher Hb than is observed in Tibetans. Other than ethnic variation, there appears to be a direct correlation with altitude; a study in North India observed no cases of CMS below 3000 m, yet a prevalence of 13% above this elevation.
The underlying cause of the EE is not yet fully understood. It is generally accepted that chronic hypoxia, potentially exacerbated by a loss of ventilatory acclimatization leading to central hypoventilation, results in an erythrocytosis. The EE may be mediated by EPO, which is produced in response to hypoxia; however, the correlation between EPO levels, SpO 2 , and Hb is not consistent, suggesting that it is not the sole factor. Recent gene studies have highlighted the potential role of SENP1, a gene involved in EPO regulation. Individuals with CMS SENP1 appear to show a higher transcriptional response to hypoxia and the gene is the subject of ongoing research in this field.
Clinically, CMS may be identified by the excessive erythropoiesis (females Hb ≥19 g/dL; males Hb ≥21 g/dL) and severe hypoxemia. Symptoms of this include headache, dyspnea, fatigue or sleep disturbance, and a burning sensation in the hands and feet. Meanwhile, signs include cyanosis (particularly marked in mucous membranes), finger clubbing, and dilatation of blood vessels. Further investigations may demonstrate pulmonary hypertension and cardiac failure. Of clinical relevance are family history, obesity, and sleep apnea, which are all risk factors for the development of CMS. The gold standard tool for diagnosis is the Qinghai score for CMS. It is important to note that the diagnosis of CMS should only be made in the absence of other systemic disease that may explain hypoxemia, such as chronic airway diseases.
The optimal management of CMS is permanent relocation to a lower altitude; however, CMS will dissipate with the resumption of normoxia. When permanent relocation is not possible, sojourns to lower altitudes may help to stop Hb from hitting excessive levels. Venesection, with or without isovolumetric hemodilution, is used widely in clinical practice. However, supportive literature is of limited quality and there are concerns about a rebound effect on Hb, although these are unproven. In addition, recent evidence suggests that iron deficiency caused by venesection may worsen pulmonary hypertension and advocates the use of iron supplementation to ameliorate this effect. Numerous pharmacologic compounds have been studied with limited success including: ACE inhibitors, ventilatory stimulants, and dopaminergic antagonists. Recently, more focus has been given to acetazolamide with positive results, suggesting it is safe, able to reduce erythrocytosis, and improve pulmonary circulation, although side effects associated with long-term use remain unknown.
The acute altitude pathologies described previously have been largely studied in healthy populations and there is a limited body of evidence to characterize how existing disease may be affected by ascent to altitude. At the same time, travel to remote, high-altitude destinations is becoming easier, and people are living longer with a higher burden of chronic disease ; as a consequence, the challenges of managing chronic disease at altitude are likely to become increasingly relevant.
It is recommended that individuals with significant preexisting disease should consult an experienced altitude physician and undergo a risk assessment before travel. The assessment should consider existing conditions as well as details of the proposed trip, including ascent profile and level of exertion. The conditions most likely of concern at altitude are those affecting the cardiorespiratory system because these may cause additional difficulty in adapting to the hypoxic environment. Consideration should be given to two separate issues: whether chronic disease will predispose individuals to the development of acute altitude pathologies and whether altitude exposure will exacerbate chronic disease.
Ischemic heart disease is a pathology that may be worsened by altitude exposure. Acute altitude exposure increases cardiac output, and thus myocardial oxygen demand, while in the presence of reduced arterial oxygen content. Estimates of myocardial perfusion in healthy subjects suggest that there may be some reduction at altitude, which may be partly ameliorated by acetazolamide. However, several studies have exposed patients with stable coronary artery disease (CAD), who are deemed to be low risk and who have exercised at altitude with no adverse effects. A single study of eight patients with moderate-risk disease demonstrated that compensatory mechanisms of coronary circulation may be exhausted at even moderate altitude. Current recommendations advise cautious ascent to a maximum of 4200 m by low-risk patients, who have had a minimum of 6 months postmyocardial infarction or revascularization before travel. These recommendations also advise moderate-risk patients not to ascend above 2500 m, with only light exercise undertaken, while those at highest risk should avoid altitude entirely. Consideration should be given to acetazolamide, as it may aid coronary perfusion, but this has not been verified in patients with CAD.
Patients with heart failure may pose a significant challenge at altitude, particularly as the condition is often closely linked to other comorbidities. However, studies on patients with stable disease (New York Heart Association Class II-IV) have demonstrated moderate altitude may be tolerated without significant compromise, although those with the most severe disease were significantly limited in their exercise tolerance. Medications used to manage heart failure should also be closely reviewed. Use of diuretics must be monitored, as fluid status is likely to alter at altitude and particular care should be paid to concomitant use of acetazolamide. Studies suggest that selective β1 receptor antagonists may improve exercise performance when compared to nonselective beta blockers and are advised where practical. In summary, those with the most severe disease should abstain from travel to altitude whereas those with mild to moderate disease should proceed with caution.
In the presence of the hypobaric hypoxia found at altitude, there is concern for exacerbation of respiratory disease. Counterintuitively, however, observational data suggest that asthma is less common, and less severe, in those living at altitude. One likely reason for this is the reduced population of dust mites at altitude secondary to the cold, dry, and hypoxic conditions that result in a significant improvement in many pathologic features of asthma. However, such studies do not provide evidence regarding acute altitude exposure. There have been a number of observational studies exploring acute altitude exposure with conflicting results for physiologic measurements (e.g., peak expiratory flow, forced expiratory volume in 1 second [FEV1]), but all have demonstrated safe travel for patients with mild asthma to altitudes of up to 6410 m. Caution is advised, however, as upper airway infections, particularly in remote environments, remain a risk for asthmatic patients. Hence, while travel to altitude is safe for well-controlled asthmatics, adequate supplies of rescue inhalers and oral steroids are advised. Measures may also be taken to limit cold, dry air exposure, which often exacerbates asthma. These can be as simple as covering the nose and mouth during outdoor exposure. Those with poorly controlled or severe asthma are at high risk because of the remoteness of the environment and variability of medical facilities, and travel should be avoided where possible in this group.
Chronic obstructive pulmonary disease (COPD) poses a particular challenge at altitude. Studies of altitude dwellers with COPD demonstrate increased mortality and cor pulmonale. A number of studies simulating altitude as a model for commercial air flight have shown marked hypoxemia in response to moderately simulated altitude. A similar drop in PaO 2 was observed in mild to moderate COPD sufferers on ascent to 1920 m, with a mean PaO 2 fall from 8.8 kPa at sea level to 6.9 kPa. However, no adverse clinical events were reported in these studies and, given the chronic nature of the hypoxia, there is likely to be some degree of preexisting adaptation. Particular care must be taken in COPD patients with pulmonary hypertension. As previously discussed, the pathophysiology of HAPE indicates that preexisting pulmonary hypertension may increase susceptibility to HAPE. When assessing risk of hypoxemia at altitude, the use of formulae derived to predict PaO 2 during flight may be helpful. In general, there are limited data on the safety of travel to altitudes for patients with COPD, and no data for their travel above 3048 m. Patients with COPD, and in particular those with pulmonary hypertension, should seek medical advice prior to traveling to high altitude.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here