Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The first section of this chapter reviews cerebrospinal fluid (CSF) with respect to the anatomy of the CSF-containing spaces, physiology, and the effects of anesthetics and other influences. The second section reviews the relationship between CSF dynamics and intracranial pressure (ICP), anesthetics and drug-induced changes in CSF dynamics that increase or decrease ICP, and clinical situations wherein therapy to alter CSF dynamics may affect neurologic outcome.
The CSF is formed in the brain and circulates through macroscopic and extracellular fluid (ECF) spaces that are in continuity. The total volume of the macroscopic spaces ranges from 50 mL in infants to 140–150 mL in adults ( Table 3.1 ). Ventricular volume accounts for about 16% to 17% of macroscopic CSF volume in adults. Studies to determine the volumes of portions of the macroscopic CSF space using noninvasive imaging technology are ongoing. The ECF space surrounds the neuronal and glial elements of the central nervous system. Brain ECF volume is about 300 to 350 mL in adults exclusive of the macroscopic CSF space.
| Range ∗ | |
|---|---|
| Cerebrospinal fluid (CSF) pressure (mmHg): | |
| Children | 3.0–7.5 |
| Adults | 4.5–13.5 |
| CSF volume (mL): | |
| Infants | 40–60 |
| Young children | 60–100 |
| Older children | 80–120 |
| Adults | 100–160 |
The choroid plexuses (CPs) of the lateral ventricles extend from the inferior horn to the central part of the ventricle. The CPs in the body of the lateral and third ventricles receive their blood supply from the posterior and anterior choroidal arteries, respectively. The CPs in the temporal horns and the fourth ventricle are supplied by the superior and posterior inferior cerebellar arteries, respectively. The nervous supply to the CPs includes branches of the vagus, glossopharyngeal, and sympathetic nerves.
The ECF spaces of the brain and spinal cord, unlike those of other organs in the body, are small in diameter (180 Å). Exchange between cerebral capillaries and the ECF is limited because the capillary membrane is highly impermeable. This blood–brain barrier (BBB) consists of two elements. First, the cells of the cerebral capillary endothelium are joined by tight junctions (zonula occludens) that restrict the intercellular movement of molecules having a diameter of 20 Å or more. Second, astrocyte foot processes surround the capillaries. Evidence shows that the ECF spaces communicate with lymphatic channels.
CSF is a clear aqueous solution that, compared with plasma, contains higher concentrations of sodium, chloride, and magnesium, and lower concentrations of glucose, proteins, amino acids, uric acid, potassium, bicarbonate, calcium, and phosphate ( Table 3.2 ). Differences between the composition of CSF and an ultrafiltrate of plasma indicate that active secretion occurs during CSF formation. The concentrations of these and other substances in the macroscopic spaces vary according to the sampling site because diffusion between CSF and ECF occurs as CSF passes through the ventricles and subarachnoid spaces. Concentrations of CSF constituents are significantly altered during neuroendoscopy.
| Feature or Component | Mean Cerebrospinal Fluid (CSF) Value or Concentration ∗ | Mean Plasma Value or Concentration ∗ |
|---|---|---|
| Specific gravity | 1.007 | 1.025 |
| Osmolality (mOsm/kg H 2 O) | 289 | 289 |
| pH | 7.31 | 7.41 |
| Pco 2 (mmHg) | 50.5 | 41.1 |
| Sodium (mEq/L) | 141 | 140 |
| Potassium (mEq/L) | 2.9 | 4.6 |
| Calcium (mEq/L) | 2.5 | 5.0 |
| Magnesium (mEq/L) | 2.4 | 1.7 |
| Chloride (mEq/L) | 124 | 101 |
| Bicarbonate (mEq/L) | 21 | 23 |
| Glucose (mg/100 mL) | 61 | 92 |
| Protein (mg/100 mL): | 28 | 7000 |
| Albumin | 23 | 4430 |
| Globulin | 5 | 2270 |
| Fibrinogen | 0 | 300 |
The rate of CSF formation (
) is about 0.35 to 0.40 mL/min, or 500 to 600 mL/day in humans. Approximately 0.25% of total adult CSF volume is replaced by freshly formed CSF each minute. The turnover time for total CSF volume is 5 to 7 hours, yielding a turnover rate of about four times per day. The traditional view is that about 40% to 70% of CSF enters the macroscopic spaces via the CP, and 30% to 60% enters across the ependyma and pia. However, some recent studies suggest bidirectional fluid exchange at the BBB far exceeding choroidal CSF formation.
Unlike the capillary endothelium of other cerebral vessels, the capillary endothelium of the CP does not possess tight junctions between its cells. Instead, the capillary endothelium of the CP is fenestrated. Blood entering CP capillaries is filtered across this endothelium and forms a protein-rich fluid within the CP stroma that is similar in composition to interstitial fluid in other tissues of the body. Selected constituents of the stromal fluid are transported across the relatively impermeable CP epithelium by the combined processes of ultrafiltration and secretion. Stroma fluid enters clefts between the CP epithelial cells as a result of hydrostatic pressure and bulk flow ( Fig. 3.1 ).
