Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
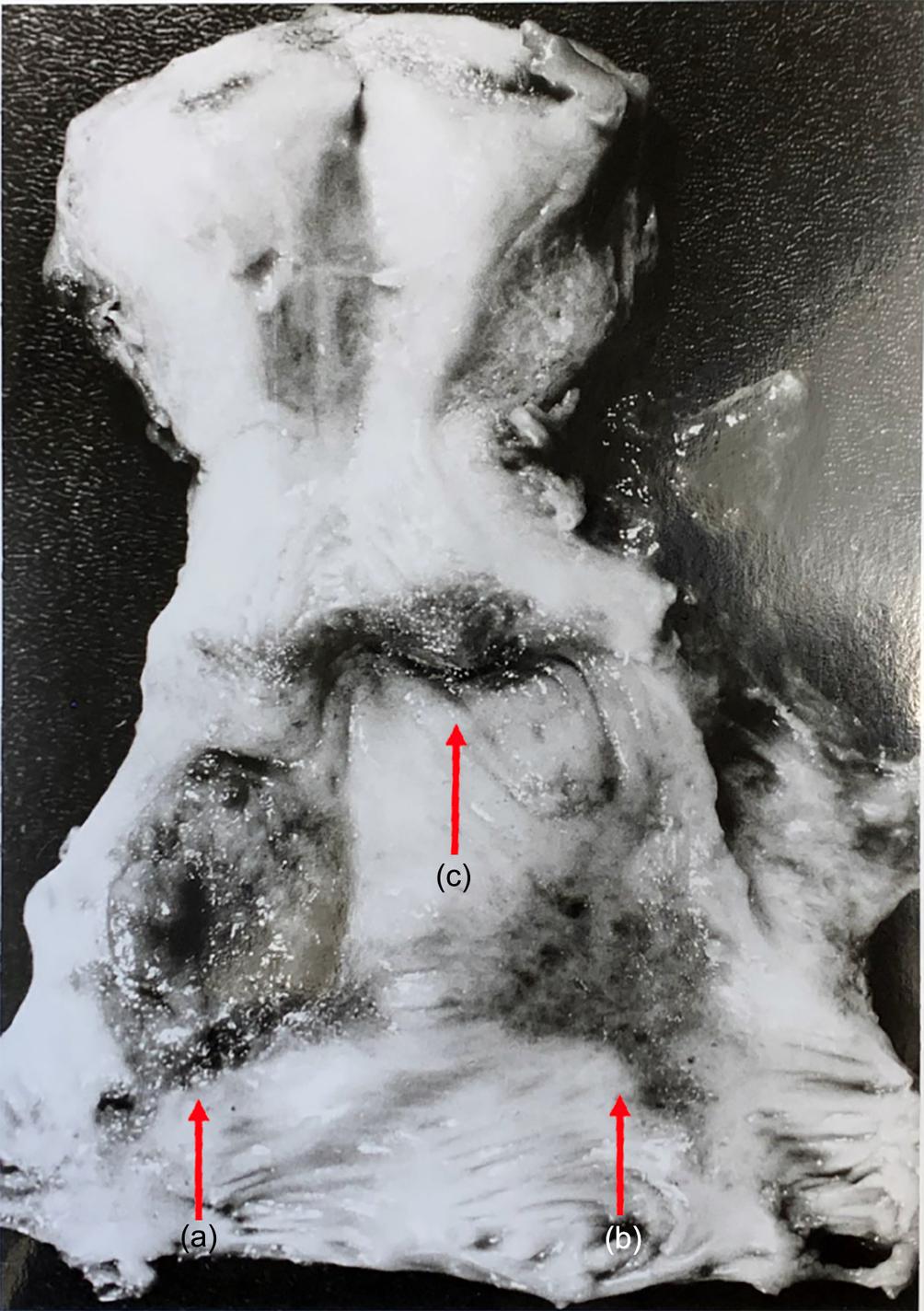
A 29-year-old G2P2 woman with a known bicornuate uterus presents to the office with irregular vaginal bleeding. A pelvic exam demonstrates a 2 cm friable lesion on the proximal vagina adjacent to the cervix ( Fig. 16.1 ). A biopsy is consistent with clear cell carcinoma of the vagina. An MRI shows no parametrial involvement or invasion into nearby structures, and a PET scan demonstrates no distant metastases. How do you proceed?
