Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Normal cellular proliferation and differentiation are essential to tissue homeostasis in all organs, including the digestive tract. The neoplastic process involves a fundamental disruption of these mechanisms, which can give rise to cancer development and metastasis with the additional acquisition of other hallmarks of cancer. As a group, malignancies of the GI tract are the leading cause of cancer-associated mortality, and it is therefore essential to understand the underlying biology that gives rise to tumor formation. This chapter reviews mechanisms of normal cell growth and the fundamental cellular and molecular alterations that facilitate malignant transformation. The basic concepts discussed in this chapter provide the framework for discussion of specific GI neoplasms in later chapters.
Tissue homeostasis is maintained by the delicate balance of cellular proliferation and differentiation, which provide new cellular elements to replace dying cells as part of normal tissue function or during tissue repair. At a fundamental level, neoplasia arises when cell proliferation escapes the homeostatic mechanisms that maintain this process in balance with senescence and programmed cell death. Cell proliferation occurs as cells divide, a process that occurs through an orderly set of steps referred to as the cell cycle ( Fig. 1.1 ). In preparation for cell division, there is a period of biosynthetic activity called the G 1 phase that is typically associated with an increase of cell size. This phase is followed by precise duplication of the genome, designated the S phase. After an intervening gap period designated as the G 2 phase, mitosis occurs during the M phase.
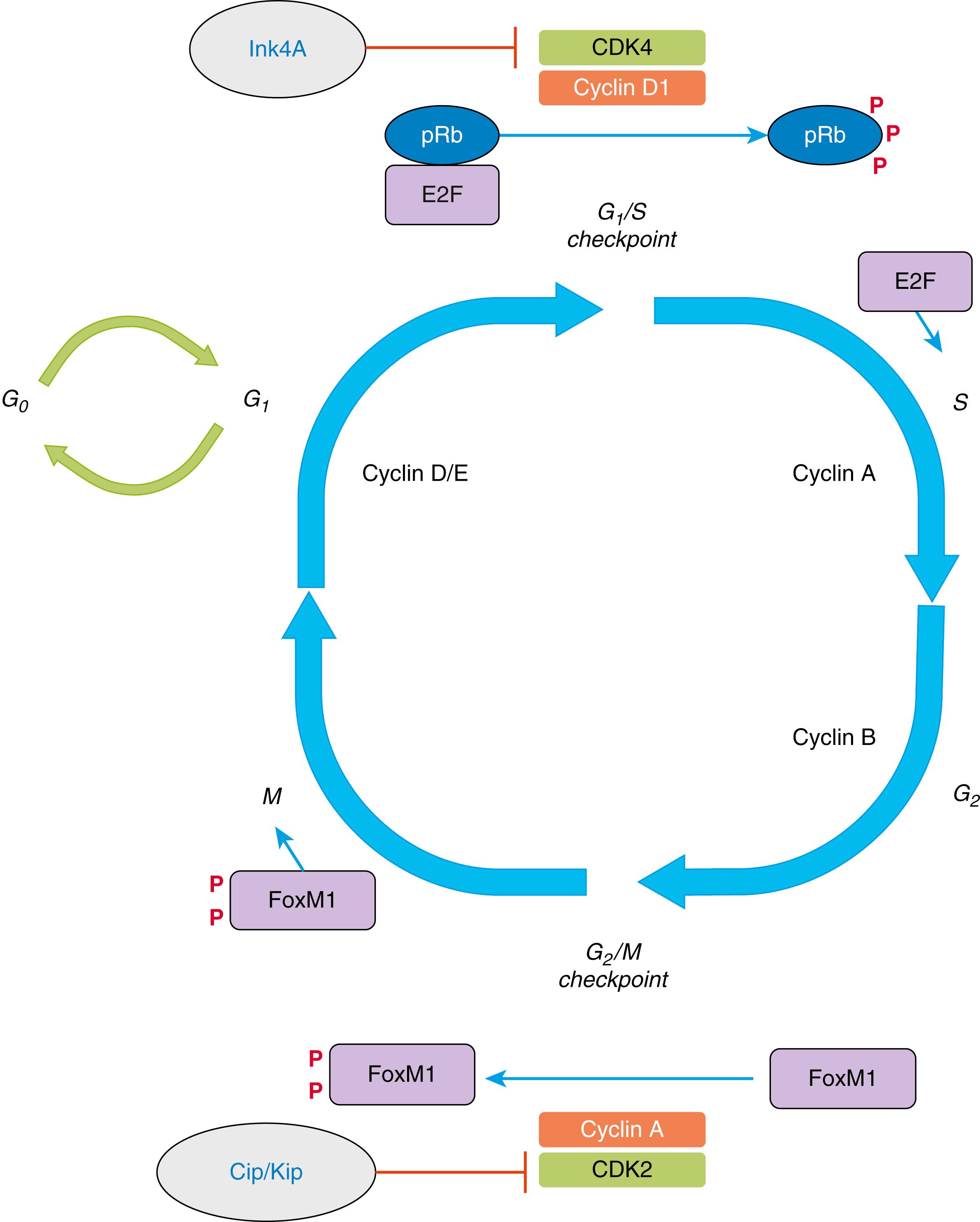
The commitment to proceed to DNA replication occurs at the G 1 /S checkpoint or restriction (R) point. Cells may exit this cycle of active proliferation before reaching the R point and enter a quiescent phase known as G 0 . Cells can subsequently reenter the cell cycle from the G 0 state (see Fig. 1.1 ). Another checkpoint exists at the boundary between the G 2 and M phases. The G 2 /M checkpoint ensures that mitosis does not proceed prior to the repair of any damaged DNA after genome replication. Impaired function of these checkpoints is frequently observed in cancers.
Regulation of cell cycle progression is achieved principally by a set of proteins known as cyclins and cyclin-dependent kinases (CDKs). These proteins are expressed in specific parts of the cell cycle and regulate the G 1 /S and G 2 /M checkpoints. During the G 1 phase, cyclins D and E are most active. Overexpression of cyclin D1 in fibroblasts results in more rapid entry of cells into the S phase, and, consistent with a role in cancer, cyclin D1 is frequently overexpressed in a number of GI and non-GI malignancies. During the S phase, cyclin A is predominantly expressed, and by the G 2 phase cyclin B is the main regulator (see Fig. 1.1 ).
