Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The very-low-birth-weight infant is hemodynamically vulnerable due to a unique set of risk factors, which include an immature myocardium with poor response to volume load and afterload, immature autonomic vasoregulation, and thus ineffective cardiovascular compensatory mechanisms, a propensity to develop specific or non-specific inflammatory responses, the imposition of positive airway pressure, systemic-to-pulmonary shunts, and variability in the degree of placental restoration of blood volume according to cord clamp timing.
In part due to the immaturity of cerebral autoregulation and the limited range of cerebral perfusion pressure in preterm neonates, the low cardiac output, state that may occur is associated with adverse outcomes, including neurological injury and potential long-term developmental impairment.
Assessment of the degree of hemodynamic impairment is difficult, with reliance on standard hemodynamic assessment tools such as capillary refill time, acidosis, and blood pressure all having limitations, especially when used in isolation.
Awareness of the risks of hemodynamic instability with appropriate treatment response may decrease the risk of some of the severe complications of prematurity.
Clinician performed cardiac ultrasound (CPU) can assist in recognizing hemodynamic compromise and allow targeted management of the underlying problems.
The birth of a very-low-birth-weight (VLBW) infant creates a unique set of circumstances that can adversely affect the cardiovascular system, resulting in cardiovascular compromise. The cardiovascular system of the fetus is adapted to an in utero environment that is constant and stable. The determinants of cardiac output, such as preload and afterload, are maintained in equilibrium without interference from the external factors that may affect a neonate born prematurely. Postnatal factors that can affect the cardiovascular function of the VLBW infant include perinatal asphyxia, sepsis, positive pressure respiratory support, unnecessary exposure to high oxygen concentration, and cord clamp time. During the immediate transitional period, these factors may alter preload and change afterload at a time of rapid transition from the fetal circulation, characterized by low systemic vascular resistance, to the postnatal neonatal circulation with higher peripheral vascular resistance. The predominantly systemic to pulmonary shunts at the atrial and ductal level through persisting fetal channels can further reduce potential systemic blood flow ( Figure 20.1 ).
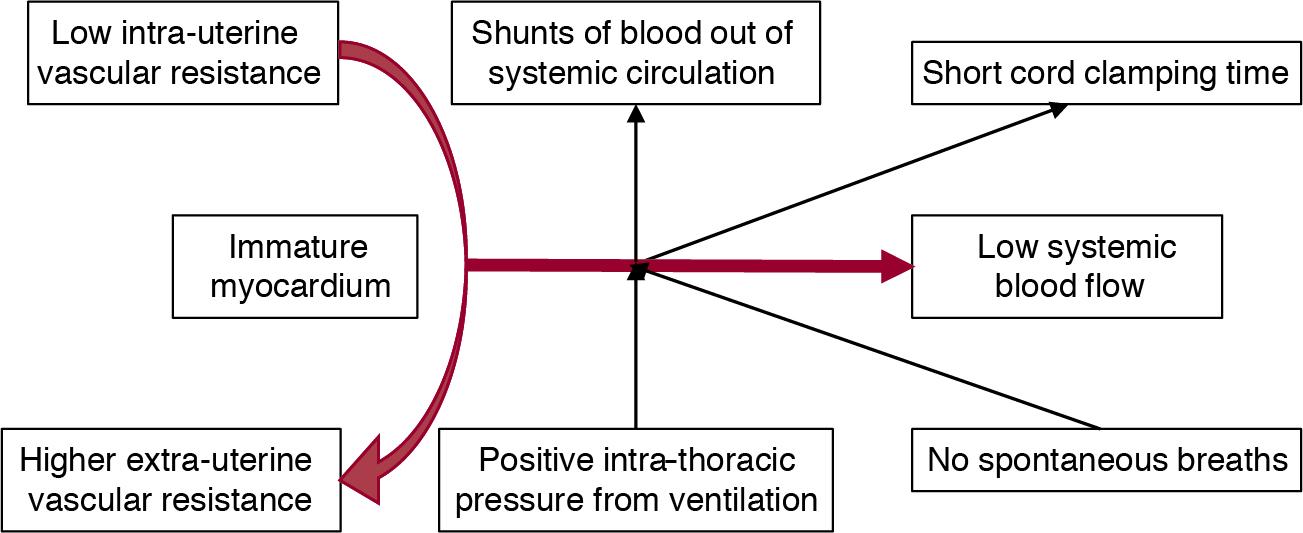
The situation is made more complex by the difficulty in assessing the adequacy of the cardiovascular system in the VLBW infant. The small size of the infant and the frequent presence of shunting at both ductal and atrial levels preclude the use of many of the routine cardiovascular assessment techniques used in children and adults to determine cardiac output. As a result, clinicians are forced to fall back on more easily measured parameters such as the blood pressure. However, blood pressure is the dependent measure among the three determinants of systemic circulation, with cardiac output and systemic vascular resistance being the independent determinants. Accordingly, changes in blood pressure do not necessarily reflect changes in the cardiac output and subsequent changes in organ blood flow and tissue oxygen delivery (see Chapters 2 and 15 ).
