Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Advanced Biliary Cancer 2
cholangiocarcinoma
cell division kinase
confidence interval
cyclooxygenase 2
computed tomography
external-beam radiation therapy
epidermal growth factor
epidermal growth factor receptor
endoscopic retrograde cholangiography
endoscopic ultrasound
fluorescence in situ hybridization
fibroblast growth factor receptor 2
FLICE-inhibitory protein
hepatitis B virus
hepatocellular-cholangiocellular carcinoma
hepatitis C virus
hepatocyte growth factor
human Mut L homolog 1
hazard ratio
human telomerase reverse transcriptase
isocitrate dehydrogenase
interleukin 6
interleukin 33
mitogen-activated protein kinase
myeloid cell leukemia 1
matrix metalloproteinase
magnetic resonance cholangiography
magnetic resonance imaging
nitric oxide
odds ratio
photodynamic therapy
primary sclerosing cholangitis
percutaneous transhepatic cholangiography
radiation therapy
stereotactic body radiotherapy
signal transducer and activator of transcription
tumor necrosis factor
tumor node metastasis
WNT1-inducible signaling pathway protein 1
Cholangiocarcinoma (CCA) is a malignancy of the bile ducts which has rising incidence. CCA is caused by malignant transformation of cholangiocytes—the epithelial cells that line the bile ducts—and/or possibly biliary stem cells and hepatocytes. CCA accounts for 10% to 20% of all hepatobiliary neoplasms and is the second most common primary liver tumor. Anatomically, CCA is divided into three types (intrahepatic, perihilar, and distal; Fig. 47-1 ) based on its location along the biliary tree, each having discrete epidemiologic and clinical features and probable independent etiopathogenetic origins. Despite recent progress, a better understanding of CCA biology is needed. This increase in knowledge is likely to improve early detection and hopefully therapy for CCA.
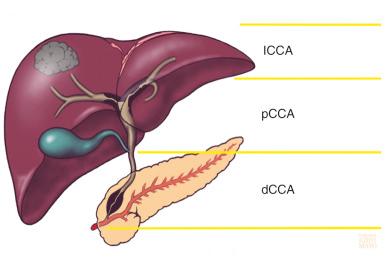
The incidence of CCA has increased over past decades. Approximately 5000 new cases of CCA are diagnosed every year in the United States. However, it should be noted that in several registries perihilar CCAs are classified as intrahepatic CCAs (perihilar CCAs involve the right and left hepatic ducts and their confluence and extend from the insertion of the cystic duct up to the second-degree bile ducts). We believe this classification to be erroneous and consider perihilar CCAs a distinct subtype given their clinical and biologic characteristics. Mixed hepatocellular-cholangiocellular carcinoma (HCC-CCA) is the newly recognized phenotype of CCA. Mixed HCC-CCA expresses markers of hepatocellular and biliary differentiations and represents less than 1% of liver tumors. In this text we focus on the main subtypes of CCA (intrahepatic, perihilar, and distal).
The epidemiology of intrahepatic as opposed to perihilar and distal CCA is different. Possible misclassification between these three kinds of CCA may affect reported epidemiologic parameters. In the United States, based on the Surveillance, Epidemiology, and End Results registries, the age-adjusted incidence rate of intrahepatic CCA increased slightly from 0.92 per 100,000 in 1992-1995 to 0.93 per 100,000 in 2004-2007 ( p = 0.07). In contrast, the incidence of extrahepatic CCA increased significantly from 0.70 per 100,000 in 1992-1995 to 0.95 per 100,000 in 2004-2007 ( p < 0.001). These data took into consideration prior misclassification of perihilar CCA as intrahepatic CCA. Because of the epidemiologic disparity, we discuss the epidemiology of intrahepatic, perihilar, and distal CCA independently.
The incidence of intrahepatic CCA varies across the world. It is highest in northeast Thailand (96 per 100,000 men and 38 per 100,000 women), probably due to the high prevalence of liver-fluke infestations. In the past, the average age of diagnosis of intrahepatic CCA was in the mid-50s, but there has been a recent shift in age of diagnosis towards the mid-60s. This observation might relate to: (1) development of CCA in the context of ever-increasing chronic liver disease in the aging population; and (2) improved diagnosis, follow-up, and management of risk factors (i.e., primary sclerosing cholangitis [PSC], choledochal cysts) in younger individuals. Caucasians and African-Americans have a comparable age-adjusted incidence of intrahepatic CCA. In contrast, the incidence among Asians is twice as high as that of Caucasians. However, Caucasians are the only ethnic group in which a gradual increase in the age-adjusted incidence of intrahepatic CCA is reported.
