Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiotoxicity in the context of anticancer therapy requires a deep appreciation for the differences and potential interactions between patient risk factors for cardiac disease and the cardiovascular risk profile for an anticancer drug.
Understanding the structural, functional, and physiologic changes in the cardiovascular system that occur over the lifetime of the patient with cancer is critical to cancer therapy delivery and long-term care.
There is a clear need for additional evidence-based guidelines tailored to cardio-oncology that are informed and in part derived from existing oncology and cardiology treatment guidelines.
Appropriate nonclinical strategies and models can enhance early capture of safety signals and assessment of human risk.
Anthracycline cardiotoxicity can occur over a broad spectrum of time; recent data challenge the paradigm of irreversible and late toxicity.
ErbB2-targeted therapy, primarily trastuzumab, is associated with a clinically significant incidence of cardiac dysfunction and heart failure (HF). Cardiac dysfunction, as defined by declines in left ventricular ejection fraction (LVEF) is reversible in the majority of, but not in all, cases.
Vascular endothelial growth factor (VEGF)–targeted therapies are associated with several cardiovascular toxicities, including hypertension, left ventricular dysfunction, and HF. Of these, hypertension is the most prevalent adverse effect.
Proteasome inhibitor therapy, primarily carfilzomib, is associated with a number of cardiotoxicities, including hypertension and HF.
Immune checkpoint inhibitors are associated with a low but clinically significant incidence of cardiomyopathy and HF, secondary to myocarditis.
Common types of radiation -induced cardiotoxicity include pericarditis, cardiomyopathy, coronary artery disease, and valvular disorders.
The Common Terminology Criteria for Adverse Events (CTCAE) grading criteria for cardiac adverse events do not necessarily correspond with specific prognostic stages for heart disease because an acute adverse event that occurs in the course of treatment may not follow the same trajectory as is typically seen with heart disease progression.
Acute and subacute cardiac events are captured during the course of treatment.
Late or delayed toxicities may not be identified during a trial, but years later through surveillance efforts.
Circulating biomarkers can be used to aid in the diagnosis of cardiac injury and dysfunction, have been studied as surrogate measures of toxicity, and also can be used as prognostic measures of clinical outcomes, particularly in HF.
Echocardiography remains the mainstay of routine cardiac structure and function assessment before, during, and after cancer therapy and is incorporated in many clinical algorithms.
Advantages of echocardiography include widespread availability and lack of ionizing radiation.
Disadvantages include greater variability in the estimation of key parameters, in part related to limitations in image quality, particularly when acoustic windows are poor; and variability in interobserver and intraobserver interpretation.
A number of epidemiologic studies and clinical trials have suggested that cardiovascular risk factors, such as hypertension, diabetes, obesity, hyperlipidemia, and a prior history of cardiovascular disease, play an important role in the development of cardiotoxicity, particularly as it pertains to the development of cardiomyopathy.
Mitigation of cardiotoxicity and the implementation of cardioprotective strategies can be considered before, during, or after therapy.
Strategies include altering dosage and administration of agents and delivering additional cardioprotective pharmacotherapies.
Multiple strategies are under investigation, including behavioral interventions.
The management of cardiomyopathy once it ensues largely follows conventional therapy for the management of HF and cardiomyopathy, including the use of β-blockers, renin-angiotensin-aldosterone system antagonists, and diuretic therapy as indicated.
More than 15 million Americans with a history of cancer are alive.
Recent surveillance studies have demonstrated late cardiac effects of cancer treatment and the potential for competing health outcomes in cancer survivors.
There is a critical need to evaluate cardiovascular risk factors in patients with cancer before they undergo cancer therapy to manage those complications during and after cancer treatment.
A deeper scientific and molecular understanding of cancer treatment–induced cardiac toxicities will be the basis for predicting patient risk, developing effective management strategies, and ultimately designing drugs with improved cardiovascular safety profiles.
Systemic cancer treatment has evolved substantially over time. Although traditional cytotoxic chemotherapy is still widely in use, there have been significant increases in the development and use of molecular strategies that target signaling pathways involved in cancer growth. However, perturbation of these signaling pathways can result in off-target adverse effects on the cardiovascular (CV) system, often referred to broadly as cardiotoxicity.
Cardiotoxicity encompasses a number of potentially diverse CV adverse events, which may manifest as abnormalities in cardiac function (left ventricular ejection fraction [LVEF] declines, diastolic dysfunction, cardiomyopathy, and heart failure [HF]); valvular disease (stenosis, regurgitation); pericardial disease (pericarditis, pericardial effusion); arrhythmias (atrial arrhythmias, ventricular arrhythmias, prolonged Q-T intervals); abnormalities in cardiac structure (cardiomyocyte death, myocardial fibrosis, left ventricular [LV] cavity dilation); worsening pulmonary hypertension; and microvascular and macrovascular abnormalities (myocardial ischemia, endothelial dysfunction, accelerated atherosclerosis, coronary vasospasm, peripheral arterial disease). Moreover, cancer therapies can induce significant hypertension, dyslipidemia, and even metabolic syndrome, which are central risk factors for the development of cardiomyopathy and HF, coronary artery disease (CAD), and myocardial infarction. Any of these effects are superimposed on physiologic and structural changes that accompany aging. Finally, metastatic cancer itself can result in systemic biologic perturbations, including a prothrombotic state and increased inflammation, that can result in additional adverse CV effects.