Hypotension occurs in up to 30% of VLBW infants, with 16% to 52% of these infants receiving treatment with volume expansion and up to 39% receiving vasopressors (see Chapter 3 for details). More recent surveys of the management of extremely-low-birth-weight (ELBW) infants have demonstrated treatment (fluid bolus or vasopressor administration) rates for hypotension up to 93% at 23 weeks’ gestational age (GA) and up to 73% of infants at 27 weeks’ GA. Most treatment was commenced in the first 24 hours of postnatal life. Similarly, low systemic blood flow in the first 24 hours is seen in up to 35% of VLBW infants, but not all of these infants will have hypotension initially, though many will develop it. Recent studies of the incidence of low superior vena cava (SVC) flow show a reduced incidence (5–21% of ELBW infants), possibly reflecting changes in obstetric and delivery room management, respiratory care, and perhaps fluid management of the neonate. There is a wide variation in the assessment and management of cardiovascular compromise both among institutions and individual clinicians. , As with many other areas in medicine where there is variation in practice with multiple treatment options, the lack of good evidence for both when to treat cardiovascular compromise and whether treatment benefits the long-term outcome of infants underlies this uncertainty. This chapter explores the importance of the unique changes involved in the transitional circulation during the first postnatal day and how they impact the presentation, assessment, and management of neonatal cardiovascular compromise.
The definition of normal blood pressure and hypotension is discussed in detail in Chapter 3 . This chapter will focus on the relevance of these definitions to the VLBW infant. Hypotension can be defined as the blood pressure value where vital organ (brain) blood flow autoregulation is lost. If effective treatment is not initiated at this point, blood pressure may further decrease and reach a “functional threshold” when neuronal function is impaired, and then the “ischemic threshold” resulting in tissue ischemia with likely permanent organ damage. Neither the blood pressure causing loss of autoregulation nor the critical blood pressure resulting in direct tissue damage has been clearly defined for the VLBW neonate in the immediate postnatal period. The distinction between the three levels of hypotension is important because, although loss of autoregulation and cellular function may predispose to brain injury, reaching the ischemic threshold of hypotension by definition is associated with direct tissue damage . Finally, these thresholds may be affected by several factors, including gestational and postmenstrual age, the duration of hypotension, and the presence of acidosis and/or infection. Furthermore, these thresholds are thought to be specific to the individual patient and are affected by the neonate’s ability to compensate in order to maintain adequate oxygen delivery to the organs (see Chapters 1 and 7 ).
Although the normal autoregulatory blood pressure range in the VLBW infant is not known, in clinical practice there are generally two definitions of early hypotension in widespread use:
Mean blood pressure less than 30 mmHg in any gestation infant in the first postnatal days. This definition is based on pathophysiologic associations between cerebral injury (white matter damage or intraventricular hemorrhage) and mean blood pressure less than 30 mmHg , and to a lesser degree on more recent data looking at maintenance of cerebral blood flow (CBF) measured by near-infrared spectroscopy (NIRS) and single-photon emission computed tomography(SPECT) over a range of blood pressures, suggesting a reduction in CBF when a particular (28–30 mmHg) mean blood pressure threshold is reached. It is important to note that although the 10th centile for infants of all gestational ages is at or above 30 mmHg by the third postnatal day, in more immature infants the 10th centile of mean blood pressure is lower than 30 mmHg during the first 3 days. Additionally, there are very few infants with GA <25 weeks included in these studies. Therefore it is too simplistic to use a single cut-off value for blood pressure across a range of gestation and postnatal ages.