The mortality related to intrahepatic CCA is also increasing worldwide. In fact, the percentage increase in mortality from intrahepatic CCA was greater than that observed for hepatocellular carcinoma. In the United States, the age-adjusted mortality rate for intrahepatic CCA increased from 0.07 per 100,000 in 1973 to 0.69 per 100,000 in 1997. Mortality rates from intrahepatic CCA in American Indian and Native Alaskan groups reach 1.3 per 100,000 and 1.4 per 100,000, respectively. Overall, the 5-year survival of patients with intrahepatic CCA remains disappointingly low and practically unchanged over the decades. This lack of progress occurs despite earlier detection and employment of aggressive surgical approaches (i.e., hepatectomy, extended lobectomy, vascular reconstruction, and portal vein embolization).
The incidence of perihilar and distal CCA also differs across the globe. In the United States, the age-adjusted incidence of extrahepatic CCA has been reported to be 1.2 per 100,000 for men and 0.8 per 100,000 for women. As mentioned earlier, the overall incidence of extrahepatic CCA is increasing. Correspondingly, cumulative mortality rates have also increased by 39%. Mortality rates are higher in men compared with women (1.9 per 100,000 and 1.5 per 100,000, respectively).
Most patients diagnosed with CCA do not have or have not been exposed to known risk factor(s) associated with the disease ( Table 47-1 ). Primary sclerosing cholangitis (PSC) is a definite risk factor for CCA. The risk for developing CCA in a patient with PSC is approximately 1.5% per year after diagnosis of the cholestatic liver disease. Interestingly, among PSC patients who develop CCA, approximately 50% are found to have malignancy of the bile duct within 2 years of the diagnosis of PSC. This risk lowers to 7% for the 2- to 10-year period after diagnosis of PSC.
|
Liver flukes, namely Opisthorchis viverrini and Clonorchis sinensis, are strongly associated with CCA. These liver worms inhabit the bile ducts and sporadically the gallbladder. Individuals become infected with these parasites by eating undercooked fish. In patients with choledochal cysts (i.e., congenital cystic dilatation of bile ducts) the lifetime risk of developing CCA is approximately 10% to 15%, and the median age of diagnosis is 34 years. Intrahepatic biliary stones (i.e., hepatolithiasis) are frequent in Asia but rare in western countries. Studies have reported the association of hepatolithiasis with peripherally located intrahepatic CCA. Thorotrast is a colloidal suspension of 232 ThO 2 , which mainly emits alpha-particles and was used as a contrast agent in radiology from the 1930s to the 1950s. Thorotrast causes microsatellite instability and subsequently CCA, probably via clonal expansion of cholangiocytes and inactivation of human Mut L homolog 1 (hMLH1) by hypermethylation. In addition, toxins like dioxin and polyvinyl chloride have been postulated to contribute to development of CCA. Recently, chronic hepatitis B virus (HBV), hepatitis C virus (HCV), and cirrhosis have been added to the list of risk factors for development of intrahepatic CCA. HCV has a stronger association with intrahepatic CCA in western countries and Japan. A HBV association with CCA has been demonstrated in Asian countries. Metabolic syndrome, diabetes mellitus, and obesity were also associated with intrahepatic CCA in recent epidemiologic studies. Common features among several of the risk factors of CCA include chronic inflammation of the bile ducts and cholestasis. As discussed later in this chapter, both these events are likely to contribute to the malignant transformation of biliary epithelia.