Each cyclin forms a complex with a CDK and function as catalysts for CDK activity in a cell cycle–dependent fashion (see Fig. 1.1 ). The cyclin-CDK complexes regulate cell cycle progression through phosphorylation of key target proteins. For example, cyclin D1–dependent progression from G 1 to S phase is the result of cyclin D1/CDK4 phosphorylation of the tumor suppression pRb, the product of the retinoblastoma gene, as well as the Rb family members p130 and p107. These proteins sequester E2F transcription factors that promote expression of factors required for S phase, and their phosphorylation by CDK4 leads to their functional inhibition. Thus, loss of Rb expression also accomplishes more rapid progression to S phase and is another genetic lesion seen in many tumors. An analogous circuit is found in the G 2 /M transition, where cyclin A/CDK2 mediates the activation of another transcriptional regulator, FoxM1, required for the expression of factors involved in mitosis.
The cell cycle is also regulated by multiple CDK inhibitors, which are classified into various classes and are referred by multiple names. CDK4 and CDK6 are inhibited by members of the Ink4 family of inhibitors known as p16 INK4a (encoded by the Cdkn2a gene), p15 INK4b ( Cdkn2b ), p18 INK4c ( Cdkn2c ), and p19INK4d ( Cdkn2d )]. Thus these factors also impinge on Cyclin D1/CDK4 regulation of pRb, and consequent E2F activity and S phase entry. p16 INK4A loss in cancer results in greater activation of CDK4 and is frequently inactivated in GI cancers, a finding consistent with its function as a tumor suppressor gene. Members of the Cip/Kip family of CDK inhibitors are known as p21 Cip1 ( Cdkn1a ), p27 Kip1 ( Cdkn1b ), and p57 Kip2 ( Cdkn1c )] and are more promiscuous and interfere with multiple cyclin/CDK complexes, including CDK2.
Apoptosis is a form of programmed cell death that is genetically programmed and executed by specific proteases known as caspases. Similar to other protease cascades, such as the coagulation system, caspases become active upon cleavage of an inactive pro-form, typically through the action of another caspase or as a result of focal accumulation of inactive caspases. Apoptosis is an important mechanism that counterbalances cell proliferation; thus, escape from normal apoptotic mechanisms plays a critical role in oncogenesis. Morphologically, apoptosis is characterized by distinctive features that include chromatin compaction, condensation of the cytoplasm, nuclear fragmentation, and marked alterations at the plasma membrane, resulting in compacted apoptotic bodies that are eventually phagocytosed and eliminated.