Mean blood pressure less than the gestational age in weeks during the first postnatal days, which roughly correlates with the 10th centile for age in tables of normative data. , This statistical definition has also been supported by professional body guidelines such as the Joint Working Group of the British Association of Perinatal Medicine. Again this rule of thumb applies mainly in the first 24–48 hours of extrauterine life; after this time, there is a gradual increase in the expected mean blood pressure such that most premature infants have a mean blood pressure greater than 30 mmHg and thus above gestational age by postnatal day 3 ( Chapter 3 ).
The current definitions are not related to physiologic endpoints such as maintenance of organ blood flow or tissue oxygen delivery. However, most but not all studies using 133 Xe clearance, SPECT, or NIRS to assess changes in CBF found that the lower limit of the autoregulatory blood pressure range may be around 30 mmHg even in the 1-day-old ELBW neonate. , , Again there is little data published for infants <25 weeks’ GA. Indeed, preterm neonates with a mean blood pressure at or above 30 mmHg appear to have an intact static autoregulation of their CBF during the first postnatal day. It is reasonable to assume that, although the gestational age–equivalent blood pressure value is below the CBF autoregulatory range, this value is still higher than the suspected ischemic blood pressure threshold for the VLBW patient population.
A confounding finding to the straightforward-appearing blood pressure-CBF relationship has been provided by a series of studies using superior vena cava (SVC) flow measurements to indirectly assess brain perfusion in the VLBW neonate with a focus on the ELBW infant in the immediate postnatal period. , The findings of these studies suggest that, in the ELBW neonate, blood pressure in the normal range may not always guarantee normal vital organ (brain) blood flow. Although, caution is needed when interpreting these findings as only approximately 30% of the blood in the SVC represents the blood coming back from the brain, in the preterm neonate ( Chapter 2 ), animal and additional human data support this notion (see Chapter 7 ). In the compensated phase of shock redistribution of blood flow from the non-vital organs (e.g., muscle, skin, kidneys, intestine, etc.) to the vital organs (brain, heart, adrenals), as well as neuroendocrine compensatory and local vascular mechanisms ensure that blood pressure and organ blood flow to the vital organs are maintained within the normal range. With progression of the condition, shock enters its uncompensated phase, and blood pressure, vital organ perfusion, and oxygen delivery also decrease. Since the immature myocardium of the ELBW neonate may not be able to compensate for the sudden increase in peripheral vascular resistance (LV afterload) immediately following delivery, cardiac output may fall. , Yet, despite the decrease in cardiac output, many ELBW neonates maintain their blood pressure in the normal range by redistributing blood flow to the organs that are vital at that particular developmental stage (see Chapter 7 ). As referred to earlier, data suggest that the rapidly developing cerebral cortex and white matter of the ELBW neonate have not yet reached vital organ assignments with appropriately developed vasodilatory responses when perfusion falls. , , However, by the second to third postnatal days, forebrain vascular response matures and normal blood pressure is more likely to be associated with normal brain and systemic blood flow. , The molecular mechanisms by which the vasculature of the cerebral cortex and white matter of the ELBW neonate mature rapidly and become “high-priority” vascular beds soon after delivery are unknown. ,
The traditional understanding of the changes occurring in the transitional circulation of the preterm infant suggests that atrial and ductal shunts in the first postnatal hours are of little significance and are bi-directional or primarily right to left in direction as a result of the higher pulmonary vascular resistance expected in the newborn premature infant. In contrast to this understanding, longitudinal studies using bedside noninvasive ultrasound show significant variability in the time taken for the preterm infant to transition from the in utero right ventricle (RV)–dominant, low-resistance circulation to the bi-ventricular, higher-resistance postnatal circulation. Shortly after delivery, the severing of the umbilical vessels, the inflation of the lungs with air, and the associated changes in oxygenation lead to a sudden increase in the resistance in the systemic circulation and a lowering of resistance in the pulmonary circulation. Cardiac output now passes in a parallel fashion through the pulmonary and the systemic circulation except for the blood flow shunting through the closing fetal channels. The role of placental transfusion and the timing of cord clamp and how this affects transitional hemodynamics are discussed in Chapter 6 .