Recently, there has been considerable progress in understanding the molecular pathogenesis of CCA. It is widely accepted that malignant transformation of biliary epithelium takes place amidst a milieu of chronic inflammation of the bile ducts along with persistent cholestasis. This environment causes increased production of cytokines and reactive oxygen species, resulting in protracted cellular (i.e., cholangiocyte) stresses and accrual of irreversible DNA damage. Subsequently, cholangiocytes undergo malignant transformation, namely, attain molecular changes and develop cellular and subcellular characteristics that are otherwise lacking in normal conditions. Table 47-2 shows the proposed contributing pathways. The molecular alteration of cholangiocytes, which leads to carcinogenesis is a multifaceted process of interrelated events. A recent integrative molecular analysis of 119 intrahepatic CCAs identified two gene signatures, an “inflammatory” class and a “proliferation” class. The former demonstrated activation of pathways responsible for overexpression of cytokines and signal transducer and activator of transcription 3 (STAT3). The proliferation subtype (62% of cases), demonstrated alterations in expression of oncogenes KRAS, RAS, MAPK, MET, and BRAF, among others. CCA subtypes exhibit differing biologic behavior that is likely dependent on genetic alterations. Pathogenic pathways contributing to intrahepatic CCA are not necessarily pertinent to perihilar or distal CCAs. For example, alterations in isocitrate dehydrogenase 1 (IDH1) and DNA repair genes are observed in intrahepatic CCA (18% of intrahepatic CCA), whereas aberrations of the ERBB2 oncogene occur in extrahepatic CCA (25% of tumors). Provocatively, the most recent studies with genetic models demonstrated the potential contribution of differentiated liver cell subtypes, including hepatocytes, to cholangiocarcinogenesis. This might explain why viral hepatitis and cirrhosis are risk factors for CCA. Despite impressive advancements, the tempo of the research is limited by difficulties in sample acquisition from perihilar CCAs. These tumors are highly desmoplastic and therefore obtaining adequate cells for studies is challenging. Some of the major genetic aberrations observed in CCA are discussed later in this chapter.
| Contribution in Carcinogenesis | Molecular Mechanisms | References |
|---|---|---|
| Autologous proliferation signaling | IL-6, gp80/gp130 up-regulation | Sugawara et al. |
| HGF/ c-met up-regulation | Yokomuro et al., Lai et al. | |
| EGF/c-erbB-2 up-regulation | Ito et al., Kiguchi et al. | |
| COX-2 up-regulation | Chariyalertsak et al., Endo et al., Yoon et al. | |
| KRAS mutations | Kang et al., Tannapfel et al. | |
| Loss of antigrowth signaling | p53 mutations | Kang et al. |
| p21/WAF mutations | Furubo et al. | |
| Mdm2 up-regulation | Furubo et al. | |
| p16 INK4 mutation | Tannapfel et al., Ahrendt et al. | |
| Evasion of apoptosis | FLIP up-regulation | Que et al. |
| NO inhibition of caspases | Torok et al. | |
| Bcl-2 up-regulation | Harnois et al. | |
| Bcl-xL up-regulation | Okaro et al. | |
| Mcl-1 up-regulation | Yoon et al. | |
| COX-2 up-regulation miR-29 down-regulation |
Nzeako et al. Mott et al. |
|
| Unlimited replicative potential | Telomerase activity | Itoi et al. |
| Angiogenesis | VEGF overexpression | Benckert et al. |
| Tissue invasiveness and metastasis | E-cadherin down-regulation | Ashida et al. |
| α-catetin and β-catetin down-regulation | Ashida et al. | |
| Matrix metalloproteinase (MMP) up-regulation | Terada et al. | |
| Human aspartyl (asparaginyl) β-hydroxylase overexpression WISP1v overexpression |
Lavaissiere et al., Ince et al., Maeda et al. Tanaka et al. |
|
| Epigenetic modifications | IDH1 , IDH2 hot spot mutations | Churi et al., Saha et al. |
| Survival signaling | FGFR2 fusions; Notch, Hedgehog pathway overexpression | Razumilava et al., Ang et al., Borad et al. |
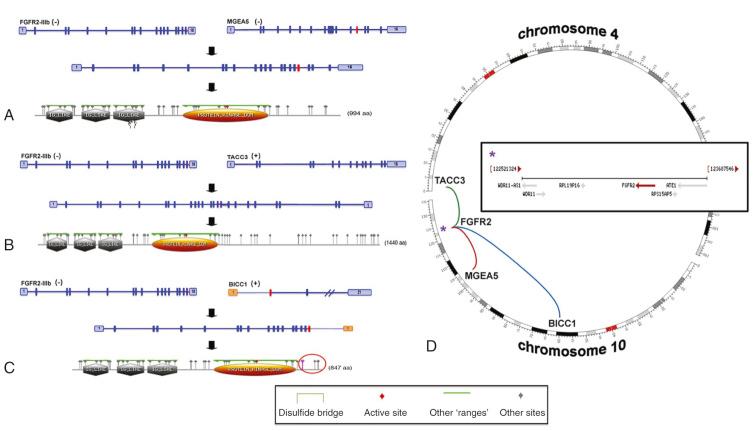
Dysregulation of cell proliferation, differentiation, and morphogenesis can lead to malignant transformation. For instance, the fibroblast growth factor receptor (FGFR) is a transducer of signals for cell differentiation and mitogenesis. Several FGFR2 gene fusions have been described in CCA, including FGFR2-BICC1, FGFR2-MGEA5, and FGFR2-TACC3 ( Fig. 47-2 ). It was proposed that FGFR fusion partners mediate oligomerization, which triggers activation of the respective FGFR kinase. The FGFR2 tyrosine kinase fusion occurs in up to 17% of intrahepatic CCAs and is associated with a more indolent clinical course. FGFR inhibitors are available for use in a clinical practice and were utilized in patients with CCA as the first example of targeted therapy for biliary cancer. The FGFR2-MGEA5 fusion treated with ponatinib and the FGFR2-TACC3 fusion treated with pazopanib and subsequently ponatinib demonstrated clinical effectiveness in patients with intrahepatic CCA. However, consecutive resistance development and off-target effects are still drawbacks of these therapies.