Apoptosis may be triggered by internal or external stimuli. Internal stimuli of apoptosis may include nutrient deprivation, hypoxia, DNA damage, or other stressors, including specific toxins, chemical signals, and pathogens. Apoptosis routinely occurs during normal development to facilitate tissue patterning. Similarly, a number of stress situations, including tissue inflammation, can trigger apoptosis. Apoptosis may also be stimulated by specific cell surface receptors belonging to the tumor necrosis factor receptor superfamily, including tumor necrosis factor R1 and Fas, which are referred to as death receptors ( Fig. 1.2 ).
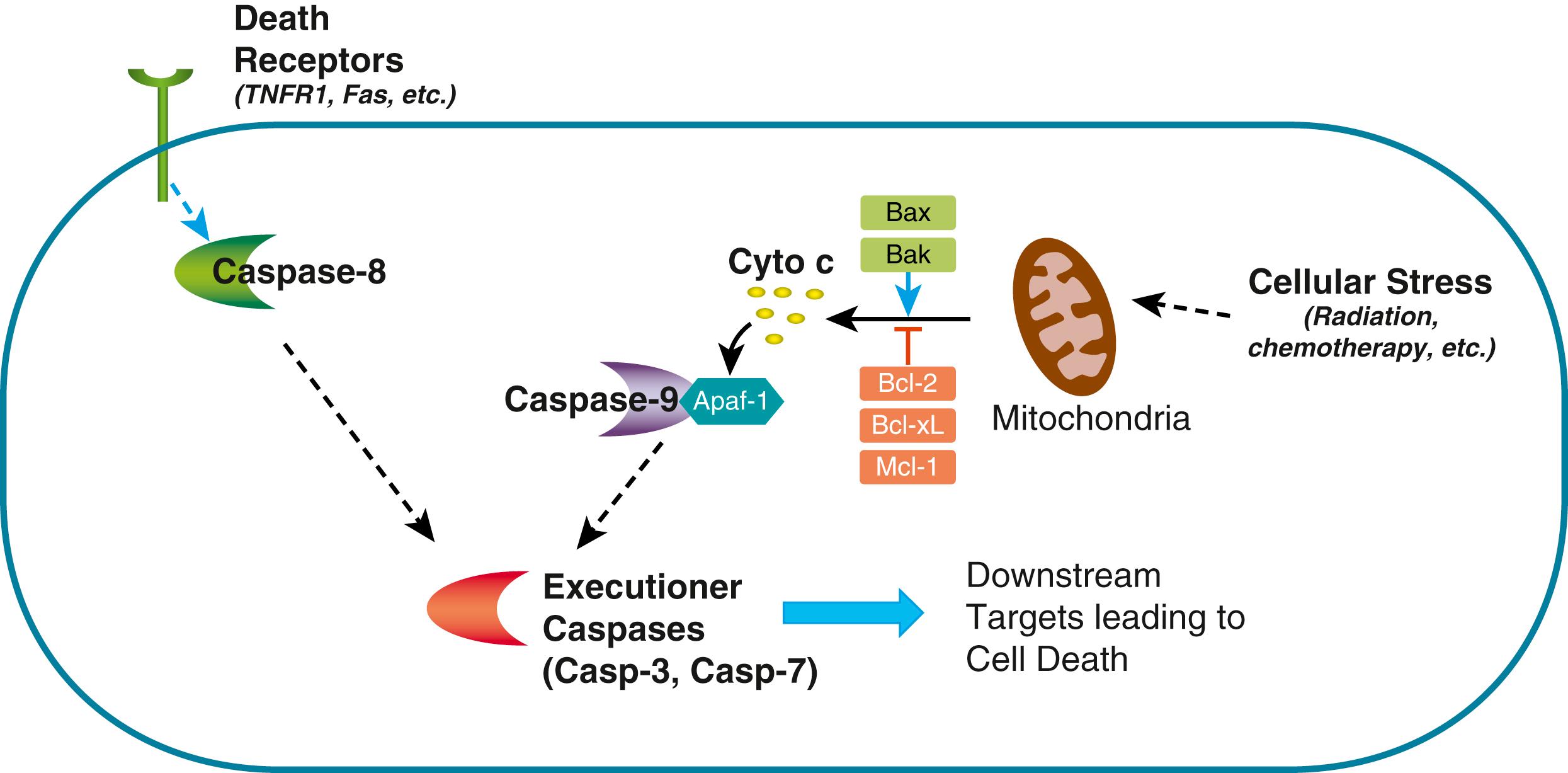
At the intracellular level, the last common event in all forms of apoptosis is the activation of so-called executioner caspases, caspase 3 and 7, which mediate the cleavage of a large number of downstream targets that eventually precipitate cell death. Proapoptotic signals frequently converge at the level of the mitochondria, where they destabilize the mitochondrial membrane and collapse the electrical gradient required for aerobic respiration (see Fig. 1.2 ). Besides the effects that result in cellular energetics, this process leads to the release into the cytosol of proteins normally present in the intermembrane space of the mitochondria, including cytochrome c, a component of the respiratory chain. In the cytosol, cytochrome c helps in the assembly of a multiprotein complex known as the apoptosome, which contains Apaf1 and facilitates the activation of caspase 9, which can directly activate caspases 3 and 7. On the other hand, death receptors activate executioner caspases through receptor initiated intracellular signaling events that result in the upstream activation of caspase 8.
The mitochondrial membrane permeabilization events that lead to apoptosome formation are controlled by proteins of the Bcl-2 family. On the one hand, Bax and Bak help form the pore, whereas Bcl-2, Bcl-xL, and Mcl-1 inhibit pore formation. The stoichiometric ratio between proapoptotic and antiapoptotic members of the Bcl-2 family can determine the balance between cell survival and cell death. In cancer, alterations in the balance of proapoptotic and antiapoptotic factors, including member of the Bcl-2 family, are common events.
Senescence is the process by which cells permanently lose their ability to divide. Senescence may occur in response to the stress induced by activation of oncogenes or DNA damage or after a fixed number of cellular divisions (replicative senescence). Associated with the exit from the cell cycle, senescence is associated with a secretory phenotype that includes a variety of proinflammatory factors. As a physiologic event, senescence limits dysregulated or excessive proliferation. However, when dysregulated, senescence can also contribute to aging and depletion of stem cells. During carcinogenesis, senescence is frequently bypassed or lost.