Understanding the structural, functional, and physiologic changes in the CV system that occur over the lifetime of the patient with cancer is critical to cancer therapy delivery and long-term care. Such an understanding forms a basis for determining the best strategies to mitigate CV toxicity and manage delivery of the most appropriate cancer treatment. This need has resulted in the burgeoning field of cardio-oncology, a growing collaboration between oncologists and cardiologists, and an increased attention to and awareness of the inherent and induced CV risk factors and disease in cancer patients and survivors. This chapter provides an overview of the basic mechanisms, epidemiology, and clinical manifestations of CV injury resulting from specific cancer therapies that are commonly used. Methods for monitoring and detection of cardiotoxicity and the use of cardioprotective strategies are also reviewed.
Cardiotoxicity can occur at any time during or after cancer therapy, depending on the pathophysiologic basis of the toxicity, inherent biologic variability, physiologic reserves of the patient, and secondary stressors. Acute cardiac injury occurs within hours to days of treatment and can manifest as acute LV systolic dysfunction—that is, a cardiomyopathy that may be asymptomatic or present as symptomatic HF with doxorubicin, arrhythmia with ibrutinib, coronary artery spasm with 5-fluorouracil (5-FU), hypertension with vascular endothelial growth factor (VEGF) inhibitors, or transient conduction abnormalities with paclitaxel. Early-onset cardiotoxicity occurs within 1 year after treatment. Patients may develop arrhythmia, left-ventricular dysfunction, and HF.
Late-onset (or delayed) cardiotoxicity may become clinically evident years (possibly even 10 to 20 years) after the initial exposure and may represent a progression of injury that was not readily clinically evident, worsened by the cumulative effects of underlying patient CV risk factors. As an example, this is observed with the interaction of treatment factors such as anthracyclines and radiation, and CV risk factors such as hypertension, atherosclerosis, and obesity. The actual onset of clinical toxicity may be unclear given incomplete follow-up and the variable characterization of CV function in patients with cancer. Although some studies have estimated the incidence of late cardiac effects such as cardiomyopathy and HF to be about 1.6% to 5%, others have suggested an incidence of clinically overt HF of 7% to 15%. A true temporal classification of late-onset (or delayed) cardiotoxicity is dependent on evidence of a clear relationship between occult asymptomatic cardiotoxicity and the subsequent development of overt disease. For example, imaging and serologic biomarkers of subclinical myocardial damage may precede echocardiographic indices of systolic and diastolic dysfunction.
Cardiac toxicity can be reversible or irreversible, with reversibility referring to recovery of cellular or organ function. Myocardial changes such as myocardial cell loss (by necrosis or apoptosis), myofibrillar loss, and mitochondrial degradation are generally considered irreversible in the context of cellular or tissue injury. For example, late anthracycline cardiotoxicity is often perceived to be “irreversible,” given evidence for cardiomyocyte death, and thus led to the classification of “Type 1” or irreversible cardiotoxicity (in contrast to “Type 2” cardiotoxicity with trastuzumab, viewed generally at least to some extent as reversible and occurring during therapy). The Type 1 and Type 2 classification has fallen out of favor, given a perceived oversimplification of the underlying pathophysiology and natural history of cardiotoxicity. Recent evidence suggests that cardiac function may recover and “reverse” in the setting of anthracycline cardiotoxicity. Moreover, trastuzumab cardiotoxicity is not always reversible. Finally, there may be common mechanisms to cardiotoxicity observed with both strategies.
A functional definition of reversibility would mean the clinical signs and symptoms associated with cardiac dysfunction have resolved. For example, evidence for HF and profound myocardial dysfunction documented by radiography, echocardiography, and signs and symptoms of HF (e.g., lower extremity edema, jugular venous distention, pulmonary congestion, ascites, fatigue, dyspnea at rest or exertion, paroxysmal nocturnal dyspnea, orthopnea) that resolves after the discontinuation of treatment would be considered reversible. A number of compensatory mechanisms to maintain cardiac output and systemic vascular resistance can occur transiently and subsequently reverse LV dysfunction. However, over time and with additional exposure to the therapy or other insults, these compensatory mechanisms may be inadequate, and irreversible cardiomyopathy and worsening symptomatic HF may occur ( Fig. 42.1 ).
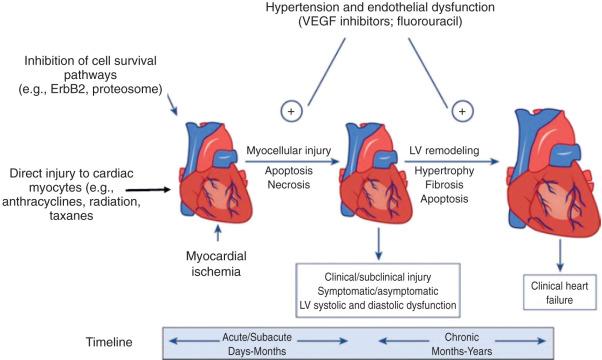
The epidemiology, basic mechanisms, clinical manifestations, and risk factors for cardiotoxicity for certain classes of agents are discussed in the following sections. Table 42.1 provides a broad overview of the CV effects reported with commonly used agents.