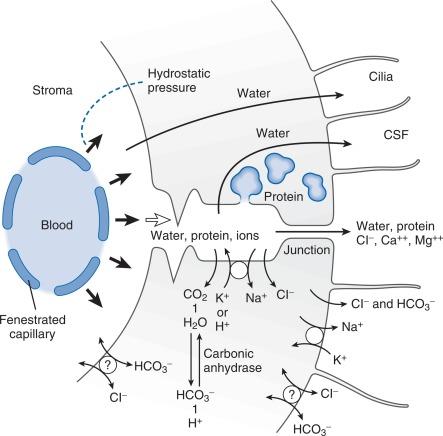
Sixty percent of extrachoroidal CSF formation results from oxidation of glucose (into water and carbon dioxide) by the brain, and 40% from ultrafiltration from cerebral capillaries. In neurons and glial cells water resulting from oxidation of glucose passes into the brain ECF. In most of the cerebral vasculature, passage of large and polar molecules across the “blood–ECF” interface is restricted by capillary tight junctions and specialized heterolytic vesicles within endothelial cells. Water, electrolytes, glucose, amino acids, urea, lipid-soluble materials, and a number of small nonelectrolytes pass more freely across this interface. Some of these substances may be actively transported by the astrocyte layer that envelops the capillary endothelium, whereas others may diffuse into the brain ECF. Osmotic forces appear to play a major role in water movement. Pericapillary spaces provide less restricted passage of water and electrolytes than most of the cerebral vasculature. This glucose-rich and protein-poor “lymph” diffuses through the ECF space toward the macroscopic CSF spaces ( Fig. 3.2 ).
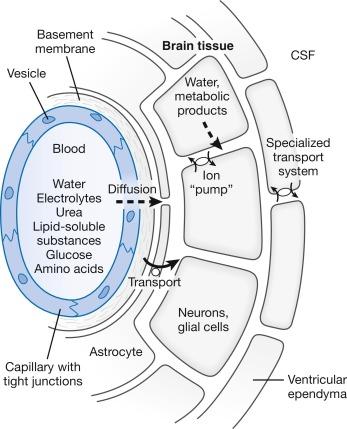
The concentration of glucose in CSF at the CP or in mixed samples is approximately 60% of that in blood. This ratio remains constant unless blood glucose rises to more than 15 to 20 mM (270 to 360 mg/dL). Glucose in blood enters CSF by facilitated transport, so that glucose crosses the blood–CSF barrier more quickly than would be predicted on the basis of its lipid solubility. Transport follows saturable kinetics, with the rate being directly related to serum glucose concentration and independent of the serum-to-CSF glucose concentration gradient. Movement of glucose in the opposite direction, from the cerebral ventricles into the surrounding brain and blood, occurs via ouabain-sensitive sodium-potassium ATPase and ouabain-insensitive fluxes and diffusion.
Protein entry into CSF from blood at the CP and extrachoroidal sites is limited, so CSF protein concentrations are normally 0.5% or less of the respective plasma or serum concentrations. The permeability of the blood–CSF barrier to albumin increases with age and does not differ between genders. If a structural barrier between the brain ECF and the macroscopic CSF space is absent, proteins entering the brain ECF drain into the macroscopic CSF space by bulk flow. Once in CSF, proteins are transported along with CSF through the macroscopic pathways and are cleared from the CSF space into dural venous sinuses. This “sink effect” of flowing CSF keeps the CSF and brain protein concentration low and far from equilibrium with blood. In normal infants and adults, CSF protein concentrations are lowest in the ventricles (about 26 mg/100 mL), intermediate in the cisterna magna (about 32 mg/100 mL), and highest in the lumbar sac (42 mg/100 mL). Under normal conditions, 60% of protein entry into CSF occurs at the CP, and 40% at extrachoroidal sites.
The negative correlation between
and increased ICP is weak; the relationship between
and cerebral perfusion pressure (CPP) is somewhat stronger. Increase of ICP to 20 mmHg produces no change in
as long as CPP remains above ~ 70 mmHg. When CPP falls below ~ 70 mmHg, whether from arterial hypotension or because of the combination of arterial hypotension with increased ICP,
diminishes. These
results are consistent with reported effects of changes in CPP on cerebral blood flow (CBF), lateral ventricle CP blood flow (CPBF), and fourth ventricle CPBF. A decrease of CPP to 70 mmHg by arterial hypotension, combined with increased ICP, reduces CBF and CPBF. A drop of CPP to 50 mmHg causes a further decline in CPBF when CPP is reduced by an even greater increase in ICP, but not when CPP is reduced solely by arterial hypotension.