Replicative senescence is triggered shortening of telomeres, repetitive sequences at the end of chromosomes that protect genomic integrity. Telomeres shorten with each cell division, and when they reach a critically short length, they initiate DNA damage signaling and cellular senescence. This phenomenon can be routinely seen in vitro when primary cells undergo repeated rounds of replication, eventually acquiring critically short telomeres. To prevent senescence from being triggered by sustained replication, cancer cells activate the telomerase enzyme, which adds additional telomeres to the end of chromosomes.
Cellular proliferation is achieved through transition of cells from G 0 arrest into the active cell cycle (see Fig. 1.1 ). Although progression through the cell cycle is controlled by the regulatory mechanisms just described, overall proliferation is also modulated by external stimuli. Growth factors that bind to specific transmembrane receptors on the cell surface are especially important. Also acting through transmembrane cell surface receptors, extracellular matrix and cell-cell adhesion molecules (i.e., integrins, cadherins, selectins, proteoglycans) can also have a significant impact on cell proliferation. Alterations in cell-matrix or cell-cell interactions are particularly important in contributing to the invasive phenotype of malignant cells.
After ligand binding, the cytoplasmic tails of these transmembrane receptor proteins activate intracellular signaling cascades that alter gene transcription and protein expression. Based on the nature of the intracellular signaling cascades that these receptors initiate, they can be classified into three major categories: (1) tyrosine kinases, (2) serine and threonine kinases, and (3) G protein–coupled receptors (GPCRs).
The receptors for many peptide growth factors contain intrinsic tyrosine kinase activity within their intracellular tail. After ligand binding, tyrosine kinase activity is stimulated, leading to phosphorylation of tyrosine residues in target proteins within the cell. Most receptors also autophosphorylate tyrosine residues present in the receptors themselves to magnify signaling, and, in some cases, this also causes attenuation of their own activity to effect an intramolecular feedback regulatory mechanism. The receptors for many peptide growth factors, including the receptor for EGF and related growth factors, belong to this receptor class.