The most well-established risk factor for clear cell carcinomas of the vagina and cervix is in utero exposure to diethylstilbestrol (DES). DES is a synthetic estrogen that is administered orally. Beginning in the late 1940s, physicians in the United States began prescribing DES as a means to decrease adverse pregnancy outcomes, including preterm delivery, recurrent pregnancy loss, and hypertensive disorders of pregnancy. It was prescribed most commonly between 1947 and 1971. In1970, the first case series of six women with clear cell carcinoma of the vagina was published. In 1971, a subsequent case control study showed an increased odds of having been exposed to DES in utero in the women with clear cell carcinoma compared with the control group. At this point, the Food and Drug Administration (FDA) changed the label for DES to no longer include prevention of miscarriage, and added pregnancy as a contraindication. Use of DES during pregnancy declined significantly following.
In the Registry established to study these tumors, 695 women diagnosed with clear cell carcinoma of the vagina and cervix were reported, and 416 of these patients had known in utero exposure to DES. Because of its rarity, the exact quantification of increased odds of developing clear cell carcinomas of the vagina and cervix attributable to DES exposure is uncertain, but the majority of cases have been associated with in utero DES. However, the significant proportion of women in the Registry without a known in utero DES exposure implies that there are probably other mechanisms by which these clear cell carcinomas may develop. In a study using the Central Netherlands Registry, a similar proportion of women developed clear cell carcinomas of the vagina and cervix without having had in utero exposure to DES as compared with the United States registry.
Less is known about the relationship between clear cell carcinoma of the cervix or vagina and race and ethnicity. Only a small number of women in the United States registry were Black women (7%). A smaller proportion of Black women in the Registry had known DES exposure in utero, but this may have been due at least in part to decreased availability of maternal pregnancy histories compared with the white women included in the Registry.
Mullerian abnormalities also have been associated with clear cell carcinomas of the vagina and cervix. Some anomalies are related to DES exposure and abnormal Mullerian embryological development. Common anomalies include small, T-shaped uteri and abnormal appearing cervixes (cockscomb) and upper vaginas, which can be associated with infertility. However, non-DES-related anomalies have also been linked to clear cell carcinomas of the vagina and cervix, including Mullerian anomalies (bicornuate uterus, vaginal septum) and urinary tract abnormalities such as renal agenesis. Chromosomal abnormalities have also been linked to development of these rare cancers, as have endometriotic implants in the vagina and cervix which have undergone malignant transformation into clear cell carcinomas. Many cases are likely to be sporadic.
After the association with DES was demonstrated, registries were established to follow women with known diagnoses of clear cell carcinoma arising in the vagina or cervix. Women were included regardless of their DES exposure status. Through these registries, more accurate risk estimates could be made. In women who had DES exposure in utero, the risk of developing clear cell carcinoma of the vagina or cervix by the age of 34 was estimated to be about 1 in 1000. Of the 720 women with clear cell carcinoma who were included in the Registry, 400 (56%) patients had vaginal cancer, 182 (25%) had cervical cancer, and 138 (19%) had cancer involving both the vagina and cervix. After the FDA listed pregnancy as a contraindication for the use of DES, the incidence of clear cell carcinoma of the vagina and cervix declined. However, it is estimated that approximately 50 cases each year are still diagnosed.