| Categories of Drugs | Cardiovascular Effects Reported | Reference |
|---|---|---|
| ANTHRACYCLINES | ||
| Target intercalation into DNA and disruption of topoisomerase II–mediated DNA repair; generation of free radicals and their damage to cellular membranes; DNA and proteins. | Chlebowski, 1979 ; Tjuljandin, 1990 ; Anderlini, 1995 ; Tong, 1980 | |
| Doxorubicin, epirubicin, idarubicin, daunorubicin | Acute cardiotoxicity (transient arrhythmias, especially sinus tachycardia with Q-T prolongation) reported; LVD manifesting as acute toxicity within 2 weeks of ending treatment; subchronic toxicity developing within 1 year of exposure; and late-onset toxicity manifesting many years after exposure | |
| DNA CROSS-LINKERS AND FREE RADICAL–MEDIATED STRAND BREAKS | ||
| Busulfan (bifunctional DNA alkylation) | Edema, tachycardia, hypertension (all grades), vasodilation, cardiac tamponade; left HF, complete atrioventricular (AV) block, endomyocardial fibrosis | |
| ANTIMICROTUBULE AGENTS | ||
| Exert their effect by binding to and subsequently polymerizing and stabilizing microtubules, thus preventing mitosis and resulting in apoptosis. Suppress both the growth and shortening phases of microtubule dynamic instability. Block cells in the G 2 /M phase of the cell cycle, and such cells are unable to form a normal mitotic apparatus. Docetaxel also decreases Bcl-2 and Bcl-xL gene expression. | Perez, 1998 ; Kenmotsu, 2015 ; Bollag, 1995 | |
| Paclitaxel, docetaxel (Taxotere) | LVD, cardiac ischemia; Q-Tc prolongation, edema, vasodilation, hypotension, HF (higher incidence with trastuzumab), bradycardia; syncope, phlebitis | |
| Eribulin | Q-T prolongation | |
| Ixabepilone | Adverse CV effects poorly specified in one study, where 7 of 48 patients were noted to have cardiac adverse effects, which were all grade 1 or 2 | |
| ANTIMETABOLITES | ||
| Target DNA, incorporation of metabolites into DNA, the inhibition of DNA polymerase β, dihydrofolate reductase, thymidilate synthase, or ribonucleotide reductase. | Senturk, 2009 ; Schimmel, 2004 ; Chanan-Khan | |
| Fludarabine | Edema (mainly peripheral edema) | |
| Capecitabine | Ischemia, vasospasm, pericarditis, HF, cardiogenic shock | |
| Cytarabine | Pericarditis with effusion, rare incidence of vasculitis | |
| Pentostatin and cladribine | Angina pectoris, dysrhythmias, AV block, cardiac arrest, HF, hemorrhage, hypertension or hypotension, pericardial effusion, pulmonary embolus, sinus arrest | |
| Methotrexate | Pericarditis, pericardial effusion, hypotension, thrombotic events, vasculitis | |
| ALKYLATING AGENTS | ||
| Target DNA, binding to DNA, producing a variety of interstrand or intrastrand cross-links called adducts, that alter DNA structure or function. A common site of alkylation is the N-7 position of guanine. | Gottdiener, 1981 ; Goldberg, 1986 ; Kandylis, 1989 ; Tascilar, 2007 ; Lebedinsky, 2011 ; Bueren-Calabuig, 2011 ; Feuerhahn, 2011 ; Stephens, 2000 ; Ito, 2010 ; Zhu, 2011 ; Hecht, 2000 | |
| Cyclophosphamide | LVD at high dosages, HF within 3 weeks of administration, hemorrhagic myopericarditis that is rare | |
| Ifosfamide | Arrhythmias (including premature atrial contractions, premature ventricular contractions, supraventricular tachycardia, atrial fibrillation, atrial flutter), supraventricular tachyarrhythmia, HF (dose 10–18 g/m 2 ) | |
| Cisplatin | Thromboembolic disorders, HF, hypertension, orthostatic hypotension, myocardial ischemia or infarction, bradycardia | |
| Trabectedin | Cardiac ischemia | |
| Thalidomide | Thromboembolic events, hypotension, edema, bradycardia, orthostatic hypotension | |
| ANTIBIOTICS | ||
| Target DNA and RNA, formation of reactive intermediates resulting in selective degradation of DNA and oxidative degradation of all major classes of cellular RNAs. | ||
| Mitomycin C | HF, edema, thrombophlebitis | |
| Bleomycin | Edema, Raynaud phenomenon, myocardial ischemia or infarction | |
| HDAC INHIBITORS | ||
| Target histone deacetylases (HDACs), leading to induction of gene expression or destabilization and degradation of growth factors. | Piekarz, 2009 ; Atadja, 2009 ; Richon, 2000 ; Glaser, 2003 | |
| Romedepsin | Increased HR (average 11 beats/min) following infusion, ST segment flattening and depression, supraventricular arrhythmia, hypotension, edema, ventricular arrhythmia | |
| Panobinostat | An early phase I study using an intensive intravenous panobinostat administration schedule led to initial safety concerns surrounding possible Q-Tc interval prolongation Analyses of electrocardiograms of patients with solid tumors and hematologic malignancies enrolled in subsequent phase I/II studies suggest that QT-cF prolongation is not an issue with intermittent weekly administration |
|
| Vorinostat (suberoylanilide hydroxamic acid [SAHA]) | Isolated rare events of Q-Tc prolongation in previous vorinostat studies were observed Administration of a single supratherapeutic dose of the HDAC inhibitor vorinostat is not associated with prolongation of the Q-Tc interval |
|
| VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) INHIBITORS | ||