Other receptors on the cell surface possess kinase activity directed toward serine or threonine residues rather than tyrosine. These receptors also phosphorylate a variety of cellular proteins, leading to a cascade of biological responses. Multiple sites of serine and threonine phosphorylation are present on many growth factor receptors, including the tyrosine kinase receptors, suggesting the existence of significant interactions among various receptors present on a single cell. The transforming growth factor (TGF)-α receptor complex is one important example of a serine-threonine kinase–containing transmembrane receptor.
Many receptors are members of the so-called 7-membrane–spanning receptor family. These receptors are coupled to guanine nucleotide binding proteins, also known as G proteins, and thus, the receptors are referred to as G protein–coupled receptors. G proteins undergo a conformational change that is dependent on the presence of guanosine phosphates. Activation of G proteins can trigger a variety of intracellular signals, including stimulation of phospholipase C and the generation of phosphoinositides (most importantly, inositol 1,4,5-triphosphate) and diacylglycerol through hydrolysis of membrane phospholipids, as well as modulation of the second messengers cyclic adenosine monophosphate and guanosine monophosphate. Somatostatin receptors exemplify a GPCR prevalent in the GI tract.
Binding of growth factors and cytokines to cell surface receptors typically produces alterations in a variety of cellular functions that influence growth. These functions include ion transport, nutrient uptake, and protein synthesis. However, the ligand-receptor interaction must ultimately modify one or more of the homeostatic mechanisms discussed to affect cellular proliferation.
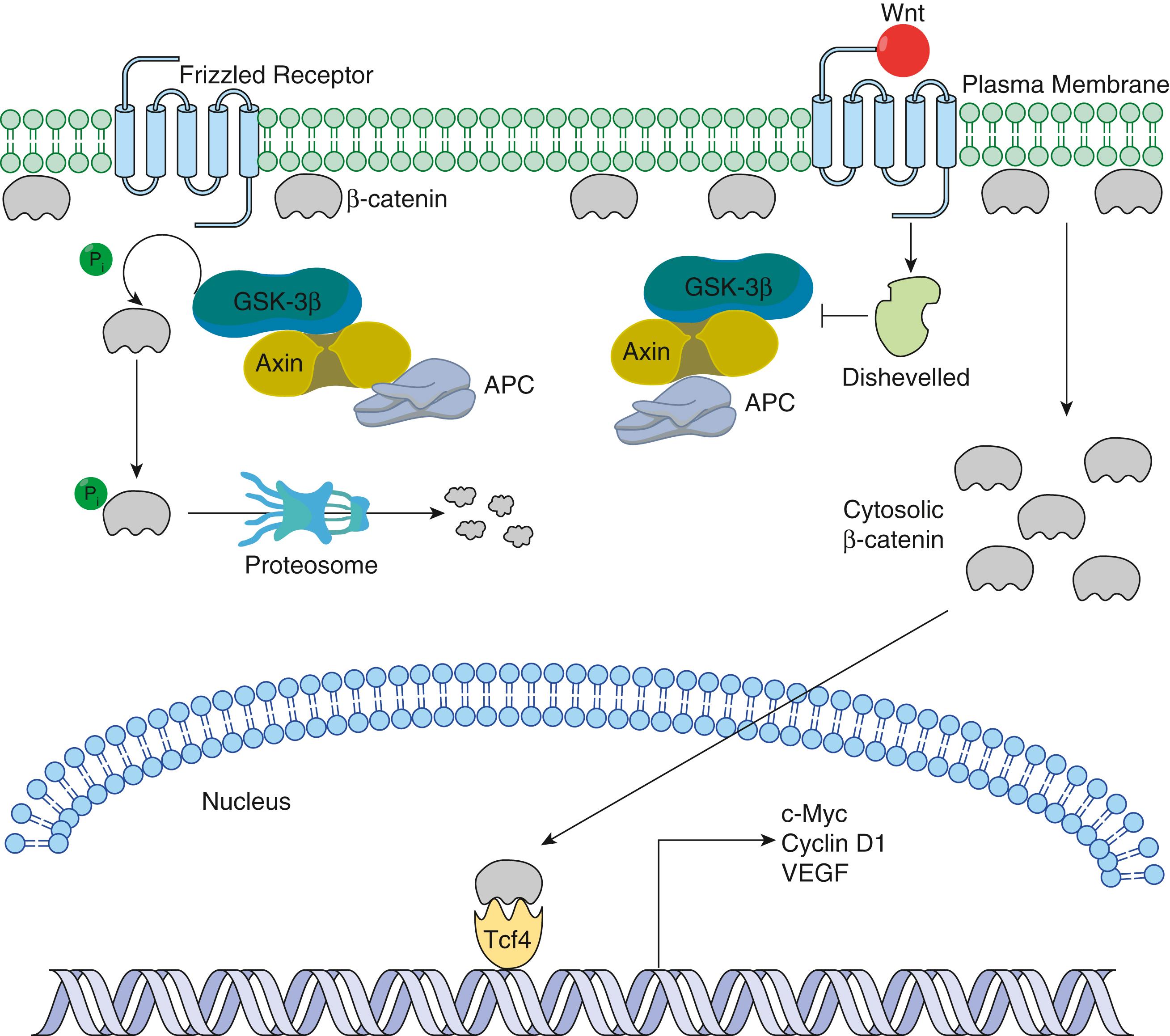
The Wnt pathway is one important example of a signaling pathway that regulates a diverse number of homeostatic mechanisms to control proliferation of intestinal epithelial cells ( Fig. 1.3 ). Evolutionarily conserved among several species, Wnt signaling, as a rule, regulates proliferation in the stem cell niche and is essential for epithelial homeostasis in the GI tract. From a signaling perspective, its actions are largely the result of the accumulation of α-catenin in the nucleus, where it binds with the transcription factor Tcf-4 to activate a set of target genes. In normal