The hydrostatic pressure of CSF formation, 15 cm H 2 O, produces CSF flow where it is freshly formed. Cilia on ependymal cells generate currents that propel CSF toward the fourth ventricle and its foramina into the subarachnoid spaces. Respiratory variations and vascular pulsations of the cerebral arteries and CP cause ventricular excursions, supplying additional momentum for CSF movement. The pressure differences between mean CSF pressure, 15 cm H 2 O, and superior sagittal sinus pressure, 9 cm H 2 O, provides a 6 cm H 2 O pressure gradient for passage of CSF across the arachnoid villi. The high velocity of blood flow through the fixed diameter of the sinuses and the low intraluminal pressure that develops at the circumference of the sinus wall where the arachnoid villi enter cause a “suction-pump” action that may explain how the circulation of the CSF continues through a wide range of postural pressures.
Radioisotope studies show that labeled CSF moves from the ventricles to the basal cisterns within a few minutes and collects along the superior sagittal sinus area at 12 to 24 hours. The labeled fluid enters the low cervical–high thoracic region at 10 to 20 minutes, the thoracolumbar area at 30 to 40 minutes, the lumbosacral cul de sac at 60 to 90 minutes, and the basal cisterns at 2 to 2.5 hours. About 20% to 33% of the labeled CSF reaches the intracranial cavity within 12 hours. More recent studies emphasize the “mixing and swirling” and “to-and-fro” movement of CSF. , The central canal within the spinal cord alters these oscillatory flows. CSF circulation concludes with reabsorption across arachnoid villi into the superior sagittal sinus and spinal dural sinusoids located on dorsal nerve roots ( Fig. 3.3 ).
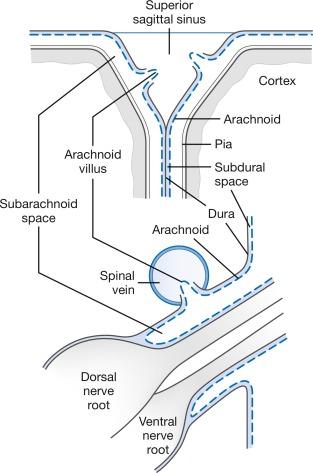
CSF passes from the subarachnoid space into venous blood through microscopic arachnoid villi and macroscopic arachnoid granulations. Intracranial arachnoid villi are located within the dural wall bordering the superior sagittal sinus and venous lacunae, and spinal arachnoid villi are located within the dural wall bordering dural sinusoids on dorsal nerve roots. Traditional estimates were that under usual conditions, 85% to 90% of CSF is reabsorbed at intracranial sites, and 10% to 15% at spinal sites. Newer studies add the role of CSF drainage into lymphatic pathways and CSF reabsorption throughout the entire CSF-interstitial fluid interface. , , The arachnoid villus or granulation is composed of arachnoid cells protruding from the subarachnoid space into and through the wall of an adjacent venous sinus ( Fig. 3.4 ). Under normal conditions, an endothelium composed of arachnoid cells joined by tight junctions covers the villus. In adults, this endothelial covering may be multilayered.
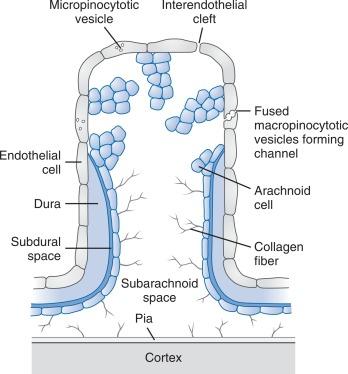
The endothelium covering the villus acts as a CSF–blood barrier that limits the rate of passage of CSF and solute into venous blood. The rate at which CSF passes through the subarachnoid space and arachnoid villi and across the endothelium is determined by (1) the transvillous hydrostatic pressure gradient (CSF pressure–venous sinus pressure) and (2) a pressure-sensitive resistance to CSF outflow at the arachnoid villus. Because the endothelium is highly permeable, transvillous osmotic differences probably do not play a major role in determining CSF movement through arachnoid villi. CSF may exit the villus by passing between or through endothelial cells. CSF may pass through endothelial cells via pinocytotic vesicles and transcellular openings formed by chains of fused vesicles extending from one surface of the epithelium to the other. These vesicles transport macromolecular tracers, as well as fluid, from CSF to blood. Although micropinocytotic vesicles appear to be the primary route of CSF transport at resting CSF pressure, both pathways contribute to the total resistance to CSF outflow.
The rate of reabsorption of CSF (
) increases as the pressure gradient across the villus (CSF pressure − venous sinus pressure) increases. Resistance to reabsorption of CSF (R a ) remains close to “normal” as CSF pressure increases to more than 30 cm H 2 O. Thereafter, with further increases in CSF pressure, R a declines. An increase in the size and number of endothelial vesicles was reported when CSF pressure was increased from 9 to 30 cm H 2 O. At CSF pressures greater than 30 cm H 2 O, growing numbers of transcellular channels were present concurrent with progressive increases in steady-state pressures and decreases in R a .
Under normal conditions, there is relatively little bulk flow across cerebral capillaries and through the parenchyma of the brain. Molecules in the brain ECF move through that space primarily by diffusion. The rate at which molecules exit the brain ECF relates to their molecular size, tissue concentration gradients, and the ability of the molecules to cross the BBB and reenter the vascular system.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here