| Target VEGFR1, VEGFR2, PDGFRα, and PDGFRβ, bFGF receptors, stem-cell factor (SCF) receptor (KIT), RET, TIE2, fetal liver tyrosine kinase receptor 3 (FLT3) compounds generally directed against the endothelium, inhibiting either endothelial growth factors or the receptors of such factors. They all have antiangiogenic effects. | Awad, 2014 ; Morabito, 2009 | |
| Bevacizumab | Heat failure, hypertension, ischemia | |
| Cabozantinib | Hypertension | |
| Regorafenib | Hypertension, myocardial ischemia and infarction | |
| Axitinib | Hypertension | |
| Ponatinib (main target Bcr-Abl, Bcr-Abl T315I) | HF, myocardial ischemia, peripheral ischemia (stroke, peripheral vascular disease) | |
| Sunitinib | Hypertension is present in approximately 60% of patients; Q-T interval prolongation, decreased LVEF, LVD, HF, increased BP, HF linked to CV comorbidities, arterial thrombosis reported | |
| Nilotinib (main target Bcr-Abl) | Q-T interval prolongation, sudden death (possibly ventricular repolarization related), ischemia, peripheral ischemia | |
| Sorafenib | Q-T interval prolongation, sudden death (possibly ventricular repolarization related), HF (cardiomyopathy), ischemia, arterial thrombosis | |
| Pazopanib | Cardiac dysfunction (HF and decreased LVEF), Q-T prolongation, cases of torsades de pointes, hypertension | |
| Vandetanib | Q-T interval prolongation, torsades de pointes, acute cardiac failure, hypertension | |
| MEMBRANE RECEPTORS OF TUMOR CELLS | ||
| Target HER2 or EGFR. | Awad, 2014 | |
| Trastuzumab, pertuzumab, lapatinib T-DM1 (HER2) | Reduction in LVEF, cardiomyopathy, HF These effects are often reversed if the drug is withdrawn, but may also require HF treatment Anthracyclines exacerbate cardiotoxicity with trastuzumab |
|
| T-DM1 (HER2) | In a phase II study, there was no symptomatic HF, but patients in each T-DM1 group showed reduction in LVEF One patient in the T-DM1 group had LVEF <40% In a phase III comparative study, only 1.7% of patients in the T-DM1 group experienced decreased LVEF at least 15% below the baseline value Grade III LVEF developed in only one patient (0.2%) |
|
| Necitumumab (EGFR with gemcitabine or cisplatin) | Venous thromboembolic events (VTEs) and arterial thromboembolic events (ATEs), including fatal cases, were observed with necitumumab in combination with gemcitabine and cisplatin An increased frequency of cardiorespiratory arrest or sudden death was observed with necitumumab in combination with gemcitabine and cisplatin compared with patients treated with chemotherapy alone Cardiorespiratory arrest or sudden death |
|
| Osimertinib (EGFR T790M mutation) | Q-Tc interval prolongation, cardiomyopathy occurred in 1.4% and was fatal in 0.2% of 813 Tagrisso patients LVEF decline >10% and a drop to <50% occurred in 2.4% of (9/375) Tagrisso patients |
|
| INTRACELLULAR PATHWAYS OF TUMOR CELLS | ||
| Target various kinases, mainly RAF/MER/ERK, anaplastic lymphoma kinase (ALK), BCR-ABL kinase. | Morabito, 2009 | |
| Cobimetinib (BRAF V600E or V600K mutation) | Symptomatic and asymptomatic decline in LVEF can occur Risk of cardiomyopathy is increased in patients receiving combination with vemurafenib compared with vemurafenib as a single agent |
|
| Trametinib | The early trials of trametinib reported that 8% of patients experienced LVEF dysfunction; however, few events were thought to be specifically related to trametinib and a recorded drop in LVEF was rarely symptomatic and was not associated with increased risk of Q-Tc prolongation as a single agent | |
| Vemurafenib, dabrafenib | Q-T interval prolongation, cases of vemurafenib-induced pericarditis | |
| Ibrutinib | Atrial fibrillation, 10-fold increase in rate observed | |
| Imatinib, nilotinib, dasatinib, bosutinib | Peripheral arterial disease, metabolic syndrome, pulmonary hypertension, fluid retention | |
| Crizotinib, ceritinib (ALK; other targets IGF-1R, INSR, STK22Dn, c-MET) | Q-T interval prolongation, bradycardia | |
| IMMUNOMODULATORY AGENTS | ||
| Antibodies directed against immune-related tumor or T-cell antigens. | Smith, 2003 | |
| Rituximab (CD20) | Cardiac arrhythmias, symptomatic polymorphic ventricular tachycardia | |
| Alemtuzumab (CD52) | HF or arrhythmia that mostly improved after alemtuzumab discontinuation; patients with mycosis fungoides (MF) or Sezary syndrome may have a higher risk of cardiac complications | |
| Ipilimumab (CTLA4) | Autoimmune myocarditis, cardiomyopathy, HF, cardiac fibrosis, and cardiac arrest Preexisting cardiac pathologic condition or peripheral arterial disease was present in some patients, but all patients were free of symptoms when starting therapy |
|
| Nivolumab (PD-1) | HF, cardiomyopathy, heart block, myocardial fibrosis, and myocarditis | |
| Pembrolizumab (PD-1) | Rare occurrence of autoimmune myocarditis with severely impaired left ventricular function with dyssynchrony | |
| IL-2 | Hypotension (capillary leak syndrome), 71% ventricular tachycardia, coronary artery thrombosis, myocarditis | |
| MAGE-A3a3a TCR cells | Cardiac myonecrosis with an unusual lymphoid predominant infiltrate into the myocardium, a cross-reactive epitope derived from the human protein titin, presented in the context of HLA-A*01 | |
| Donor-derived anti-CD19 CAR T cells | Mainly sepsis-induced cardiomyopathy, tachycardia, and hypotension associated with cytokine release syndrome | |