Under normal conditions, cholangiocytes retain tissue homeostasis despite exposure to proliferation signals. However, chronic biliary inflammation causes local interleukin 6 (IL-6) and hepatocyte growth factor (HGF) production, which are initially derived from periductal stromal cells (i.e., stellate cells). IL-6 is a powerful mitogen involved in cholangiocyte proliferation, as reported in both animal and human studies. IL-6 binds to its plasma membrane receptor, forming the active heterodimer, gp80/gp130 that in turn stimulates cellular transcription through the mitogen-activated protein kinase (MAPK)/signal transducer and activator of transcription (STAT) pathway. Interestingly, malignant, but not normal, cholangiocytes also produce high levels of IL-6 and overexpress the gp80/gp130 heterodimer. A new genetic model of CCA suggests that biliary mitogen interleukin 33 (IL-33) imposes its carcinogenic effect via the IL-6 axis. HGF also promotes cholangiocyte growth via its plasma membrane receptor, c-met. In addition, CCA cells achieve an endogenous capacity to produce HGF and up-regulate its c-met receptor. Hence, through the IL-6 and HGF pathways malignant cholangiocytes maintain autologous proliferating mechanisms.
Another mechanism that contributes to cholangiocarcinogenesis is the signaling activation of epidermal growth factor (EGF) and its receptor (EGFR). Interaction of EGF with EGFR leads to activation of the MAPK pathway. The c-erb-B2 protein, a homolog of EGFR, is a tyrosine kinase that is activated in CCA. Indeed, constitutive expression of c-erb-B2 in gallbladder epithelium leads to adenocarcinoma. When a patient with a mutation in the gene encoding the direct negative regulator of EGFR, ERRFI1, was treated with an EFGR inhibitor, erlotinib, a rapid response with tumor regression was observed.
Cyclooxygenase 2 (COX-2), an isoform that catalyzes the formation of prostaglandins from arachidonic acid, is induced by mitogens and cytokines and is involved in the pathogenesis of CCAs. COX-2 is overexpressed in malignant, but not normal, cholangiocytes. The complex and interrelated processes of carcinogenesis in bile ducts is indicated by the fact that IL-6, HGF, and EGF stimulate COX-2 expression in cholangiocytes. The exact mechanism by which COX-2 causes CCAs is uncertain, but probably involves inhibition of apoptotic pathways.
KRAS is an oncogene that plays an important role in mitogenic cellular signals. Mutations of this gene have been detected in over 20% of biopsy-proven CCAs. KRAS mutations have been associated with perihilar CCAs and periductal tumor extension.
In addition to biliary inflammation as a precipitant of cholangiocarcinogenesis, CCA demonstrates an inherent tropism for bile. For example, bile acids have been reported to transactivate the EGFR and to promote the expression of COX-2 in cholangiocytes.
