Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Otitis media is one of the most common diseases of childhood and may result in complications, including acute and chronic mastoiditis, petrositis, skull base osteomyelitis, intracranial infection, and the sequela of early childhood auditory deprivation.
Acute otitis media (AOM) may result in persistent otitis media with effusion (OME), which is currently recognized as the leading cause of childhood hearing loss.
Aural cholesteatomas are epidermal inclusion cysts of the middle ear or mastoid and are classified as congenital or acquired.
Cholesteatomas can be eradicated from the temporal bone only by surgical resection.
The mechanism of bone resorption in chronic otitis media with or without cholesteatoma is by the transient, intermittent activation of multinucleate osteoclasts.
Osteoclastic bone resorption in chronic otitis media is stimulated by a variety of factors, including inflammatory cytokines, localized pressure, keratin, and bacterial toxins.
Tympanosclerosis is tympanic membrane or middle ear hyalization. It results from chronic inflammation or trauma, and it often leads to conductive hearing loss by ossicular fixation.
Petrous apicitis is an extension of infection from the mastoid air cell tract into a pneumatized anterior or posterior petrous apex.
The management of petrous apicitis is directed toward control of the infection with topical and systemic antibiotics, and surgical drainage through one of several anatomic approaches to the petrous apex.
Otitis media is the most common disease of childhood, with the exception of viral upper respiratory infections. The acute bacterial infection of the middle ear occurs in 80% of children between the ages of 1 and 6 years, and it is the disease most frequently managed with antibiotics in the United States. In addition, there is significant racial and socioeconomic variation in the prevalence of otitis media in the United States. Although rare, the infectious and noninfectious complications of otitis media in childhood may result in serious morbidity. The infectious complications, including acute and chronic mastoiditis, petrositis, and intracranial infection, still occur despite widespread use of antibiotics. The noninfectious sequelae, including chronic perforation of the tympanic membrane, ossicular erosion, labyrinthine erosion, and tympanosclerosis, are major causes of hearing loss throughout the world.
Furthermore, some cases of acute otitis media (AOM) result in persistent otitis media with effusion (OME), which is recognized as the leading cause of childhood hearing loss. Although eustachian tube dysfunction alone may lead to effusion of the middle ear, there is substantial evidence that most cases of OME occur as a sequelae of AOM or at least share the same etiologic factors. In addition, data suggest that reflux of gastric contents may be associated with OME in children. The detection of Helicobacter pylori in middle ear effusions supports this hypothesis. Specific causes can be identified in many cases of adult-onset otitis media, such as paranasal sinus disease, nasopharyngeal tumors, and postirradiation sequelae.
In most children, AOM and OME subside spontaneously or after medical intervention. The percentage of children with OME who eventually have complications is unknown. The sequelae of otitis media can be considered in two broad categories: (1) direct destructive effects of the localized process and (2) the effects of auditory deprivation during early childhood. Otitis media may be complicated by acute or chronic perforation of the tympanic membrane, acute mastoiditis, middle ear atelectasis, adhesive otitis media, tympanosclerosis, ossicular erosion or fixation, petrous apicitis, cholesteatoma, chronic otomastoiditis, labyrinthitis, facial paralysis, and intracranial infection. There is evidence that sensorineural hearing loss may result from chronic otitis media with or without cholesteatoma. There is also evidence that the auditory deprivation associated with childhood otitis media may lead to indirect sequelae such as language and speech delays.
AOM is much less common in adults. However, chronic otitis media with and without cholesteatoma is a significant problem in the adult population. Most adults with chronic otitis media began having problems in childhood. Other factors in adulthood, such as irradiation for cancer of the skull base or head and neck cancer, may lead to osteoradionecrosis and otitis.
It has been observed that patients with a history of chronic OME have more sclerotic mastoids with decreased pneumatization compared with healthy subjects. There are two theories to explain this observation: (1) the hereditary theory, which states that children with hypoaeration of the mastoid are prone to OME, and (2) the environmental theory, which states that chronic OME results in hypopneumatization of the mastoid. Although measurable correlations between mastoid hypocellularity and OME and between the length of mastoid process or the degree of pneumatization and an abnormal eardrum have been proven, a cause-and-effect relationship is not clear. Available evidence generally supports the concept that chronic inflammation in early childhood may lead to new bone formation within the middle ear and mastoid and, subsequently, decreased size of mastoid air cells.
Middle ear atelectasis ( Fig. 140.1 ) is thought to result mainly from longstanding eustachian tube dysfunction. One of the principle functions of the eustachian tube is ventilation of the middle ear and mastoid. Intermittent opening of the eustachian tube allows exchanging of gases and equalization between the atmospheric air pressure in the nasopharynx and middle ear space. The middle ear gases also are exchanged with the middle ear mucosa. Bilateral diffusion between the middle ear cavity and the blood may be an important factor in middle ear atelectasis, because the gas composition of the middle ear basically resembles that of venous blood.