| Mitoxantrone (broad range of actions and effects on many different types of immune cells) | Cumulative dose-related cardiotoxicity, primary cardiotoxicity via destruction of myocytes | |
| PROTEASOME INHIBITORS | ||
| Targets 26S proteasome resulting in Bcl-2 phosphorylation associated with G 2 –M phase cell cycle arrest and the induction of apoptosis; may dysregulate intracellular calcium metabolism resulting in caspase activation. | Field-Smith, 2006 | |
| Carfilzomib (irreversible inhibitor) | Hypertension, HF, dyspnea, increases in pulmonary pressure; may be reversible when carfilzomib is discontinued | |
| Bortezomib (reversible inhibitor) | Variable reports; systematic review and meta-analysis demonstrated that the use of bortezomib does not significantly increase the risk of all-grade and high-grade cardiotoxicity | |
| OTHER CATEGORIES | ||
| Pasquali, 2010 | ||
| All- trans retinoic acid (ATRA) | Tretinoin treatment has been associated with pleural or pericardial effusions, hypotension, and dysfunction in myocardial contractility | |
| Etoposide (topoisomerase inhibitor) | Hypotension, HF, myocardial ischemia and infarction | |
| Estramustine | Fluid retention, hypertension, electrocardiographic changes, myocardial infarction, and thromboembolic disease | |
| Arsenic trioxide | Ventricular premature contractions, Q-Tc prolongation, TdP, cardiac dysrhythmias, edema with pericardial effusion (during acute promyelocytic leukemia differentiation syndrome) | |
| Interferon-α | Hypertension or hypotension, arrhythmias | |
Anthracyclines (doxorubicin, daunorubicin, epirubicin) and related molecules (mitoxantrone) have played an integral chemotherapeutic role in the successful treatment of breast cancer, leukemia, lymphoma, and sarcoma in pediatric patients, adolescents, and adults. Unfortunately, the oncologic successes of anthracycline chemotherapy have been accompanied by dose-dependent cardiotoxicity that has resulted in clinically important consequences.
Despite a recognition of anthracycline cardiotoxicity since the 1970s, the molecular and cellular mechanisms involved in its pathogenesis are not completely understood. The generation of reactive oxygen species and increased oxidative stress is the most widely accepted mechanism of toxicity. Recent data have raised the intriguing hypothesis that this is dependent on topoisomerase IIβ, a target of anthracyclines that is expressed in adult mammalian cardiomyocytes. After doxorubicin exposure, cardiomyocytes from wild-type mice exhibit abnormalities in the p53 tumor suppressor gene, β-adrenergic signaling, and apoptotic pathways. In contrast, cardiomyocytes from a cardiomyocyte-specific Top2β knockout mouse (Top2β Δ/Δ ) exhibited substantially fewer changes and preserved cardiac function.
It is likely that anthracyclines engage multiple mechanisms of cardiotoxicity, with additional hypotheses including significant mitochondrial DNA (mtDNA) depletion and mtDNA rearrangements, increased calpain-dependent titin proteolysis, impaired calcium handling and contractility, depletion of cardiac progenitor cells, and impairment of prosurvival signaling pathways via inhibition of neuregulin and ErbB. On histopathology, anthracycline-associated abnormalities include mitochondrial and cytoplasmic vacuolization and myofibrillar and sarcomeric disarray. Biochemical evidence has also demonstrated elevations in circulating cardiac troponins with anthracycline exposure, indicative of cardiomyocyte injury and cell death.
Clinically, acute anthracycline cardiotoxicity manifests as arrhythmias and transient changes in cardiac function. This occurs in less than 1% of patients. Subacute or early-onset chronic progressive cardiotoxicity occurs in 1.6% to 8% of treated patients and typically within the first year. This manifests as declines in LVEF, cardiomyopathy, and HF.
Late anthracycline cardiotoxicity is often perceived to be “irreversible,” but recent data challenge the notion of irreversible cardiotoxicity with anthracyclines. In one study of 201 patients with a reduced LVEF (≤45%) secondary to anthracyclines, 42% of the patients recovered their LVEF completely with institution of angiotensin-converting enzyme (ACE) inhibitors and/or β-blockers. Thirteen percent had partial recovery of their LVEF. The percentage of responders decreased as time to the initiation of cardiac medications increased. Every patient who experienced complete recovery had medications instituted within 6 months of recognition of cardiac dysfunction.
Another study from the same group of investigators included 2625 patients treated with anthracycline therapy and further challenged the notion of “late” as well as irreversible cardiotoxicity. Patients were followed with serial echocardiograms every 6 months after the completion of chemotherapy for a median of 5.2 years. In this cohort, 98% of the cardiotoxic events occurred within the first year after chemotherapy completion. Cardiotoxicity rates were on the order of 9% and occurred at a median time of 3.5 months after the end of chemotherapy. Recovery of LVEF, either full or partial, was achievable in 11% and 71% of participants, respectively.
