Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The main causes of acute kidney injury (AKI) are associated with decreased kidney perfusion. A decrease in oxygen delivery severe or prolonged enough to impair cellular function can cause tubular or vascular endothelial dysfunction. The mismatch between oxygen delivery and demand is more prominent in certain regions of the kidney because variations in blood flow are characteristic of the kidney circulation. Because the kidneys receive up to 25% of the cardiac output, any decrease in mean arterial pressure may affect kidney perfusion. The most common causes of AKI are severe transitory decline in kidney perfusion, as with hemorrhage, shock, and burns, prolonged moderate decline, as with vomiting, diarrhea, and poor oral intake, impaired cardiac output, decreased vascular resistance, and kidney vasoconstriction.
Acute tubular necrosis (ATN) describes the pathologic result from severe or prolonged hypoperfusion or toxic injury determining lethal injury. However, the most common findings described in AKI—detachment of renal tubular epithelial (RTE) cells from the basement membrane, effacement and loss of the brush border in proximal tubular segments, formation of tubular casts derived from sloughed cells and tubular debris, interstitial edema, and congestion of the peritubular capillaries—are not necessarily present in human biopsy samples. ATN is often focal, with its extent varying according to the severity and duration of the ischemic insult. Sublethal injury that does not incur histologic changes is an important component in AKI, leading to decline in GFR and kidney blood flow that can be rapidly reversed. The extent of the recovery to a sublethal injury is determined by the degree to which cells can restore normal function and promote regeneration. In addition, the degree of glomerular filtration rate (GFR) reduction and these pathologic alterations in AKI are often not correlated, demonstrating the complex interplay of vascular and tubular processes that determine kidney dysfunction in AKI.
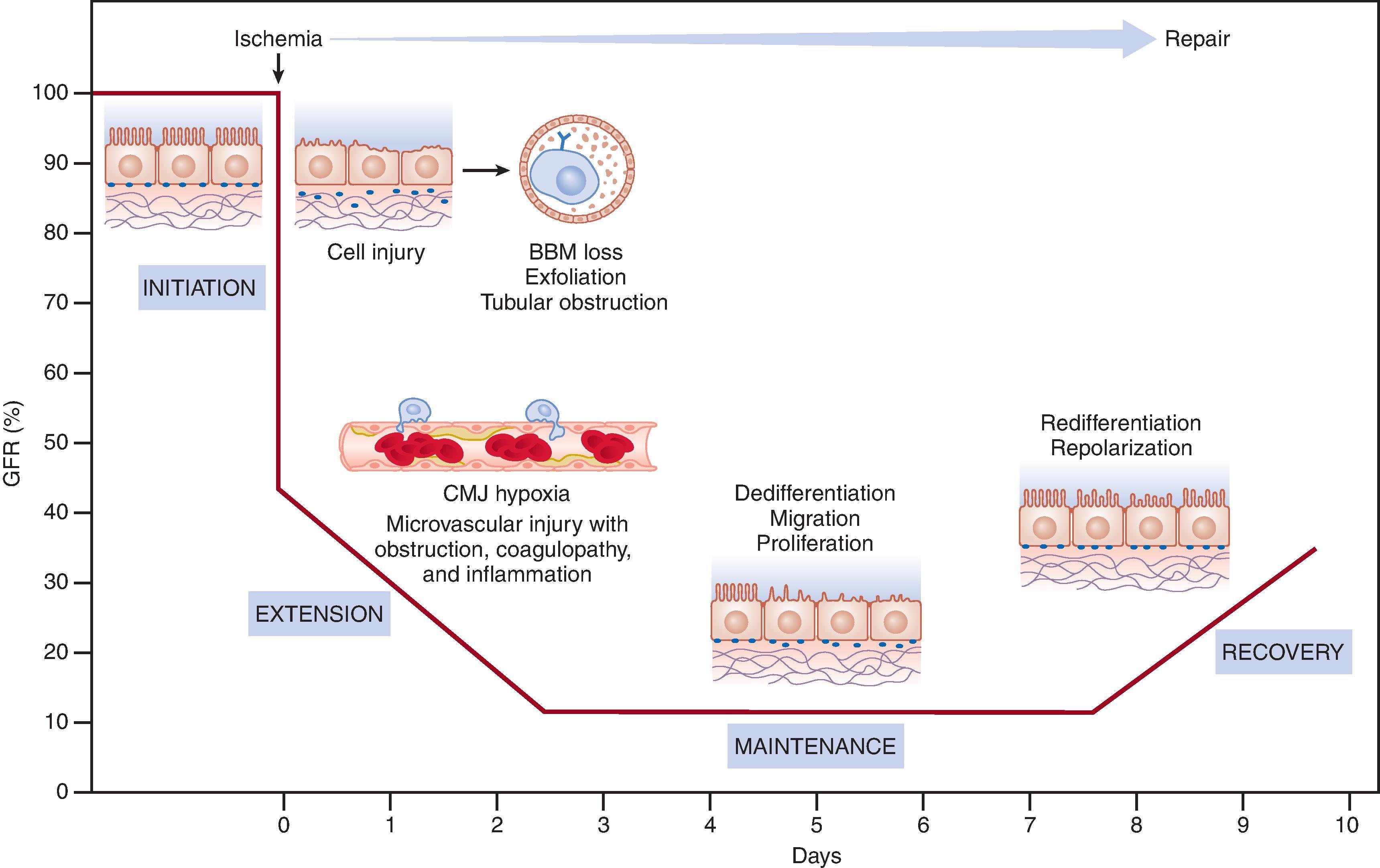
The classification of AKI into initiation, extension, maintenance, and recovery phases can be helpful for understanding the interactions of inflammation with tubular and vascular factors in determining the extent of injury. The initiation phase occurs when kidney blood flow decreases to a level that results in ATP depletion, or a toxin induces acute cell injury and/or dysfunction. The severity and duration of ischemia or toxic damage define the degree of the endothelial and/or tubular cell dysfunction. During this initiation phase, upregulation of cytokines triggers the inflammatory cascade, further worsening kidney perfusion. In addition, several endothelial mechanisms may affect kidney perfusion, including the regulation of intrinsic vascular constriction and the response to vasoconstrictors or vasodilators. In the extension phase, the subsequent necrosis and apoptosis define the extent of injury and fall in GFR. Although the inflammatory cell infiltration can be detected within 24 hours following ischemia and leukocytes may appear within 2 hours, this period is the most favorable phase to allow a therapeutic intervention to contain the amplification of the inflammatory response and prevent further deterioration of kidney function. In the maintenance phase, blood flow normalizes, GFR stabilizes, and the process of cell repair, migration, and proliferation initiates. In the following recovery phase, cellular differentiation continues and epithelial polarity is reestablished.