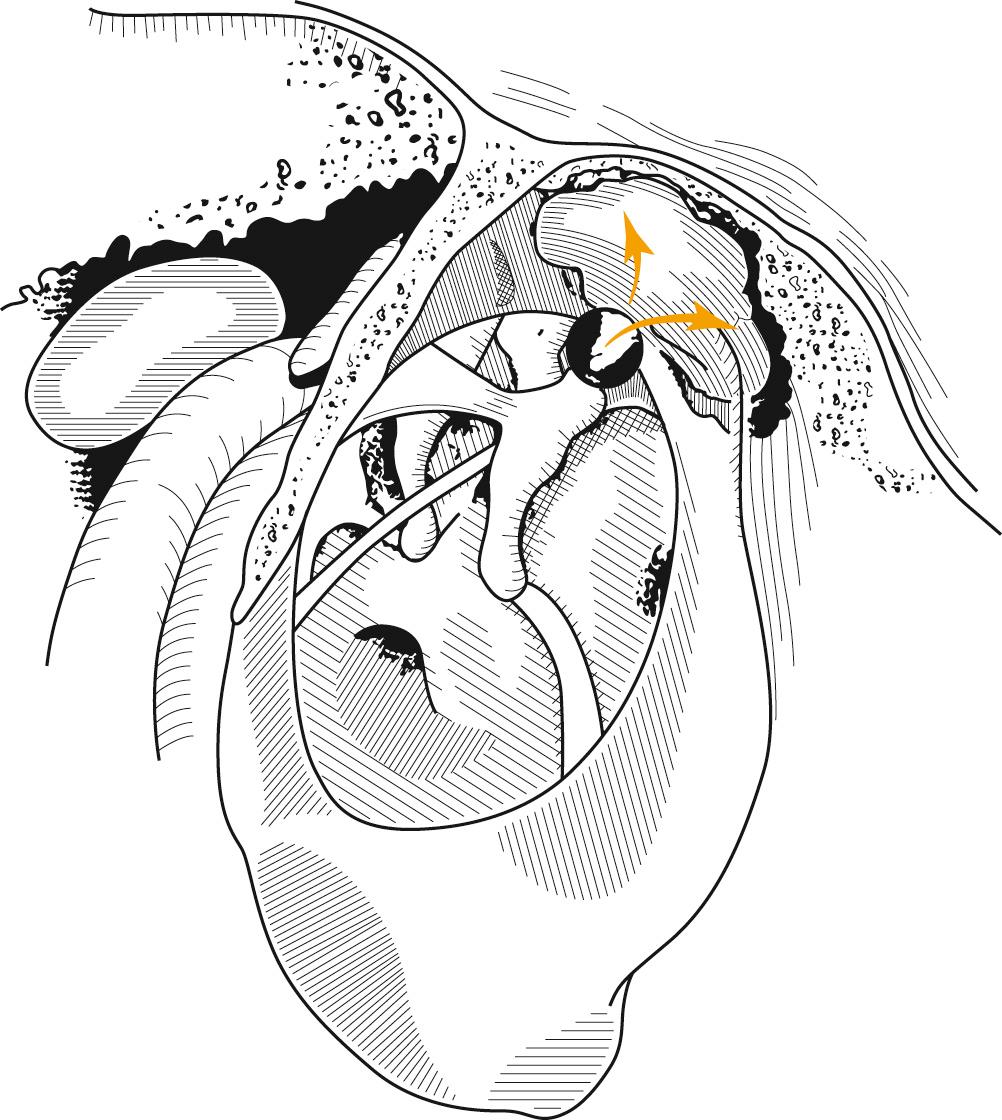
If atelectasis develops, the tympanic membrane becomes retracted onto the promontory and the ossicles of the middle ear. In atelectatic ears, the middle ear space is partially or completely obliterated, but the tympanic membrane is not adherent to the medial wall of the middle ear, and the mucosal lining of the middle ear is intact. In contrast, adhesive otitis media exists when the middle ear space is totally obliterated and the tympanic membrane is adherent to the ossicles and promontory; mucosal surfaces of the medial side of the tympanic membrane and the middle ear lining are lost. Retraction of the tympanic membrane may lead to erosion of the long process of the incus and the stapes suprastructure ( Fig. 140.2 ). Not all patients with chronic OME develop atelectasis; in most patients with OME, retraction of the tympanic membrane is limited. It may be that repeated bouts of AOM lead to weakening and thinning of the membrane, which allows atelectasis. Sadé and Berco demonstrated destruction of the collagen-containing fibrous layer of the tympanic membrane in some ears with recurrent infection. It is interesting to note that collagen destruction within the tympanic membrane may lead to another complication of OME, namely, tympanosclerosis. Sadé and Berco and Tos described a useful classification of tympanic membrane retraction: stage I, retracted tympanic membrane; stage II, retraction with contact onto the incus; stage III, middle ear atelectasis; and stage IV, adhesive otitis media ( Fig. 140.3 ).


Middle ear atelectasis may be reversible with the judicious use of ventilating tubes. Sadé showed that ventilating tubes improved the state of atelectatic ears. Graham and Knight reported three cases in which atelectatic tympanic membranes were restored to their normal position by administration of nitrous oxide during anesthesia and insertion of a ventilating tube. However, thinning of the lamina propria of the tympanic membrane makes retention of a tympanostomy tube rather tenuous, leading to early extrusion and failure of ventilation. Others have suggested that laser myringotomy may be efficacious.
Atelectasis and adhesive otitis media usually coexist with OME, although OME may resolve in these ears, allowing aeration of the attic and mastoid but leaving a collapsed middle ear. In extreme cases, when hearing loss or ossicular erosion occurs, a myringoplasty for the reinforcement of atelectatic tympanic membrane may be indicated. Cholesteatomas may originate from deep retraction pockets in which desquamated keratin debris will not be cleared into the ear canal. These retraction pockets may occur in the pars tensa or pars flaccida of atelectatic ears and should be considered precursors to cholesteatomas (see discussion of cholesteatoma).
Aural cholesteatomas are epidermal inclusions of the middle ear or mastoid. Cholesteatomas may appear as cystic structures or the presence of keratinizing epithelium within the middle ear cleft. They contain the desquamated debris (principally keratin) from their keratinizing, squamous epithelial lining. Cruveilhier first described aural cholesteatoma as a “pearly tumor” of the temporal bone. The term “cholesteatoma” was coined by Johannes Müller in 1838, because the white-yellow keratin flakes found within cholesteatomas grossly resemble cholesterol crystals. Actually, cholesteatomas contain a small amount of cholesterol.
Cholesteatomas of the temporal bone may be congenital or acquired. Acquired cholesteatomas usually arise as a sequelae of OME, AOM, or both. Some cholesteatomas are thought to be the result of chronic eustachian tube blockage, and others may result from trauma to the temporal bone with implantation of epidermis into the middle ear or mastoid. An understanding of the pathogenesis and pathophysiology of aural cholesteatoma is particularly important because it is the destructive nature of this entity that is responsible for much of the morbidity associated with chronic otitis media. The propensity of cholesteatomas to erode bone and the lack of effective, nonsurgical management add importance to the understanding of this disease.
The diagnosis of aural cholesteatoma is made on otoscopic examination, including endoscopic and microscopic evaluation, imaging, or surgical exploration. The symptoms of cholesteatoma vary; some cholesteatomas are asymptomatic, whereas others become infected and rapidly cause bone destruction. Some patients will present with slowly progressive conductive hearing loss and, frequently, with chronic otitis and purulent otorrhea. The otorrhea from an infected cholesteatoma often is malodorous because of the frequent infection with anaerobic bacteria. Patients with infected cholesteatomas occasionally are misdiagnosed as having external otitis. Therefore careful follow-up evaluation and thorough canal débridement of a patient with otorrhea are mandatory, because the cholesteatoma may not be evident during an acute flare-up. Some patients will have signs and symptoms of the complications of a cholesteatoma: vertigo and hearing loss caused by a labyrinthine fistula ( Fig. 140.4 fistula), facial nerve paralysis, or intracranial infection.