Since the late 1970s, anthracycline cardiotoxicity has been noted to be dose dependent, resulting in specific guidelines to limit cumulative dosing. Although individual variation in tolerability exists, the risk for developing cardiomyopathy increases exponentially for cumulative doses greater than 400 mg/m 2 with an average incidence of 5.1% at 400 mg/m 2 that is estimated to be 15.7% at 500 mg/m 2 . Of note, recent data also suggest that genetic variants in single nucleotide polymorphisms (SNPs) may influence this risk. Additional risk factors include age at both ends of the spectrum, including children and adults older than 70 years; radiation to the chest wall; female sex; and preexisting CV disease or CV risk factors, such as hypertension, smoking, and diabetes mellitus.
Trastuzumab, pertuzumab, and lapatinib all target the tyrosine kinase receptor HER2/ErbB2, a receptor highly expressed in many breast cancer cells. In addition to the apparent role of HER2/ErbB2 in cancer cell survival, essential roles for this signaling pathway in the heart were revealed by cardiac-restricted knockout of HER2/ErbB2 in mice that developed a dilated cardiomyopathy and an impaired ability to respond to CV stress. Trastuzumab (Herceptin) is a humanized monoclonal antibody that targets the extracellular domain of the HER2/ErbB2 tyrosine kinase receptor. In the clinical setting, trastuzumab is widely used and has demonstrated remarkable efficacy in the treatment of HER2-positive breast cancer, but there is a clinically significant incidence of cardiac dysfunction (generally reversible) and HF. Based on the role of ErbB2 in providing a protective response to cardiac stress and the cardiac phenotype of ErbB2 conditional knockout mice, cardiotoxicity of trastuzumab is presumed to be secondary to ErbB2 inhibition. Additional preclinical data support this hypothesis. An antimouse ErbB2 has been shown to impair cardiac contractility and fibrosis in mice. In addition, trastuzumab treatment promotes oxidative stress and apoptosis in the myocardium of mice, and results in elevations in biomarkers of cardiac injury cardiac troponin I (TnI), and cardiac myosin light chain 1 (cMLC1). Recent data have also suggested that disruption of ErbB2 signaling also results in endothelial dysfunction and alterations in vascular function, which may contribute to cardiomyopathy development.
The initial reports of trastuzumab-associated cardiac dysfunction were most frequently seen in patients with breast cancer with concurrent anthracycline chemotherapy. A change to sequential administration of anthracyclines followed by trastuzumab, as well as a decrease in the use of anthracyclines in patients with early-stage HER2-positive breast cancer, has resulted in a lower rate of cardiac dysfunction. Experience from large clinical trials, many of which have included combination therapy with anthracyclines and trastuzumab, demonstrates an approximate 9.8% incidence of LV dysfunction and a 2.7% incidence of severe, symptomatic HF. Retrospective analyses of registry data—for example, from the Cancer Research Network—suggest higher rates. In combination with anthracyclines, the 5-year cumulative incidence of HF or cardiomyopathy increases to 16% to 20%, with a sevenfold increased risk compared with patients not treated with chemotherapy. Retrospective analyses from the Ontario Cancer Registry, based on only HF International Statistical Classification of Diseases and Related Health Problems (ICD) codes, suggest a greater than fivefold increase in HF with adjuvant trastuzumab, although the majority of the events occurred within the first 1.5 years after chemotherapy initiation. In this study, the cumulative incidence at 5 years was 5.2%. Additional risk factors for trastuzumab-associated cardiotoxicity include older age, lower baseline LVEF, hypertension, obesity, renal dysfunction, diabetes, and arrhythmia.
Although clinical experience suggests that recovery of LVEF occurs in the majority of patients within the first year after exposure, either with temporary cessation of trastuzumab or with standard HF therapy ( Box 42.1 ), not all patients fully recover cardiac function, as defined by LVEF declines. The long-term effects of trastuzumab on cardiac function and HF remain to be determined, although clinical trial data suggest that most declines in LVEF occur during therapy.
A 55-year-old woman with a history of HER2-positive breast cancer is undergoing therapy with doxorubicin, followed by trastuzumab. She has no cardiovascular (CV) risk factors except for obesity. At baseline, her left ventricular ejection fraction (LVEF) is estimated at 55% to 60%. After completion of 240 mg/m 2 of doxorubicin, her LVEF is estimated at 55%, and subsequently declines to 40% after four cycles of trastuzumab. She denies any symptoms except for fatigue, and her physical examination does not reveal any findings of decompensated heart failure (HF). She is normotensive. What are potential management strategies?
Declines in LVEF are observed with trastuzumab therapy and potentiated by anthracycline exposure. Risk factors also include older age, hypertension, and obesity. Guidelines from the American College of Cardiology and American Heart Association typically define exposure to any cardiotoxic therapy as stage A HF, and structural abnormalities including declines in LVEF as stage B HF. In the setting of stage B HF, β-blockers and/or angiotensin-converting enzyme (ACE) inhibitors are recommended in the American College of Cardiology and American Heart Association guidelines, although these guidelines are not specific to cancer patients. The European Society of Cardiology suggests that initiating one or more guideline-based HF therapies be considered, depending on the magnitude of decrease and LVEF value. Various working groups (e.g., National Cancer Research Institute and Canadian Trastuzumab Working Group ) have suggested management strategies based on practices adapted in clinical trials. It is unclear if these practices have been widely adapted, and the American Society of Clinical Oncology cardiac dysfunction guidelines emphasize that decisions regarding therapy need to be made on an individual basis. This patient would likely benefit from one or both of these HF therapies.