The mean age at diagnosis is 22 years old, and 80% of patients are diagnosed between the ages of 15 and 30. Survival outcomes are different for women exposed to DES in utero compared with those who were not exposed, likely due to differences in tumor biology. A bimodal age distribution of incidence, one peak centered around 20 years of age and one around 60–80 years of age, has been described for non-DES-exposed women. In 2018, updated survival data were published using the United States Registry. For DES-exposed women, the 5-year survival was slightly better at 86%, compared with 81% for those without exposures. The 20-year survival is approximately 69%, and was similar for both groups. However, given the rarity of vaginal and cervical clear cell carcinomas, it is possible that some number of the women who were considered unexposed actually did have exposure to DES in utero. This theory of possible DES exposure is supported by the similar age distributions in registry patients with clear cell carcinomas of the vagina or cervix who were and were not exposed. When looking at the evolution of overall survival over time, it is thought that an increase in deaths at age 35–49 is related to late recurrences of disease.
Adenocarcinoma makes up approximately 10% of all primary vaginal carcinomas and it is more common to have metastasis or extension of tumor from uterus, cervix, or vulva. Clear cell carcinoma of the vagina is of particular interest, due to the known association with DES. The median age of diagnosis for DES-related clear cell carcinoma was 20 years, with 80% of cases between the age of 15 and 31, though they have also been reported up to age 55. Cases of clear cell carcinoma have also been reported to arise from vaginal adenosis without history of prenatal DES exposure and frequently occur in older women. Half of high grade primary vaginal adenocarcinomas may show clear cell differentiation. Primary vulvar clear cell carcinoma is extremely rare, and usually arises in association with vulvar endometriosis.
Lesions can be more exophytic with polypoid, nodular or papillary appearance or may be flat or ulcerated.
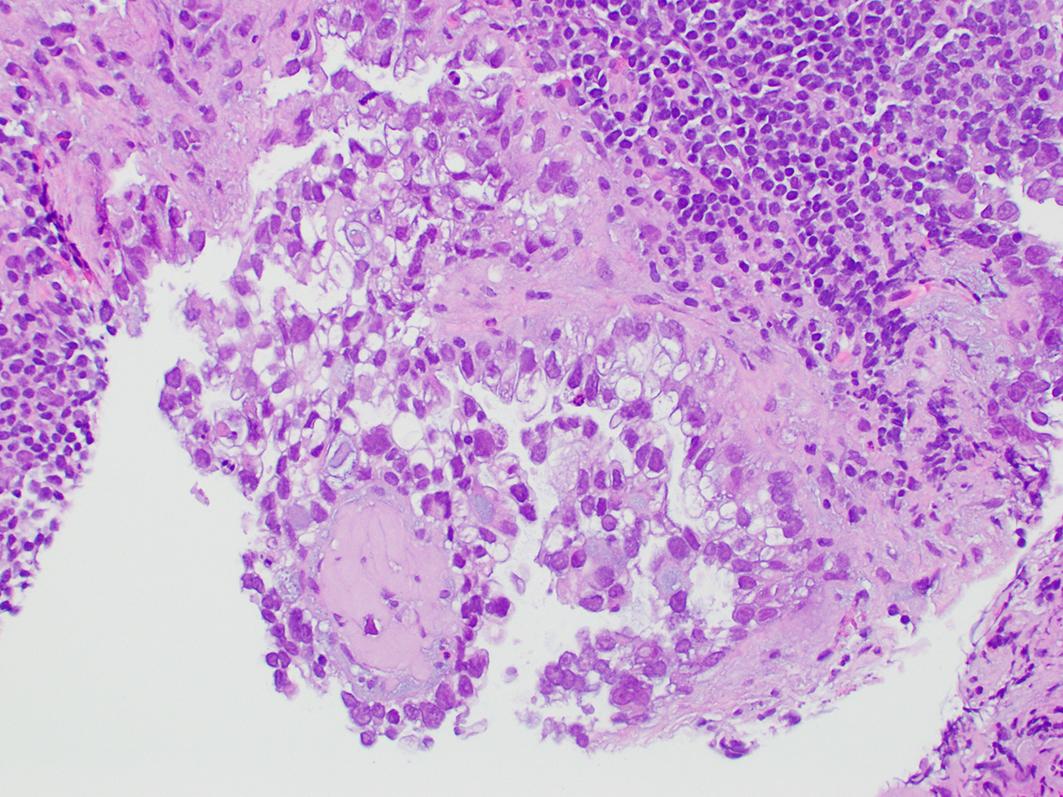
Clear cell carcinoma can have a variety of architectural patterns, including tubulocystic/glandular, papillary, and solid, and frequently show a mixture of patterns in a given tumor. The tumor cells have a varied appearance from flattened to polyhedral or cuboidal cells to “hobnail” cells with bulbous nuclei that protrude into a gland lumen or surface ( Fig. 16.2 ). Nuclear atypia is moderate to marked, though the mitotic count is usually discordant with the atypia and is often low (approximately 3–4 mitosis per 10 HPF). While the tumors typically have clear cytoplasm, sometimes it can be eosinophilic.