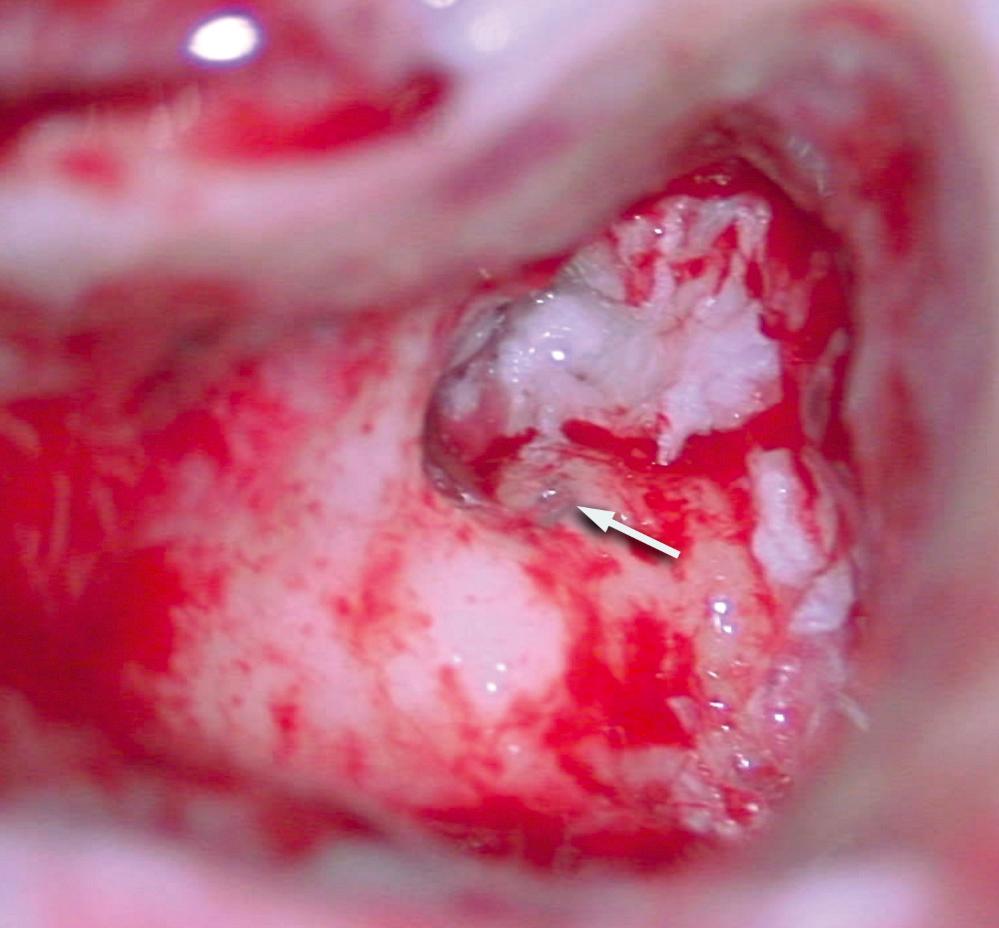
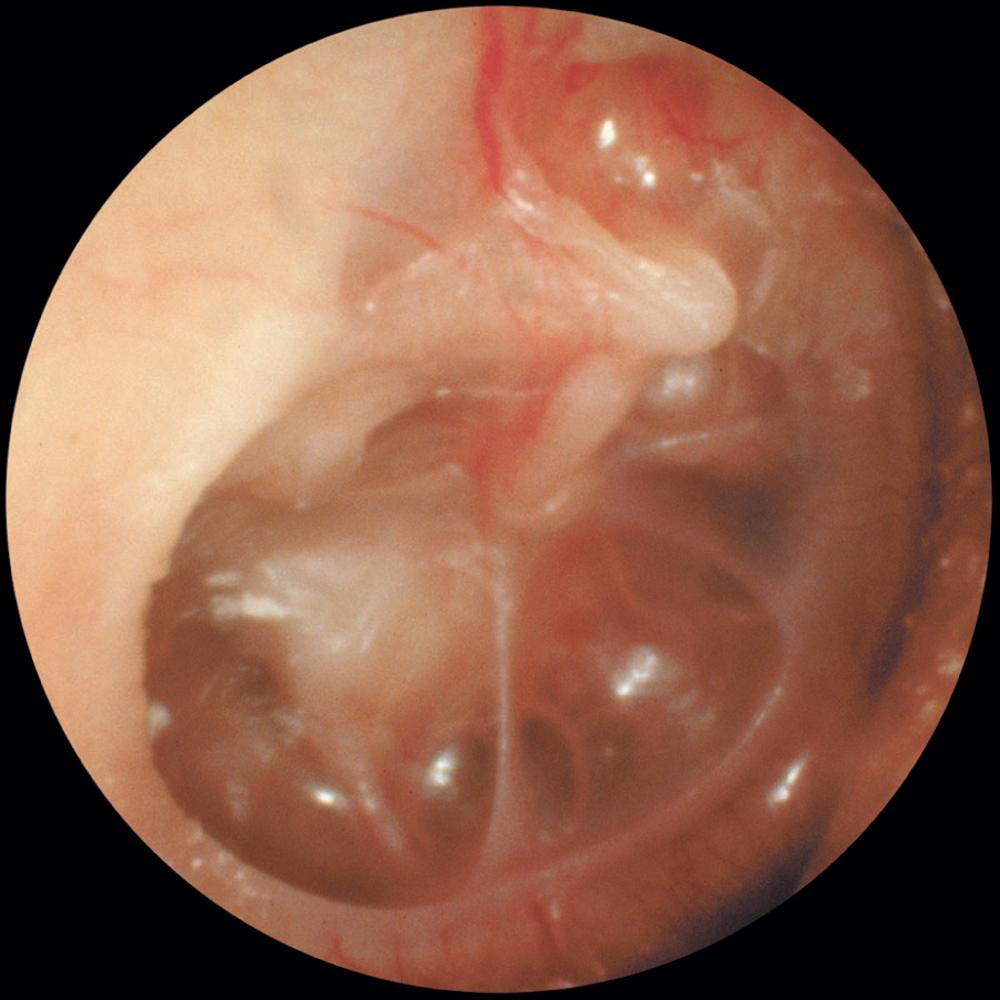
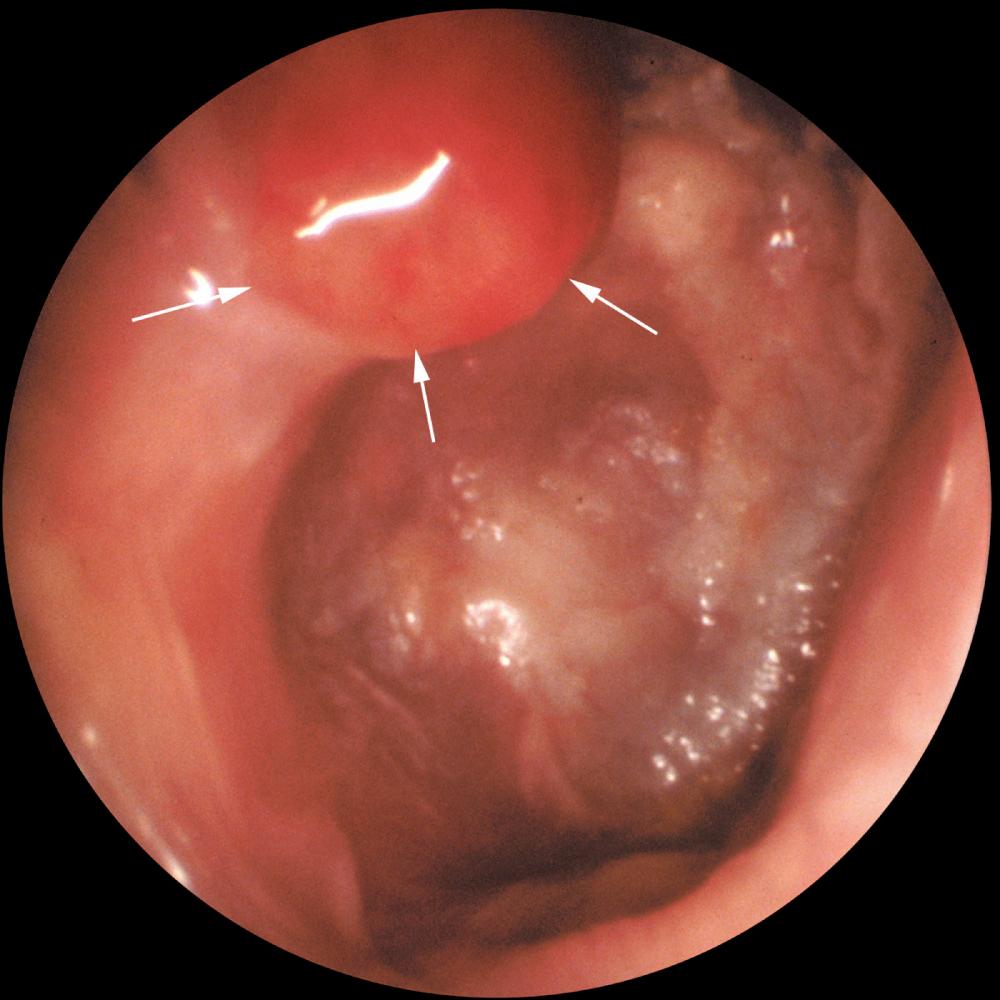
The otoscopic appearance of an aural cholesteatoma also is variable. A typical attic retraction cholesteatoma ( Fig. 140.5 ) appears as a defect of variable size adjacent to the posterosuperior portion of the tympanic membrane. The center of the defect contains keratin debris (primary acquired cholesteatoma). In other patients, keratinizing epithelium has migrated through a perforation into the middle ear ( Fig. 140.6 ; secondary acquired cholesteatoma). Cholesteatomas sometimes appear behind or within an intact tympanic membrane, so-called congenital cholesteatoma ( Fig. 140.7 ). An infected cholesteatoma will sometimes present as an “aural polyp.” These “polyps” actually are granulation tissue at the junction between an eroding cholesteatoma and bone ( Fig. 140.8 ). The presence of an aural polyp in a chronically infected ear should be considered to be a cholesteatoma until proven otherwise. Occasionally, a cholesteatoma cannot be seen otoscopically but will be discovered during tympanomastoid surgery.
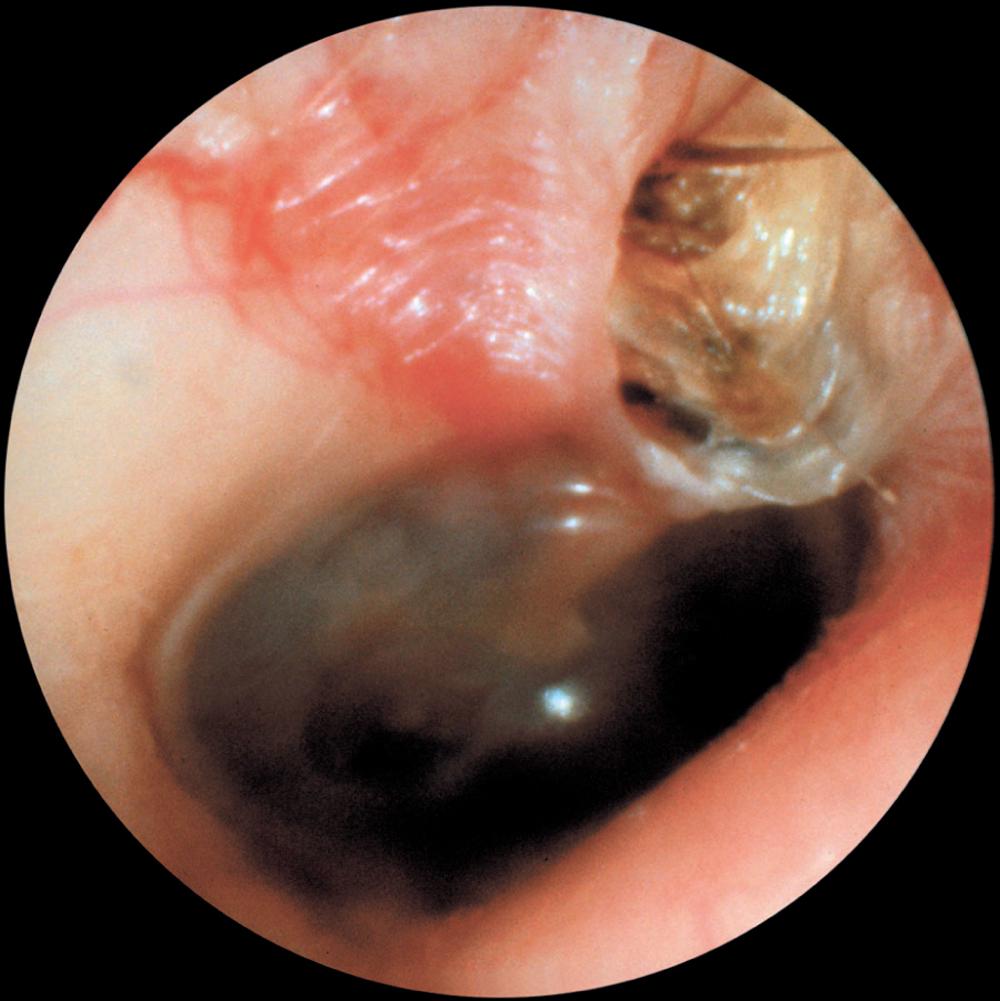

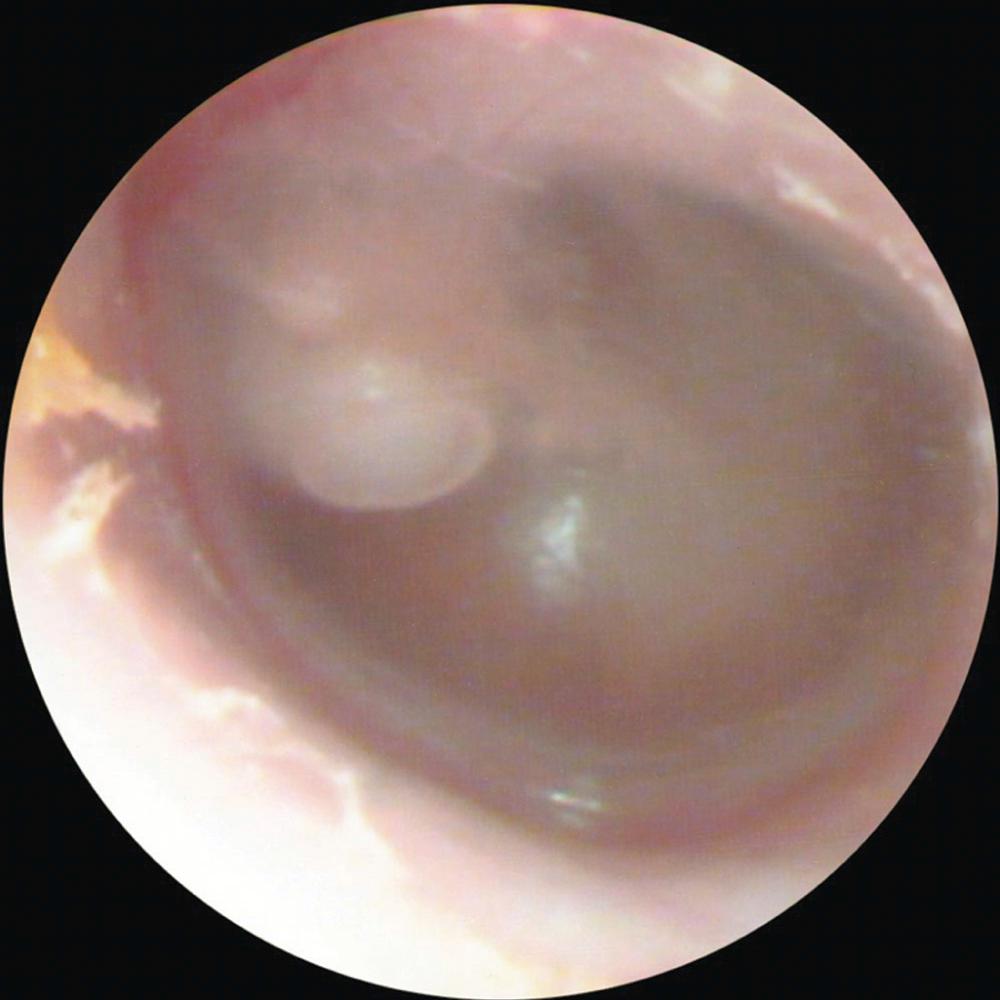
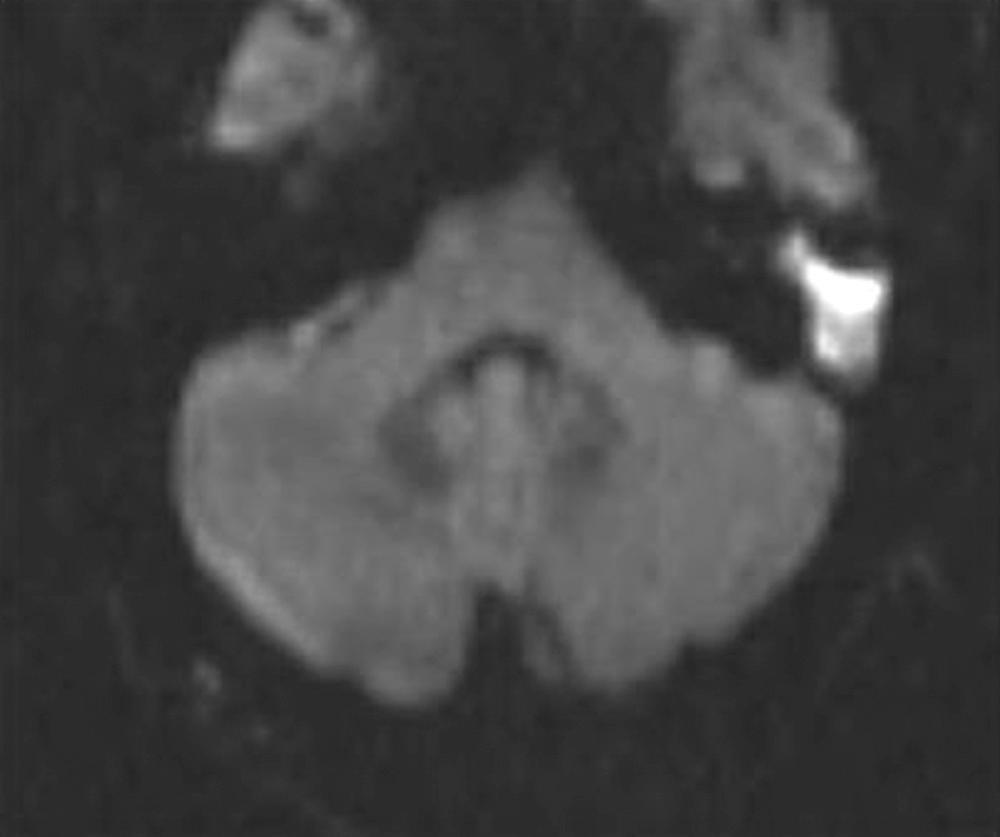