Critical pathways that inhibit cell proliferation are usually lost during cholangiocarcinogenesis. For instance, loss of heterozygocity for the tumor suppressor gene p53 is frequent in CCA with p53 genetic alterations observed in up to 35% of intrahepatic and 45% of extrahepatic CCAs. This gene alteration is also a most frequent finding in a fluke-associated CCA (44%). The p53 gene directs the cellular machinery of cell cycle and apoptosis. Regarding the cell cycle, p53 regulates the p21/WAF1 (wild-type p53 activated fragment 1) protein which binds to the cell division kinase (CDK) 4–cyclin D complex. Thus, p53 causes negative feedback of the CDK4–cyclin D complex, therefore averting phosphorylation of Rb and, as a result, release of the E2F transcription factor. Moreover, p53 can induce apoptosis by promoting Bax insertion in the mitochondrial membrane and stimulating mitochondrial depolarization and subsequent apoptosis. Inactivation of the p14/mdm/p53 pathway and the p16 (a tumor suppressor gene) via a variety of molecular mechanisms has been described in de novo CCA and in PSC-associated CCA. As shown in Table 47-2 , p53 , p21/WAF1 , and p16 mutations are involved in loss of critical cell signaling, which may allow the development of CCA.
Apoptosis is an important cellular mechanism that controls tissue homeostasis. Disarrangement of apoptosis may lead to aberrant cell proliferation. Ligand activation of the Fas/TRAIL (tumor necrosis factor [TNF]-related apoptosis-inducing ligand)/TNF-receptor family or release of cytochrome c by mitochondria causes activation of caspases, resulting in DNA fragmentation and cell destruction. As mentioned earlier, both chronic biliary inflammation and chronic cholestasis cause persistent cholangiocyte stress and DNA damage. Thus, apoptosis serves as a scavenger for cells that develop malignant transformation. Loss of the protective function of apoptosis in cholangiocytes may result in development of CCA. Cholangiocytes express the Fas receptor on plasma membrane and respond to FAS ligand stimulation with apoptosis. In a CCA cell-line there was diminished responsiveness of the Fas/FAS ligand due to alteration of the FLICE-inhibitory protein (FLIP). FLIP inhibits activation of procaspase 8, causing attenuated signaling via the Fas/FAS ligand pathway. Moreover, cholangiocytes under the effect of nitric oxide (NO) display inhibition of both caspase 3 and 9, probably via nitrosylation, and become relatively resistant to apoptosis. COX-2 induced by inflammation is antiapoptotic. Up-regulation of COX-2 inhibits Fas/FAS ligand-induced apoptosis by increasing expression of the inhibitory protein myeloid cell leukemia 1 (Mcl-1). Interestingly, bile acids, which are elevated in CCA have a positive effect on the expression of Mcl-1 protein via inhibition of proteosome degradation. Besides bile acids, inflammatory mediators could up-regulate Mcl-1. As a result, potential malignant cholangiocytes are averted from apoptosis. Importance of Mcl-1 is also emphasized by a presence of Mcl-1 genetic aberrations in 16% of cases of intrahepatic CCAs.
Cancer survival advantages can be also achieved by epigenetic changes regulated by a milieu of means including methylation. Mutations of the enzymes participating in regulation of methylation, IDH1 and IDH2, can lead to overproduction of 2-hydroxyglutarate. Accumulation of 2-hydroxyglutarate inhibits the function of α-ketoglutarate-dependent enzymes controlling histone demethylation and DNA modification. IDH1 and IDH2 mutations are observed in 10% to 23% of gastrointestinal and biliary cancers and are targetable. In addition, the serum level of 2-hydroxyglutarate can be measured and serve as a potential tumor marker.
CCAs are able to grow perpetually. In contrast, normal cholangiocytes, like other types of cells, undergo a defined number of cellular divisions prior to undergoing senescence owing to progressive telomere shortening. Telomeres are long stretches of repeat sequences present at the end of chromosomes that are involved in DNA synthesis. After multiple cell cycles, telomere shortening causes chromosomal instability and renders cells unable to divide. Preservation of telomere shortening via overexpression of the human telomerase reverse transcriptase (hTERT) allows cancers, including CCAs, to sustain chromosomal replication and therefore to maintain continual proliferation. Detectable hTERT activity and increased expression of hTERT mRNA have been reported in intrahepatic CCA.
Angiogenesis is promoted by many malignancies to ensure the adequate blood supply of oxygen and nutrients to constantly dividing tumor cells. CCAs have a rich vascular supply and intrahepatic CCAs express vascular endothelial growth factor (VEGF). Moreover, in a CCA cell line, increased expression of VEGF was dependent on transforming growth factor-β (TGF-β) stimulation. The TGF-β and its inducer SMAD, which is mutated in 16.7% of liver fluke-associated CCAs, has been associated with an increased metastatic potential.