Newer HER2 antagonists include pertuzumab and trastuzumab emtansine (T-DM1). Pertuzumab is a recombinant humanized monoclonal antibody that targets an epitope of the extracellular domain II of ErbB2 and inhibits ErbB2 homodimerization and heterodimerization. The rates of cardiac dysfunction with pertuzumab remain to be further defined. One phase II study of pertuzumab found a low incidence of LVEF declines of 10% or more to less than 50% in 3 of 66 treated patients. The phase III CLEOPATRA study did not suggest an increased incidence of cardiotoxicity in patients receiving pertuzumab in combination with trastuzumab and docetaxel, compared with trastuzumab and docetaxel alone (6.6% versus 8.6% by Common Terminology Criteria for Adverse Events [CTCAE], version 3, for LV dysfunction and HF). Trastuzumab emtansine (T-DM1), an antibody-drug conjugate that incorporates trastuzumab with the microtubule agent emtansine (DM1), is also in use for advanced HER2-positive breast cancer. Like pertuzumab, cardiotoxicity rates with T-DM1 also remain incompletely defined, although the phase III EMILIA study also did not suggest an increased risk of cardiotoxicity with this agent.
Cabozantinib, regorafenib, axitinib, sunitinib, sorafenib, vandetanib, and pazopanib are all VEGF tyrosine kinase inhibitors. Monoclonal antibodies such as bevacizumab also inhibit the VEGF signaling pathway, and additional multitargeted oral tyrosine kinase inhibitors such as ponatinib and nilotinib target BCR-ABL as well as VEGF receptors. VEGF-directed therapies have improved clinical outcomes for many solid tumors, including but not limited to metastatic renal cell cancer, pancreatic neuroendocrine tumors, thyroid cancers, gastrointestinal stromal tumors, and colorectal tumors. However, many of these agents have also been associated with several CV toxicities, including hypertension, LV dysfunction, and HF. Of these, hypertension is the most prevalent adverse effect for the multitargeted kinase inhibitors of the VEGF pathway.
Nonclinical studies indicate that VEGF is expressed in virtually every tissue, with the highest density of VEGF-expressing cells being found in tissues with fenestrated vasculature such as in the kidney. Studies in animals support the notion that VEGF may have an important effect on endothelial and vascular function and on blood pressure regulation in the adult animals, and alterations in nitric oxide (NO) have also been observed in some studies, but not all. Administration of a specific antibody against the major VEGF receptor, VEGFR2, to normal mice caused a rapid and sustained increase in BP of approximately 10 mm Hg, and significant reductions in the expression of endothelial and neuronal NO synthases in the kidney. In a swine model, sunitinib induced a compensatory increase in NO. In humans, VEGF inhibition with vandetanib was associated with decreased urinary nitrite/nitrate excretion and decreased serum levels of NO metabolites. However, no difference in flow-mediated dilation, a surrogate for NO bioavailability, was observed.
The endothelium is a major target organ for the actions of VEGF; therefore it is reasonable to expect that the CV effects of these inhibitors may be related to VEGF-related compromises in normal vascular function, such as decreases in the production of endothelium-relaxing NO and prostaglandin, enhanced production of vasoconstrictive factors such as thromboxane and endothelin, alterations in neurohormones (such as vasopressin or norepinephrine), and marked downregulation of AMP-kinase (resulting in disruption of mitochondrial function). The complexity of the pathogenic mechanism is further confounded by the fact that VEGF tyrosine kinase inhibitors typically inhibit several pathways. For example, sunitinib blocks multiple pathways in addition to vascular endothelial growth factor receptors 1 to 3 (VEGFR1 to VEGFR3) that are fundamental to CV function, including the platelet-derived growth factor receptor α/β (PDGFRα/β), c-KIT, and Fms-like tyrosine kinase-3 (Flt-3).
Inhibition of multiple kinase pathways and multiple VEGF family members may be essential to the adverse effect profile, and the development of effective means by which these events can be managed during treatment is critical. As an example, with sunitinib the overall incidence of hypertension in one meta-analysis was 21.6%, and it varied among tumor types (worse with renal cell cancer compared with other solid tumors) and sunitinib administration schedules (worse with continuous versus intermittent dosing). Clinical factors including preexisting hypertension, obesity, and older age are also associated with an increased risk of incident hypertension. Phase III trials and subsequent clinical experience have noted an incidence of hypertension ranging from 5% to 47%. In a phase III study comparing pazopanib with sunitinib, the rates of hypertension were 46% and 41%, respectively. Increases in blood pressure have been noted to occur early, within 24 hours of initiation of therapy ( Box 42.2 ).
A 52-year-old man with metastatic renal cell cancer with no significant past medical history is newly initiating therapy with sunitinib. Before sunitinib therapy is started, he has been noted to be hypertensive with a blood pressure of 142/92 at multiple clinic visits. What are important strategies to mitigate his cardiotoxicity risk?