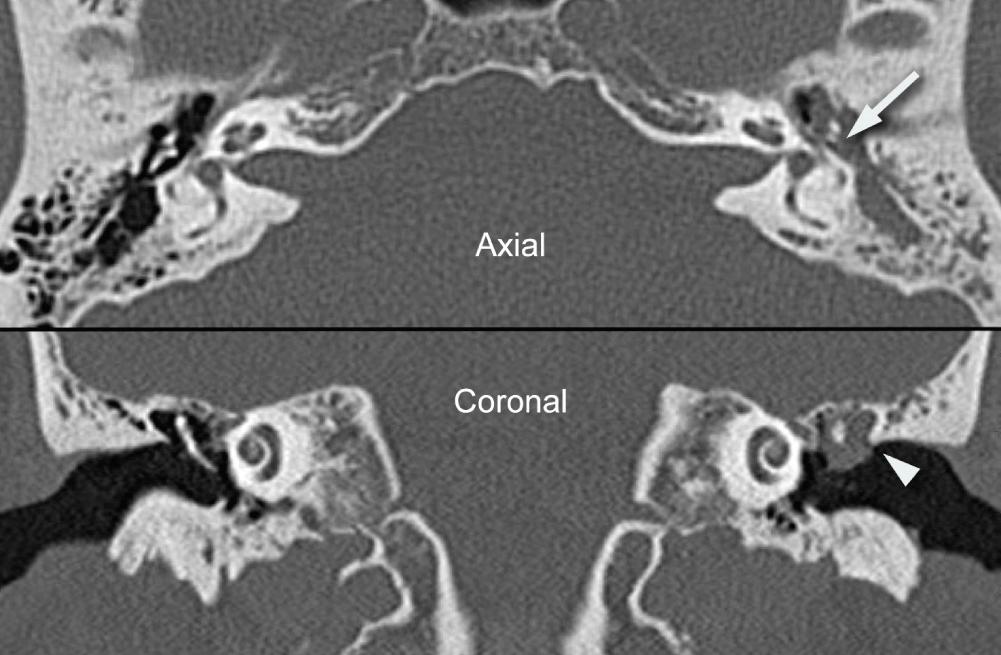
Imaging procedures, such as high-resolution computed tomography (CT) scanning and magnetic resonance imaging (MRI), may suggest the presence of cholesteatomas within the temporal bone and may be used to complement the clinical examination. Nonechoplanar diffusion-weighted MRI has been shown to be a helpful, albeit imperfect, technique for the detection of temporal bone cholesteatomas. However, a meta-analysis of published studies points out that there are false-positive and false-negative results of this imaging technique with a specificity and sensitivity of 94% ( Fig. 140.9 ). Bright signals on diffusion weighted imaging (DWI) should be correlated with low signal on apparent diffusion coefficient (ADC) maps to avoid false-positive readings. High-resolution CT scanning is useful for operative planning and is recommended for all revision and complex mastoid operations. Distinguishing fluid and granulation tissue from cholesteatoma can be difficult on CT. Erosion of the scutum on coronal imaging and demineralization of the incus are two good indicators of cholesteatoma ( Fig. 140.10 ).
The annual incidence of acquired cholesteatoma ranges from approximately 9 to 12.6 cases per 100,000 adults and from 3 to 15 cases per 100,000 children.