cells, α-catenin is largely associated with adherens junctions, and the cytoplasmic pool of this protein is rapidly degraded through a phosphorylation and ubiquitination pathway. This is mediated by the so-called destruction complex, which includes the tumor suppressor APC. When secreted Wnt ligands bind to cell surface receptors of the Frizzled family, the constitutive degradation of α-catenin is inhibited (disheveled) which results in the nuclear accumulation of this factor, and the subsequent transcriptional activation of genes that promote cell proliferation. Inhibition of the Wnt signal in mice can be achieved by deletion of Tcf-4 or overexpression of the Wnt inhibitor Dickkopf1, which results in dramatic hypoproliferation of the intestinal epithelium. Wnt signaling is most active in the base of the crypt, and as differentiation ensues, tissue homeostasis is maintained by growth-inhibiting signals that counterbalance proliferative signals and promote differentiation, including members of the TGF-α family such as BMP4. Specific members of this family have unique functions is tissue homeostasis, including promoting a differentiated and fibrogenic phenotype of mesenchymal cells, induction of specific T cell subtypes, and myriad other activities. In broad terms, the effects of TGF-α family members are mediated intracellularly through the Smad family of proteins, which are transcription factors that are activated in response to ligand-receptor binding. TGF-α induces transcription of the cell cycle inhibitors p15 INK4B and p21 CIP1/WAF1 and is a potent growth-inhibiting factor that mediates arrest of the cell cycle at the G 1 phase. Furthermore, it also enhances the inhibitory activity of p27 KIP1 on the cyclin E/CDK2 complex.
Multiple sequential genetic alterations are required for the transformation of normal intestinal epithelium to neoplasia. This multistep nature of tumorigenesis is most directly illustrated by the changes that accrue in the development of colonic neoplasia (see Chapter 127 ). The progression from normal epithelium through adenomatous polyps to malignant neoplasia is paralleled by the accumulation of genetic alterations that change key pathways that control proliferation and tissue homeostasis. Studies on the molecular pathogenesis of colon cancer have served as a paradigm for the elucidation of genetic alterations in other GI cancers, including gastric and pancreatic cancer.