This patient may experience further increases in his blood pressure once sunitinib is initiated. Guideline panel recommendations suggest that formal risk assessment for the risk factors for adverse consequences of high blood pressure be performed (e.g., evaluation of cardiovascular [CV] risk factors such as hypertension, older age, dyslipidemia, obesity, hyperlipidemia; established CV disease including myocardial infarction, ischemic stroke, peripheral arterial disease; renal disease), and that preexisting hypertension should be identified and managed before the initiation of sunitinib. Active monitoring of blood pressure, particularly in the first cycle, and institution of antihypertensive therapy in the setting of blood pressure elevations through treatment should also be performed. No recommendations currently exist regarding “optimal” pharmacologic strategies to treat hypertension, although β-blockers, angiotensin-converting enzyme (ACE) inhibitors, dihydropyridine calcium channel blockers, or diuretics may be used, depending on the individual patient. Comorbid conditions (e.g., renal insufficiency, history of nephrectomy, bradycardia) or drug interactions are part of the decision-making process. Thyroid function should also be carefully monitored during therapy, because sunitinib is associated with the development of hypothyroidism, which may also have CV consequences.
The relationship between increases in blood pressure and the development of subsequent cardiomyopathy and HF remain unknown, although there is a long-standing recognition of the importance of afterload in the pathogenesis of LV dysfunction in HF. Retrospective analyses have suggested the overall incidence of HF in sunitinib-treated patients to be 4.1%, and the incidence of significant LV dysfunction was estimated to be on the order of 10% to 13%. One limitation of the comparison of findings across studies is the heterogeneity of definitions of LV dysfunction (e.g., symptoms of cardiac dysfunction; ≥15% absolute decline compared with baseline; or ≥10% decline in LVEF compared with baseline and below the lower limit of normal). Risk factors for LVEF declines include male sex and longer duration of therapy. Multiple prospective studies have suggested that with careful monitoring, these adverse CV effects are manageable. Many questions remain, such as the choice of antihypertensive agent in controlling antiangiogenic therapy–induced hypertension and whether better understanding of the mechanisms will provide clinically useful biomarkers.
Proteasome inhibitors (PIs), such as carfilzomib and bortezomib, are frequently used in the management of multiple myeloma. The ubiquitin proteasome system (UPS) is a fundamental regulator of protein quality in all cells, including cardiomyocytes. The regulation of protein turnover is critical to the cardiomyocytes because they have very limited regenerative capacity. Cardiomyocyte survival depends on the critical balance of protein synthesis and turnover regulated by UPS. Regulation of protein degradation also occurs during the process of cardiac hypertrophy and atrophy, as well as myocardial ischemia and infarction. With this in mind, it is not surprising that proteasome inhibition, although a successful means of treating multiple myeloma, can result in cardiac dysfunction. In nonclinical studies, increased heart weight, increases in TnI, cardiac myocyte degeneration, LV enlargement, and myocardial hypertrophy were observed in cynomolgus monkeys treated with high-dose carfilzomib, an irreversible PI.
A pooled analysis of several clinical trials revealed that 22% of patients had any grade of any cardiac event, including 7% HF. Although cardiac disease in patients with multiple myeloma may be secondary to comorbidities such as chronic anemia and amyloidosis, there are still no guidelines addressing how patients' cardiac status should be assessed before they receive potentially cardiotoxic myeloma therapy. Carfilzomib treatment is associated with a poorly characterized syndrome of dyspnea, increases in pulmonary pressures, hypertension, new and worsening HF, arrhythmia, cardiac arrest, myocardial infarction, and venous thrombosis. The risk for carfilzomib-associated cardiac events increases with age greater than 75 years. Of note, studies indicate that many adverse effects were largely reversible with prompt cessation of therapy and initiation of HF treatment.
Unlike carfilzomib, bortezomib reversibly binds the proteasome. Several cases of patients demonstrating dyspnea as an initial presenting sign have been reported in the literature. However, reports of cardiotoxicity associated with bortezomib-based regimens have been variable, with incidences ranging from 0% to 17.9% in clinical trials; the incidence rates of all-grade and high-grade cardiotoxicity associated with bortezomib were 3.8% (95% confidence interval [CI], 2.6%–5.6%) and 2.3% (95% CI, 1.6%–3.5%), respectively, with a mortality of 3.0% (1.4%–6.5%). Awareness of the cardiotoxicity potential with PIs and subsequent close monitoring of patients receiving these agents is necessary to ensure that patients continue to gain the clinical benefit from them.
There are a growing number of cancer therapies focused on modulating the effects of the immune system. The immune system has been long-recognized as playing an important role in myocardial homeostasis in HF, with both beneficial and detrimental effects, influencing both myocyte growth and loss. Immune checkpoint inhibitors, such as ipilimumab, nivolumab, and pembrolizumab, have been successfully used in melanoma and lung cancer. These agents have been associated with a low but clinically significant incidence of cardiomyopathy and HF, secondary to myocarditis. Case reports of patients treated with anti-CTLA4 and anti-PD-1 monoclonal antibodies in combination have described case fatalities secondary to marked cardiac compromise, with autopsy revealing marked lymphocytic infiltration of the myocardium. Cardiotoxicity has also been described with cell-based therapies such as engineered anti-MAGE-A3 T lymphocytes, hypothesized to be secondary to cross-reactivity between T lymphocytes and the cardiac protein titin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here