The exact annual incidence of cholesteatoma is unknown. Various investigators have estimated the annual incidence per 100,000 individuals at 3 to 15 in children and 9 to 13 in adults. In human temporal bones with chronic otitis media, cholesteatoma was observed in 36% of ears with perforations and in 4% of ears without the perforated tympanic membranes.
Congenital cholesteatomas, by definition, originate from areas of keratinizing epithelium within the middle ear cleft and usually arise in children without an antecedent history of otitis. Michaels showed that a small area in the anterior tympanum in the developing fetus often contains a small area of keratinizing epithelium. He found epidermoid formation in 37 of 68 temporal bones of fetuses at 10 to 33 weeks’ gestation. Congenital cholesteatomas may originate in this region; however, congenital cholesteatomas may arise from a variety of locations within the middle ear cleft. Some observations support the concept that these cholesteatomas are inherited. For example, Al Balushi and colleagues reported cholesteatomas in the right ear of identical twin boys. Similarly, bilateral congenital cholesteatomas have been reported by a number of observers. A genetic basis for congenital cholesteatomas has not been established.
Potsic and others, in a review of 172 series of congenital cholesteatomas, have developed a useful staging system: stage I, limited to one quadrant; stage II, involving multiple quadrants without ossicular involvement; stage III, ossicular involvement without mastoid extension; and stage IV, mastoid involvement. They showed a correlation between stage and risk of residual disease; stage IV carries a 67% risk of residual cholesteatoma after surgical resection.
The pathogenesis of acquired cholesteatoma has been debated for more than a century; it is likely that cholesteatomas arise in several different ways. There are four basic theories of the pathogenesis of acquired aural cholesteatoma: (1) invagination of the tympanic membrane (retraction pocket cholesteatoma), (2) basal cell hyperplasia, (3) epithelial ingrowth through a perforation (the migration theory), and (4) squamous metaplasia of middle ear epithelium ( Fig. 140.11 ). In addition, Sudhoff and Tos proposed a combination of the invagination and basal cell theories as an explanation for retraction pocket cholesteatoma formation. Recently, Jackler and colleagues suggested a new hypothesis for the pathogenesis of cholesteatomas: the mucosal traction theory.