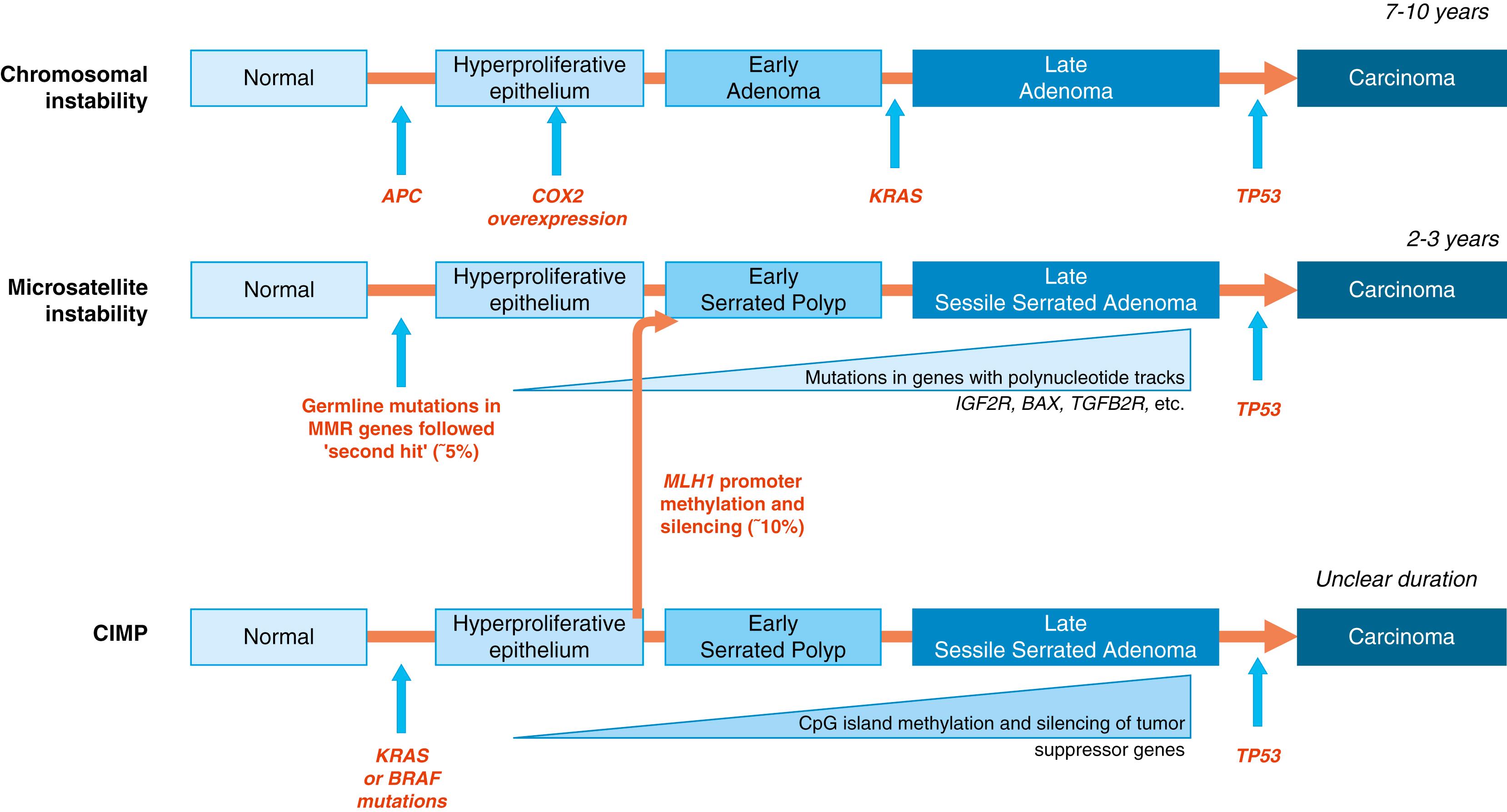
Genomic instability is observed in almost all cancers in the GI tract. This genetically unstable environment promotes the accumulation of the multiple alterations that characterize GI cancers. Instability of the genome may result from several mechanisms, including changes in the genome DNA sequence or through modifications of the nucleotides to alter their functionality, a process called epigenetic change. In colon cancer, there are now 3 well-recognized forms of genetic/epigenetic instability that promote carcinogenesis ( Fig. 1.4 ), and they have been termed chromosomal instability, microsatellite instability (MSI), and CpG island methylator phenotype (CIMP). Chromosomal instability refers to alterations in chromosomal structure resulting in large chromosomal deletions, duplications, and translocations, which in aggregate result in a state of aneuploidy. In contrast, MSI refers to frequent alterations in tracts of repetitive DNA sequences (referred to microsatellite DNA) and are often diploid or near-diploid on a chromosomal level (see later discussion on DNA repair). CIMP refers to the accumulation of an epigenetic modification, methylation of guanine residues in so-called CpG-islands, areas rich in cytidine and guanine in gene promoter sites. This modification has a potent effect on gene transcription and results in gene silencing. Other forms of epigenetic change involve the chemical modification of the histone proteins that are required for the assembly of the nucleosome and that control chromatin compaction and DNA access. Although mutations in histones themselves are rare in cancer, mutations in the enzymes that modify histones are emerging as an important group of tumor-associated mutations. It is important to note that involvement by these pathways is not mutually exclusive.