It is important to note that the degree of cell injury and dysfunction is heterogeneous in the kidney. While cells in the cortical region can remain viable and able to functionally and structurally recover, cells within the proximal tubule and the thick ascending limb in the outer medulla are more likely to suffer lethal injury. With sublethal injury, disruption of the actin cytoskeleton and loss of polarity lead to tubular back leak and obstruction. In more severe AKI, cell death occurs and can be confirmed by the presence of cellular debris and casts in the urine sediment. Two processes are associated with cell death: Necrosis results in a profound inflammatory response as a trigger, while apoptosis initiates a regulated program, leading to DNA fragmentation and cytoplasmic condensation without triggering an inflammatory response.
The remarkable efficiency of the reparative process after acute injury is attributable to the capacity of tubular epithelial cells to re-differentiate and restore the functional integrity of the kidney. New techniques of functional genomics and complementary DNA microarray have been able to demonstrate the complex pathways leading to kidney repair. Some of the genes upregulated in the reparative process are involved in cell-cycle regulation, inflammation, cell death regulation, and growth factor or cytokine production. The identification of these genes led to the discovery of early biomarkers of AKI, such as KIM-1, neutrophil gelatinase–associated lipocalin (NGAL), tissue inhibitor of metalloproteinase 2 (TIMP-2), insulin-like growth factor binding protein 7 (IGFBP7), and interleukin 18. In addition to their value for early AKI detection, these new biomarkers may also play a role in the process of injury and/or repair after AKI. The therapeutic application of these and other molecules is currently being extensively investigated and shedding light on future treatment opportunities for this syndrome.
Great progress on the definition and classification of AKI took place with the development of the Risk, Injury, Failure, Loss, and End-stage Kidney (RIFLE) classification scheme ( Fig. 31.1 ). Since then, Acute Kidney Injury Network (AKIN) and, more recently, the Kidney Disease International Global Outcomes (KDIGO) schemes have been widely used in the clinical and research setting. The criteria maintain a 48-hour time interval for absolute changes in creatinine and a 7-day time interval for relative changes in creatinine. The KDIGO group has also proposed a new term, acute kidney disease (AKD), to address the problem that changes in creatinine may evolve over periods longer than 7 days and, thus, not meet the AKI definition. AKD can occur with or without other acute or chronic kidney diseases (CKDs) and disorders (see Fig. 31.1 C).