The invagination theory of the genesis of acquired cholesteatoma is generally regarded as one of the primary mechanisms of the formation of attic, and sometimes pars tensa, cholesteatomas. These cholesteatomas are often referred to as primary acquired cholesteatomas. Retraction pockets of the pars flaccida deepen because of negative middle ear pressure and possibly repeated inflammation (see Fig. 140.2 ). As the retraction pocket deepens, desquamated keratin cannot be cleared from the recess, and a cholesteatoma results. The origin of such retraction pocket cholesteatomas is thought to be eustachian tube dysfunction (or OME) with resultant negative middle ear pressure ( ex vacuo theory). Usually, the pars flaccida, being less fibrous and less resistant to displacement, is the source of the cholesteatoma. The result of this type of cholesteatoma is an apparent defect in the posterosuperior quadrant of the tympanic membrane and erosion of the adjacent canal wall. Although these defects have the appearance of a marginal perforation, they are not perforations but rather invaginations. Sadé showed that epithelial migration patterns within attic retraction pockets are altered thus failure of epithelial migration may allow the accumulation of keratin within a retraction pocket, with subsequent enlargement merely from the accumulation of keratin within a relatively closed space. This theory has been supported by the experimental creation of retraction pockets by use of the eustachian tube obstruction and external auditory canal ligation. It is likely that the inflammatory process of repeated bouts of otitis media leads to weakening of the pars flaccida, making retraction more likely. Retraction pockets are considered as precursors to cholesteatoma. Bacteria can infect the keratin matrix, forming biofilms leading to chronic persistent infection. The presence of bacterial biofilms in the cholesteatoma matrix may lead to epithelial proliferation and invasion of the cholesteatoma.
Some observers have suggested variants of this theory, including the “selective epitympanic dysventilation syndrome” and the “mucosal traction theory.”
The epithelial invasion theory states that keratinizing squamous epithelium from the surface of the tympanic membrane invades or migrates into the middle ear from the margins of perforation in the tympanic membrane. These cholesteatomas are often referred to as secondary acquired cholesteatomas (secondary to a perforation of the tympanic membrane). This theory is supported by clinical observation and experimental evidence. Van Blitterswijk and Grote reported that cytokeratin (CK) 10, which was seen in the meatal epidermis and migrating epithelium, was preferentially expressed in the cholesteatoma matrix rather than in the middle ear mucosa. This finding suggests an epidermal (ear canal or lateral tympanic membrane) origin of cholesteatoma. Kim and others demonstrated increased CK 10 expression in the peripheral area of pars tensa of induced cholesteatoma by ear canal ligation and in the peripheral and the central area of pars tensa of induced cholesteatoma by eustachian tube obstruction. The findings of this study also support the basal cell hyperplasia hypotheses for the pathogenesis of aural cholesteatoma, with regard to hyperproliferation, migration, and an altered differentiation of keratinocytes. (See basal cell hyperplasia section.) The high level of fibronectin and tenascin and the focal disruptions of the basement membrane were reported in the middle ear cholesteatoma, which is produced in the context of migratory and hyperproliferative squamous epithelium. This theory also is supported by investigations at animal cholesteatoma models and human temporal bones. Jackson and Lim showed histologic and ultrastructural evidence that keratinizing epithelium can migrate into the cat bulla (middle ear and mastoid) by contact guidance. Palva and others have shown histologic evidence for this theory in human temporal bones. Therefore it is likely that in some tympanic membrane perforations, inflammation damages the inner mucosal lining of the tympanic membrane, allowing the outer keratinizing epithelium to migrate inward and generate a cholesteatoma. Cholesteatomas originating after temporal bone fractures may result from this mechanism; fractures within the ear canal may allow ingrowth of keratinizing epithelium by contact guidance.
Another possible mechanism for the histogenesis of cholesteatoma was first suggested by Lange. In this theory, he proposed that epithelial cells (“prickle cells”) of the pars flaccida could invade the subepithelial tissue by means of proliferating columns of epithelial cells. Nearly 40 years later, Ruedi supported this hypothesis with clinical and experimental evidence. For epithelium to invade into the lamina propria, the basal lamina (basement membrane) must be altered. Basal lamina disruptions now have been documented in human and animal cholesteatomas. Huang and others and Masaki and others provided experimental support of this theory by demonstrating that epithelial ingrowth from the tympanic membrane can be induced by instillation of propylene glycol into the middle ear of chinchillas; the resulting basal lamina breaks allow the invasion of epithelial cones into the subepithelial connective tissue and the formation of microcholesteatomas. This mechanism may explain some types of human cholesteatomas, even those occurring behind an intact tympanic membrane. According to this theory, microcholesteatomas may enlarge and then perforate secondarily through the tympanic membrane, leaving the typical appearance of an attic cholesteatoma. This sequence of events has not been documented, although the alternations in the differentiation of keratinocytes and basal cell layer of cholesteatoma matrix have been observed in several studies.
If proliferating keratinocytes invade through the basal lamina, one would expect them to express factors associated with epithelial proliferation. Indeed, a number of investigators have shown that the keratinocytes of cholesteatomas express these factors. Abnormal distribution of epidermal differentiation markers, such as filaggrin and involucrin, c-Jun and p53 proteins, and increased epidermal growth factor (EGF) receptor, has been shown in middle ear cholesteatoma matrix. Increased levels of proteins CK 13 and 16, which are markers for differentiation and hyperproliferation, were also found. Kim and others demonstrated the increased expression of CK 13 and 16 in the area of the peripheral area of pars tensa of induced cholesteatoma by ear canal ligation and in the peripheral and central area of pars tensa of induced cholesteatoma by eustachian tube obstruction. ErbB-2 protein was found to be overexpressed, and cell proliferation and apoptosis of keratinocytes were accelerated by Sakamoto and others. Caspases play a key role in apoptosis; Miyao and others suggested that caspase-8, which is activated by the induction of tumor necrosis factor-α, leads to activation of caspase-3, which activates apoptotic nucleases in cholesteatoma tissue.
It has been suggested that the fibroblasts of the subepithelial region of cholesteatomas can invade adjacent tissues. Data from Parisier and others suggested that fibroblasts in the subepithelium of cholesteatomas showed an invasive phenotype, whereas those from postauricular and ear canal skin appeared either weakly invasive or not invasive. In a similar study, Chole and others found that normal fibroblasts and fibroblasts from induced cholesteatomas did not exhibit the invasive phenotype characteristics of true neoplastic cells.
Other lines of evidence support the basal cell hyperplasia/migration theory. Increased expression of human intercellular adhesion molecules 1 and 2 has been demonstrated, suggesting a role in cell migration into tissue. The presence of heat shock proteins 60 and 70 suggested proliferation and active differentiation of basal keratinocytes in cholesteatomas. There are some reports that immune response is involved in the hyperproliferative state of cholesteatoma epithelium. Langerhans's cells may initiate immune reaction and promote proliferation of keratinizing epithelium via an interleukin-1α (IL-1α) and transforming growth factor-β (TGFβ) mechanism.
