Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The existence of low-resistance pathways between cardiac cells was postulated from experimental assessment of electronic interactions (cable analysis) between cardiac Purkinje fibers before the existence of gap junction channels was known. During the subsequent decades ion channels between cardiac cells were identified, formed by two connexon hemichannels, with each hemichannel composed of six connexin molecules. The main connexins (Cx) of the myocardium, Cx43, Cx40, and Cx45, have been defined in terms of genetic code, amino acid sequence, and molecular function. The introduction of the whole-cell dual voltage-clamp technique made it possible to directly measure intercellular electric conductance and single-channel conductance between a pair of cells obtained from enzymatic dissociation of cardiac tissue or after neoformation in cultured cells. Later, transfection techniques were used to study the biophysical properties of specific cardiac connexins in heterologous expression systems and to define the properties of pure or mixed connexons and connexins (homomeric vs. heteromeric connexons, and homologous vs. heterologous connexin channels) in terms of biophysical and diffusive properties.
Similar to ion channels, the electrical behavior of gap junction channels is sensitive to the electrical field across the channel and to variations of the intercellular milieu (Ca 2+ , pH, Mg 2+ , adenosine triphosphate [ATP]). Gap junction channels have a high turnover rate with a half-time of only a few hours. , Connexon hemichannels are transported along microtubular highways and uploaded to the periphery of the gap junction plaques by mechanisms dependent on interaction between microtubular plus end proteins and proteins of the composite junctions. The dynamic balance of gap junction channel expression at the intercalated disc (ICD) is maintained by continuous internalization. , Proteins, such as zonula occludens protein-1 (ZO-1) and CAR, take part in these processes, and they are involved in interactions with other proteins of the intercellular junction and plaque size control. The total of these behaviors indicates that gap junction channels form a highly dynamic, regulated system that is interactive with other functional protein complexes and explains why changes in composition and function can contribute importantly to disease phenotypes.
Cx43, Cx40, and Cx45 are the three major connexins of the myocardium. A fourth connexin, Cx32.1, has been described in murine nodal tissues. Cx43 is the dominating connexin in ventricular myocardium, Cx40 dominates in the ventricular conduction system, and Cx45 is present in the atrioventricular and sinoatrial nodes and in lower amounts in atrial and ventricular myocardium.
Coexpression of Cx43 and Cx45 (ventricle); Cx43, Cx40, and Cx45 (atria); and Cx40 with Cx45 (Purkinje system) raises the question of whether these connexins form mixed heteromeric connexons and/or heterotypic gap junction channels of relevance for cardiac electrical coupling and propagation. Homomeric/homotypic Cx43 and Cx40 gap junction channels have a relative large unitary electrical conductance, γ j , (approximately 100 pS and 174 pS, respectively), whereas γ j of Cx45 is considerably smaller (approximately 30 pS).
The question of the role of mixed Cx40/Cx40/Cx45 channels is especially important for atrial tissue showing a wide spectrum of electrical propagation velocities (from 0.2–1.6 m/s ). In both atria, Cx43 and Cx40 are both abundantly expressed and colocalize in ICDs; the ratio of Cx40:Cx43 expression is larger in the right than the left atrium. It has been shown in human atria that the ratio of Cx40:Cx43 expression is an important determinant of propagation velocity, with Cx40 dominance decreasing and a Cx43 dominance increasing local velocity. A very similar behavior was observed in vitro in patterned atrial strands cultured from myocytes with genetic Cx43 and Cx40 ablation. These findings are in contrast to measurements of macroscopic excitation in murine atria with Cx40 ablation in vivo, which yielded slowed or unchanged propagation. The reasons for these controversial findings are not evident, but it cannot be excluded that macroscopic measurements in whole atria with genetic Cx40 ablation are affected by malformations. The important question is whether colocalization of Cx43/Cx40/Cx45 immunosignals in the atria reflects the existence of separate gap junction channels or whether mixed Cx40/Cx43/Cx45 heteromeric connexins and/or heterotypic channels exist in the atrial myocardium. An answer to this question is important because mixed Cx40/Cx43 channels may have electrical conductances different from pure homomeric/homotypic Cx43, Cx40 channels , and thus affect propagation velocity. It is well known that Cx45 can form heteromeric connexons with Cx43 or Cx40. However, the question of whether heteromeric Cx40/Cx43 connexons or heterotypic Cx40/Cx43 gap junction channels exist in the atria has remained unanswered. In heterologous expression systems, controversial results about the existence and function of mixed Cx40/Cx43 channels systems have been reported. , ,
A further not fully resolved question relates to the role of Cx45. Cx45 is expressed in the early stages of cardiac development in all cardiac regions. , In adult hearts it is detected in the sinoatrial and atrioventricular nodes and in small amounts in atrial and ventricular tissue from human, rat and murine hearts. , , , Germline ablation of Cx43 in murine ventricular pairs produces a greater than 90% reduction in electrical cell-to-cell coupling with persistence of Cx45 expression. This indicates that Cx45 contributes very little to electrical coupling per se. However, a regulatory role has been attributed to Cx45 in cardiac failure, in which Cx45 is upregulated together with a decrease in Cx43 and a reduction in gap junction size. This may indicate that Cx45 is involved in regulation of gap junction size, as also suggested from coexpression of Cx43 with Cx45 in a rat liver epithelial cell line. Recently, it has been shown that genetic deletion of the coxsackie and adenovirus receptor (CAR) is associated with marked reduction or deletion of Cx45. Conditional ablation of CAR increased cell-to-cell dye diffusion between ventricular cells, further suggesting that Cx45 may be a significant modulator of ventricular cell-to-cell communication.