Clonal expansion is essential to tumor development. The acquisition of a mutation that may provide a growth or survival advantage to a cell is followed by clonal expansion of these mutated cells. As this population grows, and particularly with the acquisition of genetic/epigenetic instability, a second round of clonal expansion occurs as a cell within this population sustains still another genetic alteration that further enhances its growth properties. This iterative process of selection, with accumulating genetic alterations, results in malignancy. Because of the nature of the clonal expansion process, once frank malignancy has developed, it is often the case that multiple clones are present in the same tumor, with a different catalog of mutations harbored among various cancer cells. Referred to as tumor heterogeneity, this ongoing process may give certain cells selection advantages. Metastasis may be facilitated by the evolution of a subset of tumor cells that acquire the capability of traversing the circulatory system and thriving in a new environment.
Recognition of tumor heterogeneity has led to the cancer stem cell (CSC) hypothesis, which asserts that there exists a subset of tumor cells that have stem cell–like properties. CSCs are believed to be the tumor-initiating cells from which clonal expansion occurs. Moreover, it is hypothesized that eradication of these cells is a key therapeutic goal because failure to do so may result in relapse of disease. Within this CSC hypothesis, there are 2 models. The first is a hierarchical model in which CSCs serve as progenitors for all cells in in a given tumor, whereas other cells have limited long-term reproductive potential. The basic evidence for this model is the finding that only cells with specific surface markers can repopulate the tumor in xenotransplantation experiments. In the GI tract, analysis of putative CSCs demonstrate transcriptional programs and markers shared with normal intestinal stem cells, such as Lgr5 and EphB2, which identify and purify colon CSCs. The second stochastic model posits that each cancer cell has the same potential to be a CSC, but this determination is stochastically based on internal factors in addition to external environmental cues.
It has been noted that within tumors of epithelial origin, some cells acquire features of mesenchymal cells. A similar process occurs during normal embryogenesis, when polarized epithelial cells no longer recognize the boundaries imposed by adjacent epithelial cells or their basement membrane and adopt features of migratory mesenchymal cells. This phenomenon, designated epithelial-mesenchymal transition (EMT), endows cells with the ability to move through tissue planes that normally serve as boundaries for epithelial cells, such as the basement membrane, a dense matrix of collagen, glycoproteins, and proteoglycans. The transmigration of tumor cells through the basement membrane likely involves production of key proteolytic activities. Alternatively, the tumor cell may produce factors capable of activating proenzymes present in the extracellular matrix. For example, the tumor may produce urokinase, itself a protease, or plasminogen activator. Having gained access to the interstitial stromal compartment, tumor cells can then enter lymphatic and blood vessels and metastasize.
In addition to these properties, it has been recognized that cells that undergo EMT acquire not only invasive features but also CSC-like features.
One key feature of EMT is the loss of adherens junctions that normally maintain epithelial cell–cell interactions. The molecular correlate of this phenomenon is the loss of expression of E-cadherin, a critical component of the adherens junction. Mutations in E-cadherin are common in many GI cancers, particularly gastric cancer, where germline mutations in E-cadherin are also linked to hereditary diffuse gastric cancer.
Genes that become altered during the neoplastic process belong to two distinct groups: (1) oncogenes, which actively confer a growth-promoting property, or (2) tumor suppressor genes, the products of which normally restrain growth or proliferation. An important category within tumor suppressor genes includes DNA repair genes, which prevent accumulation of new mutations. Activation of oncogenes or inactivation of tumor suppressor genes contributes to malignant transformation. Although most of these genes encode for proteins, many cancer-promoting genes that harbor oncogenic and tumor suppressive functions do not encode for proteins but rather for RNAs that modulate genomic function, so-called noncoding RNAs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here