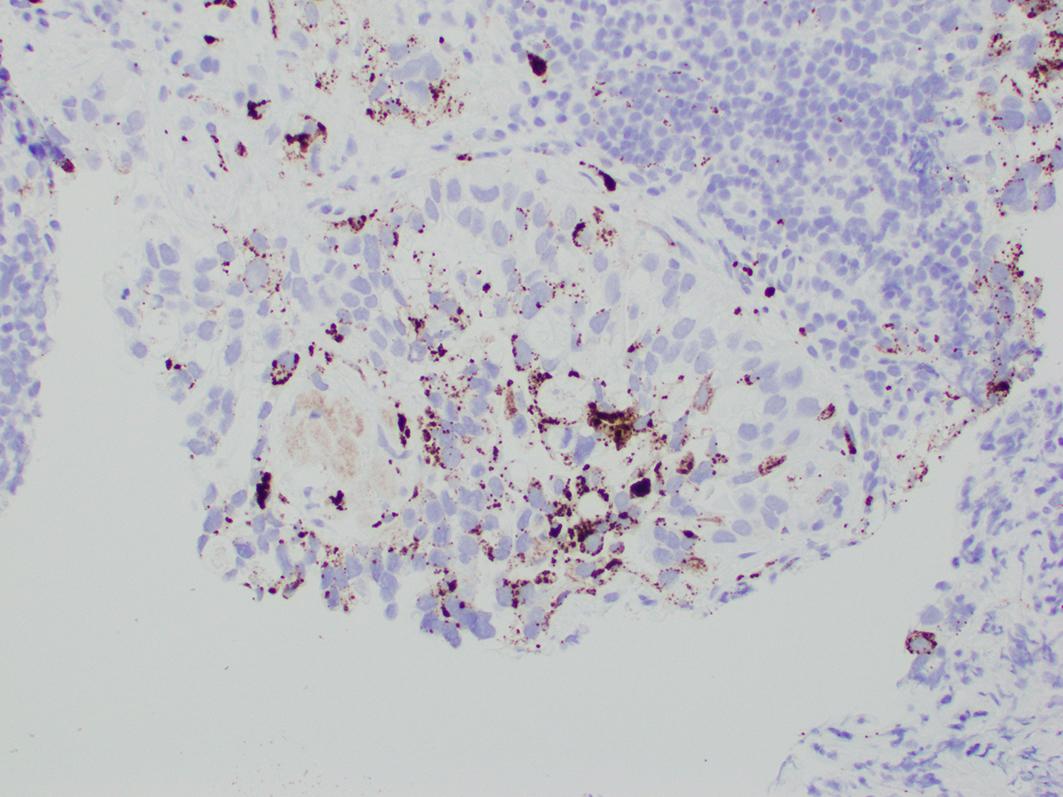
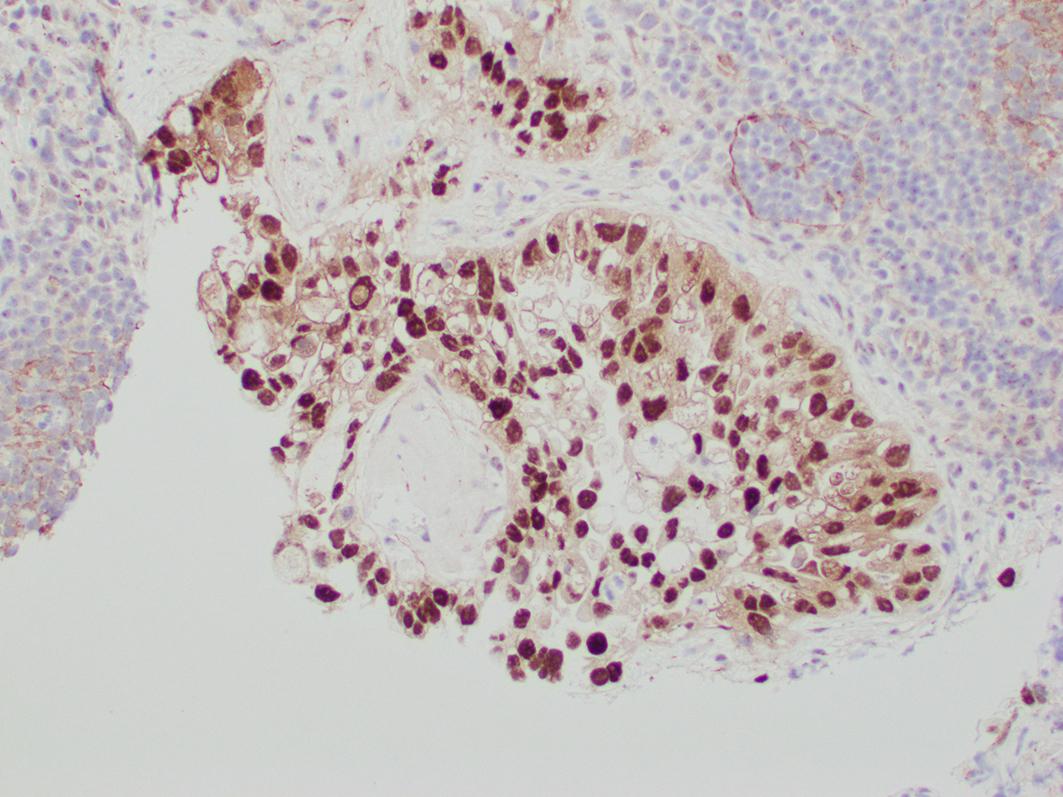
Clear cell carcinoma is typically positive for cytokeratin 7, PAX-8, HNF1-b, and Napsin, while negative or weakly positive for hormone receptors ER and PR ( Figs. 16.3–16.5 ). However, these markers are frequently not helpful in confirming the diagnosis. HNF1-b is not very specific and Napsin is not sensitive, hence negative staining for the latter does not exclude a diagnosis of clear cell carcinoma.
Benign mimics may include vaginal adenosis or mesonephric remnants, though these would lack typical nuclear atypia of clear cell carcinoma.
The differential diagnosis includes endometrioid adenocarcinoma and serous carcinoma, both of which can show clear cell change. Endometrioid carcinoma is usually diffusely positive for hormone receptors while clear cell carcinoma is negative. In some cases, distinguishing serous carcinoma from clear cell carcinoma may not be possible as both can overexpress p53 and, as mentioned earlier, the clear cell markers may not be helpful.
Extragonadal yolk sac tumors (YSTs) can be difficult to distinguish by morphology, though typically displaying Schiller–Duval bodies (papillae with central blood vessels), elevated alpha-fetoprotein (AFP) and usually a more reticular morphology. Immunohistochemical stains help, as clear cell carcinoma will be CK7 and EMA positive, while negative in YSTs. SALL4, CDX2 and villin are expressed in most cases of YST and are usually negative in clear cell carcinoma. HNF1-b is positive in both and is not a useful distinguishing marker in this differential.
Clear cell carcinomas associated with DES have genetic instability by mechanism of somatic mutations of microsatellite repeat sequences, which suggests that genes encoding DNA repair proteins may represent mutational targets of DES-induced mutagenesis. Non-DES vaginal clear cell carcinoma has been reported to harbor chromosomal imbalances, such as chromosomal gain at 20q and loss of heterozygosity at 9p along with PIK3CA mutation, which are also seen in ovarian clear cell carcinomas.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here