Wendt theorized that the simple squamous or cuboidal epithelium of the middle ear cleft could undergo a metaplastic transformation into keratinizing epithelium. According to this theory, an area of keratinizing epithelium within the middle ear would enlarge because of accumulated debris and form a pearl-like cyst in the middle ear. With intercurrent infection and inflammation, the cholesteatoma would lead to lysis of the tympanic membrane and secondary perforation, resulting in the typical appearance of an attic cholesteatoma (see Fig. 140.2 ). This theory is supported by the demonstration that biopsy specimens from the middle ear of children with OME sometimes contain islands of keratinizing epithelium. However there is no human or animal model support of this concept.
A new hypothesis on cholesteatoma pathogenesis has been proposed. The theorized mechanism is that opposing mucosal surfaces of the medial side of the tympanic membrane and the lateral surface of the ossicles propel the pars flaccida into the attic forming an invagination. This pocket is then propagated superiorly, deepening the retraction and collecting debris, by several proposed mechanisms. Firstly, the authors hypothesize that mucociliary clearance could propel the adherent mucosal surfaces upward into the attic. Secondly, net movement of the epithelial cell layer could drag the retraction pocket. Finally, they propose that adhesive forces from trapped mucous could also lead to enlargement of the retraction pocket. They point to a dearth of cholesteatomas reported in patients with primary ciliary diseases, such as cystic fibrosis and primary ciliary dyskinesia, as evidence that ciliary function may be a prerequisite for cholesteatoma formation. This theory has been challenged by others, who argue that histologic studies do not show ciliated epithelial cells around the ossicles and on the medial surface of pars flaccida. In fact, Pauna et al. studied the histology of ears with cholesteatomas, cystic fibrosis, chronic otitis media with and without retraction pockets, and controls. They found that ciliated cells in the epitympanum were rare in all groups but even rarer in ears with cholesteatoma. This finding does not support the notion that retraction pockets caused by adherence of opposing mucosal surfacing can be propagated by mechanisms related to ciliary activity.
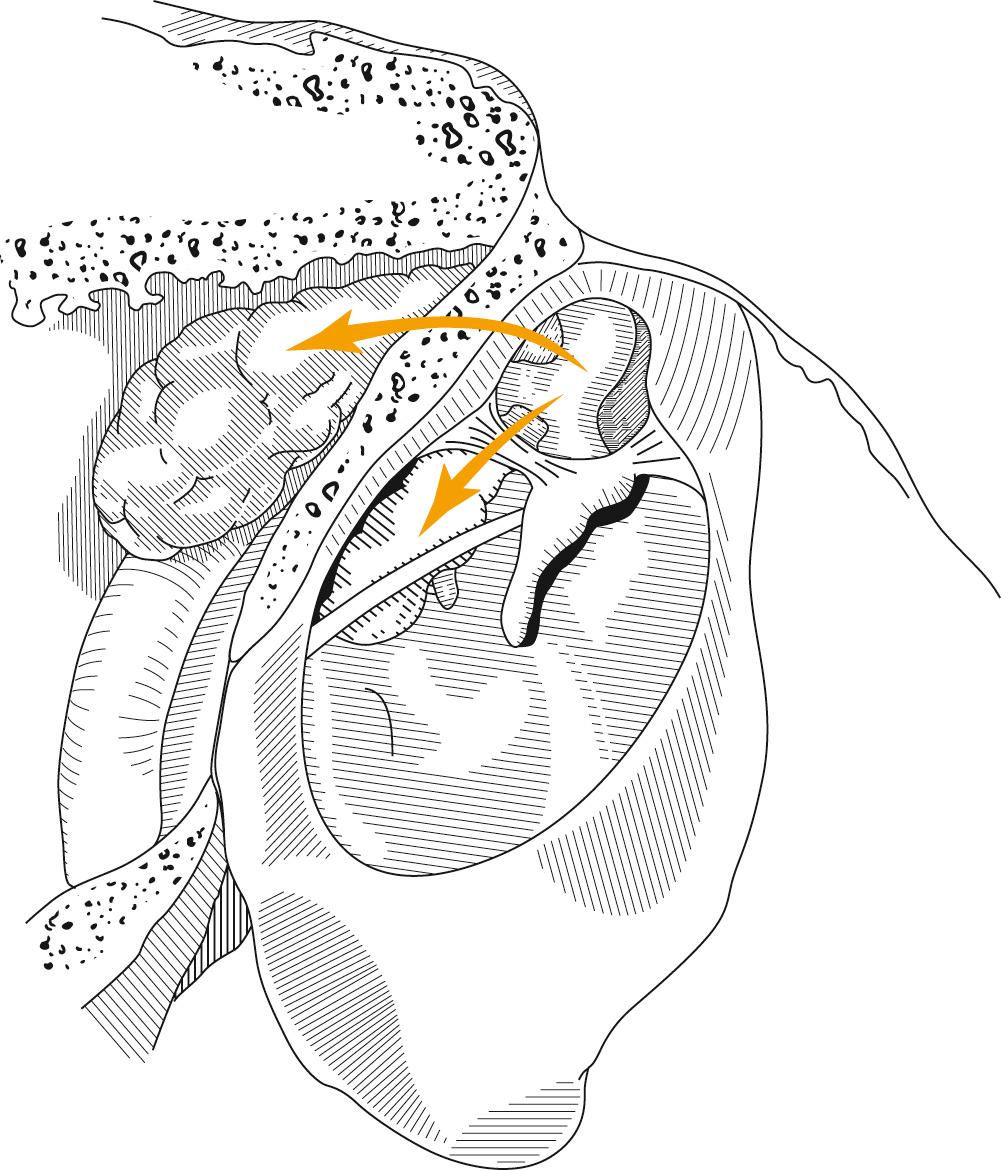
From a clinical perspective, it seems that each of these pathogenic mechanisms may account for a proportion of acquired cholesteatomas. Regardless of the pathogenesis of aural cholesteatomas, they all share certain properties. Cholesteatomas are prone to recurrent infection, and they characteristically erode the bone of the ossicles and the otic capsule. Clinicians have observed that cholesteatomas seem to be more aggressive when infected. There is currently evidence that infection increases tissue destruction in animal experiments. Aural cholesteatomas originating from the vicinity of the tympanic membrane exhibit typical growth patterns into the temporal bone. Because most acquired cholesteatomas originate by invagination of the pars flaccida, their growth is limited by the mucosal folds and suspensory ligaments of the ossicles. The pars flaccida may invaginate into the lateral-most portion of the epitympanum (Prussak space) and then into the recesses of the epitympanum posteriorly, lateral to the body of the incus, inferiorly into the middle ear by way of the pouch of von Tröltsch ( Fig. 140.12 ), or anteriorly into the protympanum ( Fig. 140.13 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here