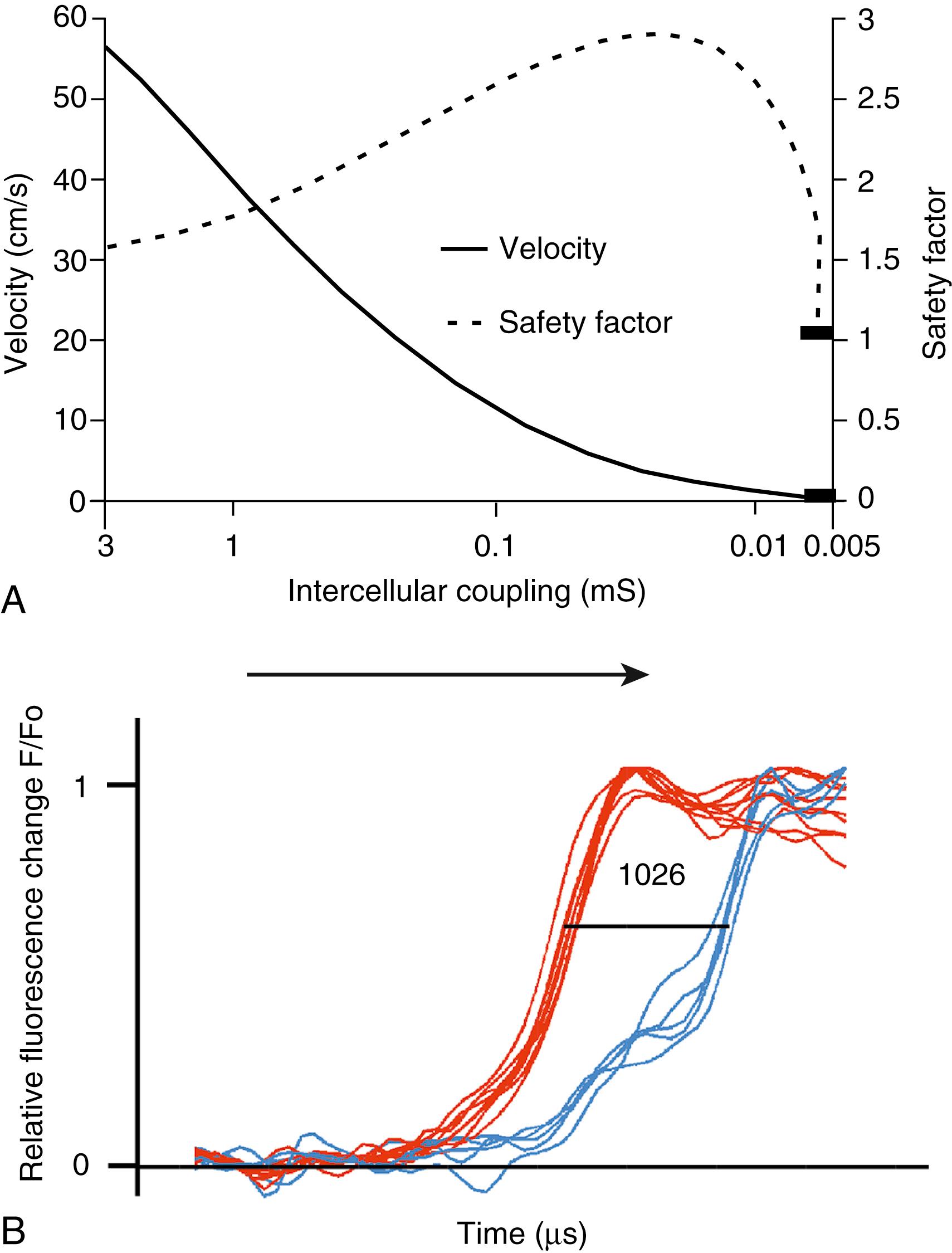
The role of gap junction channels in cardiac propagation as one of the key elements is well acknowledged. Early experimental and theoretical studies looked at the effect of a decrease in gap junction conductance on propagation velocity in a general way by implicating or inducing a uniform change of intercellular electrical conductance in the tissue. The effect of a uniform decrease in intercellular electrical conductance in best understood by consideration of the concept of conduction safety. Several formalisms describing conduction safety have been published. The formalism describing conduction safety (SF) in linear uniform tissue states that SF = ( ∫ A I c • dt + ∫ A I out • dt) / ( ∫ A I m • dt); A│Q m > 0. In simple terms, the numerator of this equation equals the electrical charge produced by a given cell in the propagating wavefront, and it consists of the sum of electrical charges needed to produce the action potential (capacitive component, ∫ A I C • dt) and the charge flowing to excite the cells downstream (axial component, ∫ A I out • dt). The denominator corresponds to the electrical charge ( ∫ A I m • dt) exciting this same cell, which is produced by the excited cells upstream of the propagating front. From this formalism it follows that propagation becomes decremental and eventually blocked, when SF decreases less than 1, or in other words, when more electrical charge is needed to excite a given cell than this same cell can produce by its machinery of excitation. The theoretical dependence of propagation velocity on cell-to-cell coupling and the associated change in SF is illustrated in Fig. 27.1A . Several distinct features specifically related to cellular uncoupling can be derived from this figure. First, changes in cell-to-cell coupling close to the normal level of coupling produce relatively small changes in propagation velocity. Second, cell-to-cell uncoupling can produce extremely slow propagation (on the order of 1 cm/s) if the conductance between cells is reduced greater than 100-fold. This behavior also predicts preservation of propagation, albeit very slow, even at extreme levels of uncoupling. This prediction made from theoretical studies has been verified in experimental work using engineered strands of neonatal rat ventricular myocytes either exposed to an uncoupling agent or using cells with germline ablation of Cx43, as depicted in Fig. 27.1B . , At such reduced