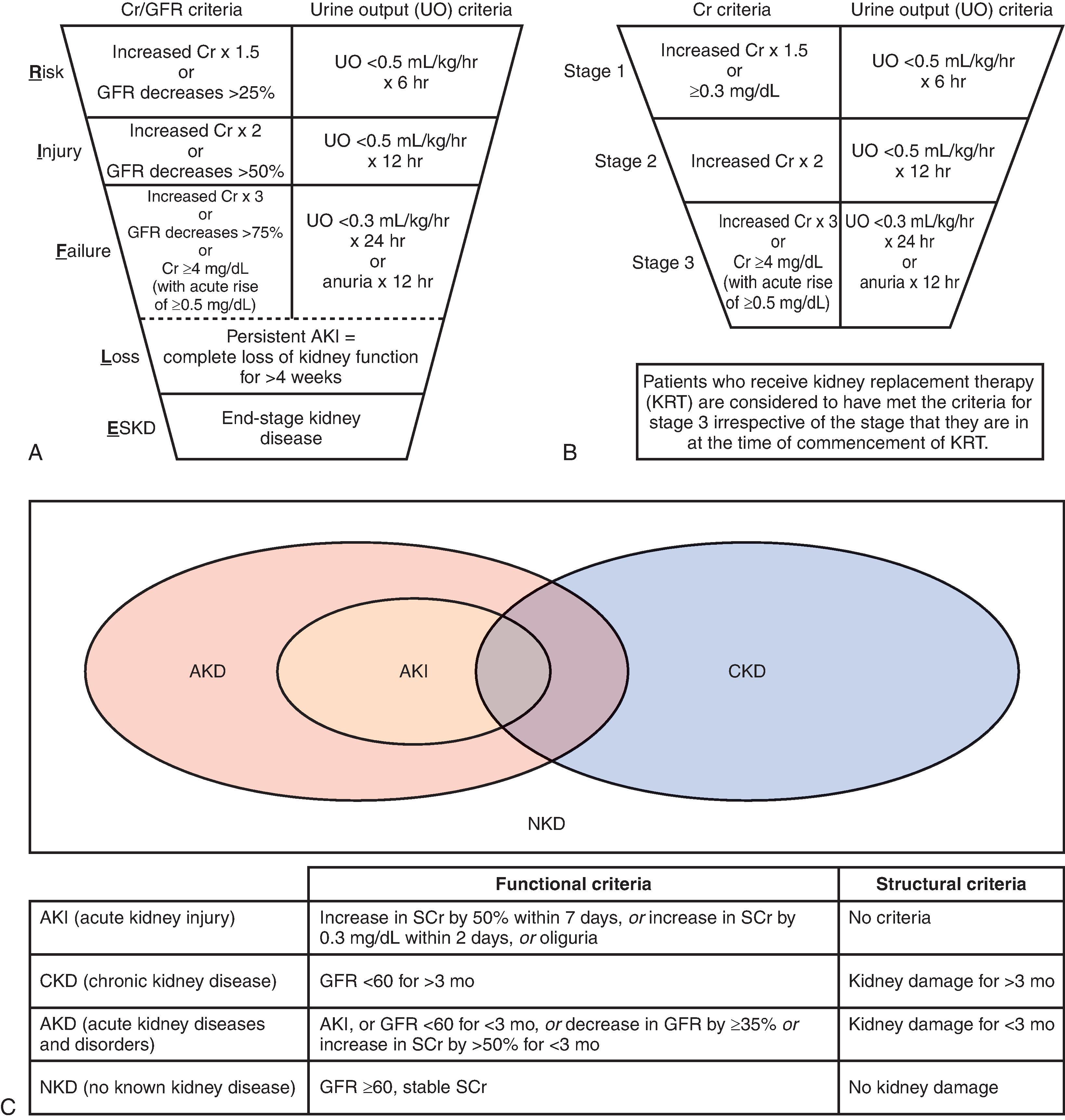
Although the use of standardized definitions has greatly improved the current knowledge of AKI, there are still shortcomings with the criteria to define AKI. The definition is still evolving, and the expansion of biomarker use will certainly provide opportunity for an improved and more refined terminology. Serum creatinine (sCr) requires comparison to a baseline or a reference value that may not always be available. Thus, to define AKI, we need two sCr values, including a “baseline” creatinine value representing the patient’s underlying kidney function.