Invasiveness of the surrounding tissue and metastasis are features of neoplasia. E-cadherin, a cell surface protein, is involved in cellular adhesion and, therefore, metastases. Intrahepatic CCAs have reduced expression of E-cadherin associated with advanced tumor histologic stage. Matrix metalloproteinases (MMPs) have been reported to be up-regulated in CCA, an event which was associated with clinical invasiveness. Moreover, the human aspartyl β-hydroxylase (HAAH), a protein involved in tumor invasion, is expressed in hepatocellular carcinoma and CCA. HAAH involves posttranscriptional hydroxylation of β-carbons on specific residues present in EGF-like domains of proteins that participate in cell migration and motility. Expression of HAAH in transfected cell lines was associated with anchorage-independent growth and tumor development in nude mice. CCA cell lines have been reported to overexpress HAAH. Another protein associated with connective tissue growth factor family, termed WISP1v (WNT1-inducible signaling pathway protein 1), is also overexpressed in CCA. Overexpression of WISP1v was linked with lymphatic and perineural CCA invasion.
MicroRNAs, small noncoding RNAs, are the new class of potent regulators of gene expression. By modulating the expression of multiple genes, including those described earlier, microRNAs participate in a variety of CCA cell processes. Thus, miR-29 is a negative regulator of antiapoptotic Mcl-1 and its expression is decreased in a CCA cell line. Cell-to-cell adhesion is regulated by miR-26a, which targets glycogen synthase kinase (GSK)-3β messenger RNA, aiming it for degradation. In CCA, microRNAs are also implicated in development of chemoresistance (miR-21, miR-200b, miR-29b, miR-205, and miR-221) and evasion of apoptosis (miR-29, miR-25, miR-31, miR-204, miR-320, miR-421, and miR-886).
Other pathways involved in cholangiocarcinogenesis include Notch signaling controlling cell differentiation, the morphogenic Hedgehog pathway, and platelet-derived growth factor (PDGF) signaling. Whereas mTOR activation in CCA was demonstrated in several studies, interestingly, it was associated with poor outcomes in patients with extrahepatic CCA but more differentiated tumors in patients with intrahepatic CCA. Tumor microenvironment provides a niche for cancer development and is enriched with cancer-associated fibroblasts. The latter participate in tumor-stroma cross talk, provide growth signals, and promote angiogenesis.
Thus, several successful strikes are made toward better understanding of CCA biology and development of new targeted therapies for CCAs. Contemporary observations of IDH gene mutations and fibroblast growth factor receptor 2 ( FGFR2 ) fusions in intrahepatic CCAs can allow application of IDH and FGFR inhibitors in CCAs in the clinic. A tumor classification based on the predominant driving oncogenic pathway rather than on only location or stage should be considered. This is an important paradigm of treatment shift in CCA from cytotoxic therapies to therapies targeting oncogenic drivers.
Most CCAs are adenocarcinomas by histopathology. They usually are well-differentiated to moderately-differentiated tubular adenocarcinomas within a dense extensive desmoplastic stroma. Other histologic variants of CCA include papillary adenocarcinoma, signet-ring carcinoma, squamous cell or mucoepidermoid carcinoma, and a lymphoepithelioma-like form.
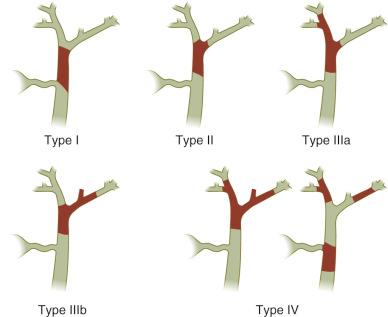
CCAs are classified into intrahepatic, perihilar, and distal (see Fig. 47-1 ). Approximately 50% of CCA cases involve the perihilar bile ducts, 25% involve the distal bile ducts, and the remaining 25% are represented by intrahepatic CCAs. The Bismuth-Corlette classification of perihilar CCA, which is used for evaluation of surgical resectability, is shown in Fig. 47-3 . Based on growth patterns, perihilar and distal CCAs can be sclerosing, nodular, and papillary. The sclerosing type is the most common and causes annular thickening of the bile ducts due to infiltration and fibrosis. Intrahepatic CCA often grows as a mass lesion, accounting for approximately one quarter of bile duct tumors, and can be confused with hepatocellular carcinoma. Intrahepatic CCAs can be solitary or multinodular. It might also be well demarcated as a mass lesion or as a diffuse infiltrating neoplasm growing along the intrahepatic bile ducts.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here