levels, propagation becomes highly discontinuous, with fast excitation of the cytoplasm and long delays at the cell border. The explanation for this behavior, which is typical for cell-to-cell uncoupling and is in contrast to the behavior during block of depolarizing ion currents (I Na , I L,Ca ), , is given by the biphasic behavior of the margin of safety of propagation. This biphasic time course of SF is determined by two processes with opposing effects. First, the axial currents flowing into a given cell from excited tissue upstream decreases because of an increase in the resistance between the cells. This so-called source effect causes the membrane capacitance of a given cell to charge more slowly and to reach the excitation threshold later than normal and therefore propagation to slow. At the same time, the excitatory electrical charge flowing downstream, because of the increased intercellular resistance between the downstream cells, is distributed over fewer cells and therefore conserved at the site of excitation within the wavefront (decrease of so-called sink effect). In other words, cell-to-cell uncoupling protects the excitatory current from downstream dissipation and therefore makes propagation safer (increase of SF). Only at extreme levels of uncoupling does failure occur and propagation is eventually blocked. The biophysical principle of source-sink interaction is not only valid for states of cell-to-cell uncoupling, but it represents a general rule independent of scale and holds also for propagation in the presence of discontinuities in tissue structure (e.g., infarct scars ). Importantly, however, very slow propagation is only preserved if sites of increased coupling resistance are closely spaced and produce an effective decrease in the electrotonic sink. If this effect is absent, such as at the transition between tissue of decreased to normal coupling, propagation block is observed. This likely explains some observations made in experimental models of heterogeneous connexin expression. Although the earlier explained concept of propagation safety holds for linear structures with homogeneous changes of cell-to-cell coupling and is helpful for the general understanding of the role of cell-to-cell coupling in propagation, more extended formalisms accounting for the two-dimensional (2D) and three-dimensional (3D) nature of cardiac tissue have been described.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here