In the absence of a baseline, the Acute Dialysis Quality Initiative (ADQI) and KDIGO have recommended back-calculation of sCr from the MDRD formula , assuming an estimated GFR of 75mL/min/1.73m 2 . This method can be used when there is no evidence of CKD. Unfortunately, in unidentified CKD, estimating the baseline sCr may mislabel a patient with AKI, when in reality the diagnosis is unidentified CKD. On the other hand, using the minimum sCr during hospitalization in those patients that recover may be helpful. However, in prolonged critical illness or sepsis, decreased creatinine generation may result in lower sCr values.
Although the use of creatinine to assess kidney function is not ideal, it is widely available, easy to measure, and has been used for more than 80 years. Many factors other than kidney function, such as age, sex, muscle mass, and catabolic rate, influence creatinine concentration. The sCr has a stable concentration, which makes it inadequate to access kidney filtration, a parameter that fluctuates continually. Thus, changes in GFR often correlate poorly with changes in sCr concentration. Three factors influence the sCr concentration: the actual GFR, fluctuations in creatinine production, and fluid balance, affecting the volume of distribution. Furthermore, in patients with normal kidney reserve, sCr may not change, despite acute tubular injury, because of compensatory increases in the function of other nephrons, creating a delay in diagnosis after injury.
In AKI, sCr generation is not equal to filtration and excretion, resulting in retention of creatinine with a rising plasma level. Until the levels of creatinine reach a plateau at a new steady state, usually 24 to 72 hours after a known injury event, sCr can overestimate kidney function. Fluid administration is another common factor delaying AKI diagnosis by sCr, as fluid accumulation increases total body water (TBW), altering the volume of distribution of creatinine and resulting in potential overestimation of kidney function ( Fig. 31.2 ). The masking of AKI severity by volume expansion may be especially problematic in settings where the creatinine is rising relatively slowly because of either lower creatinine generation, as might be expected in the elderly or patients with less muscle mass, or to more modest overall injury. Analysis of the Fluids and Catheters Treatment Trial (FACTT) noted that sCr values corrected for fluid accumulation identified a larger number of patients with AKI who had been misclassified. These patients had outcomes similar to those with AKI.
Standardized creatinine assessments with the isotope dilution mass spectrometry (IDMS) method will improve the comparison across values and will improve agreement between values across centers. However, standardizing the assays has no effect on the individual variation of sCr. Normal sCr is frequently associated with low creatinine clearance (CrCl), especially in critically ill patients.
In critically ill patients with unstable kidney function in the non-steady state, loss of kidney function does not correspond with the degree of decline in estimated GFR. In those patients, repeated measurement of CrCl may be an early indicator of AKI. For adjustment of medications, especially for toxic, antimicrobial, and chemotherapy drugs, it is fundamental to have a more reliable and accurate estimation of kidney function ( Table 31.1 ). In those circumstances, it is necessary to consider a CrCl, which can be performed in collection periods from 1 to 24 hours. The shorter the collection time, the higher the likelihood for errors caused by inaccurate time recording and incomplete urine collection. Several studies have shown that short duration (1 to 4 hours) CrCl measurements are feasible in the critically ill, and studies have validated the method compared with 24-hour clearance. The use of 4-hour CrCl in the detection of AKI is also a useful method.
| Clinical Decisions | Change in Level of GFR |
| Diagnosis | Extremes of age (elderly, children)Extremes of body size (obesity, low body mass index <18.5 kg/m 2 )Severe malnutrition (cirrhosis, end-stage kidney disease, cancer)Grossly abnormal muscle mass (amputation, paralysis, myopathy)High or low intake of creatinine (vegetarian diet, dietary supplements)Pregnancy |
| Management | Dose and monitoring for medications cleared by the kidneyDetermine safety of diagnostic tests or proceduresReferral to nephrologistsReferral for kidney transplantationPlacement of dialysis access |
Commonly used formulas to estimate GFR are the Cockcroft-Gault and the four-variable Modification of Diet in Renal Disease (MDRD) equations. Particularly in older, underweight, or overweight people, these equations may misrepresent GFR. The CKD Epidemiology Collaboration (EPI) equation performs better in these patients. The Jelliffe equation was developed to estimate GFR in non-steady state conditions; it accounts for the difference between creatinine production and excretion, using the daily changes in sCr. Creatinine production is adjusted for age and for CKD status because creatinine production generally decreases with declining kidney function. The modified Jelliffe equation accounts for the effect of positive fluid balance in variations of sCr and correlates best with measured CrCl.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here