In healthy-term infants the ductus arteriosus is functionally closed by the second postnatal day and the right ventricular pressure usually falls to adult levels by about 2–3 days after birth. , This constriction and functional closure of the ductus arteriosus is then followed by anatomical closure over the next 2–3 weeks. In contrast, in the VLBW infant there is frequently a failure of complete closure of both the foramen ovale and the ductus arteriosus in the expected time frame, probably due to structural immaturity and the immaturity of the mechanisms involved. , The persistence of the fetal channels in the setting of decreasing pulmonary pressures leads to blood flowing preferentially from the aorta to the pulmonary artery, resulting in a relative loss of blood from the systemic circulation and pulmonary circulatory overload. Contrary to traditional understanding, this systemic to pulmonary shunting can occur as early as the first postnatal hours, with recirculation of 50% or more of the normal cardiac output back into the lungs. The myocardium subsequently attempts to compensate by increasing the total cardiac output. There can be up to a twofold increase in the left ventricular (LV) output by 1 hour of age, resulting primarily from an increased stroke volume rather than increased heart rate. A significant proportion of this increased blood flow is likely to be passing through the ductus arteriosus. There is a wide range of early ductal constriction, with some infants able to effectively close or minimize the size of the ductus arteriosus within a few hours of birth while others achieve an initial constriction, followed by an increase in size of the ductus and yet another group having a persistent large ductus arteriosus with no evidence of early constriction and subsequent limitation of shunt size. Pulmonary blood flow can be more than twice the systemic blood flow as early as the first few postnatal hours, which may be enough to cause clinical effects, such as reduced systemic blood pressure and blood flow, increases in ventilatory requirements, or even pulmonary hemorrhagic edema.
In utero, the fetal communications of the foramen ovale and ductus arteriosus result in a lack of separation between the left and right ventricular outputs, making it difficult to quantitate their individual contributions. In addition to heart rate, ventricular systolic function is determined by the physiologic principles of preload (distension of the ventricle by blood prior to contraction), contractility (the intrinsic ability of the myocardial fibers to contract), and afterload (the combined resistance of the blood, the ventricular walls, and the vascular beds). The myocardium of the VLBW infant is less mature than that of a term infant, with fewer mitochondria and less energy stores. This results in a limitation in the ability to respond to changes in the determinants of the cardiac output, in particular, the afterload. , Consequently the myocardium of the VLBW infant, just like the fetal myocardium, is likely to be less able to respond to stresses that occur in the postnatal period, such as increased peripheral vascular resistance with the resultant increase in afterload. There is a significant difference in the influence of determinants of cardiac output in the newborn premature infant with a dramatically increased afterload and changes in the preload caused by the inflation of the lungs. Furthermore, the effect of lung inflation on preload is different when lung inflation occurs by positive pressure ventilation rather than by the negative intrathoracic pressures generated by spontaneous breathing. The newborn ventricle is more sensitive to changes in the afterload, such that small changes can have large effects, especially if the preload and contractility are not optimized.
Failure of the normal transitional changes to occur in a timely manner can result in impairment of cardiac function leading to low cardiac output states and hypotension in the VLBW infant. As oxygen delivery is primarily related to oxygen-carrying capacity and the oxygen content of blood and volume of blood flow to the organ, delivery of oxygen to vital organs may be impaired where there is cardiovascular impairment. Therefore the timely identification and appropriate management of early low cardiac output states and hypotension are of vital importance in the overall care of the VLBW infant.
The product of cardiac output and peripheral vascular resistance determines arterial blood pressure. The main influences on the cardiac output are the preload or blood volume and myocardial contractility. Peripheral vascular resistance is determined by vascular tone, which in the presence of an unconstricted ductus arteriosus may not only be the systemic peripheral vascular resistance but is also contributed to by the pulmonary vascular resistance. Myocardial contractility is difficult to assess in the newborn as the accepted measures of contractility in the adult, such as the echocardiographic measure of fractional shortening, are adversely influenced by the asymmetry of the ventricles caused by the in utero right ventricular dominance. In this regard, use of load-independent measures of cardiac contractility, such as mean velocity of fractional shortening or LV wall stress indices, may provide more useful information ( Chapters 9 and 10 ; Figure 20.2 ). Some studies have found a relationship between myocardial dysfunction and hypotension in the preterm infant, while others have not, even though a similar measurement method was used. Blood volume correlates poorly with blood pressure in hypotensive neonates. Due to the unique characteristics of the newborn cardiovascular system discussed earlier, systemic blood pressure is closely related to changes in the systemic vascular resistance. As systemic vascular resistance cannot be measured directly, the measurement of cardiac output or systemic blood flow becomes an essential element in understanding the dynamic changes occurring in the cardiovascular system of the VLBW infant.
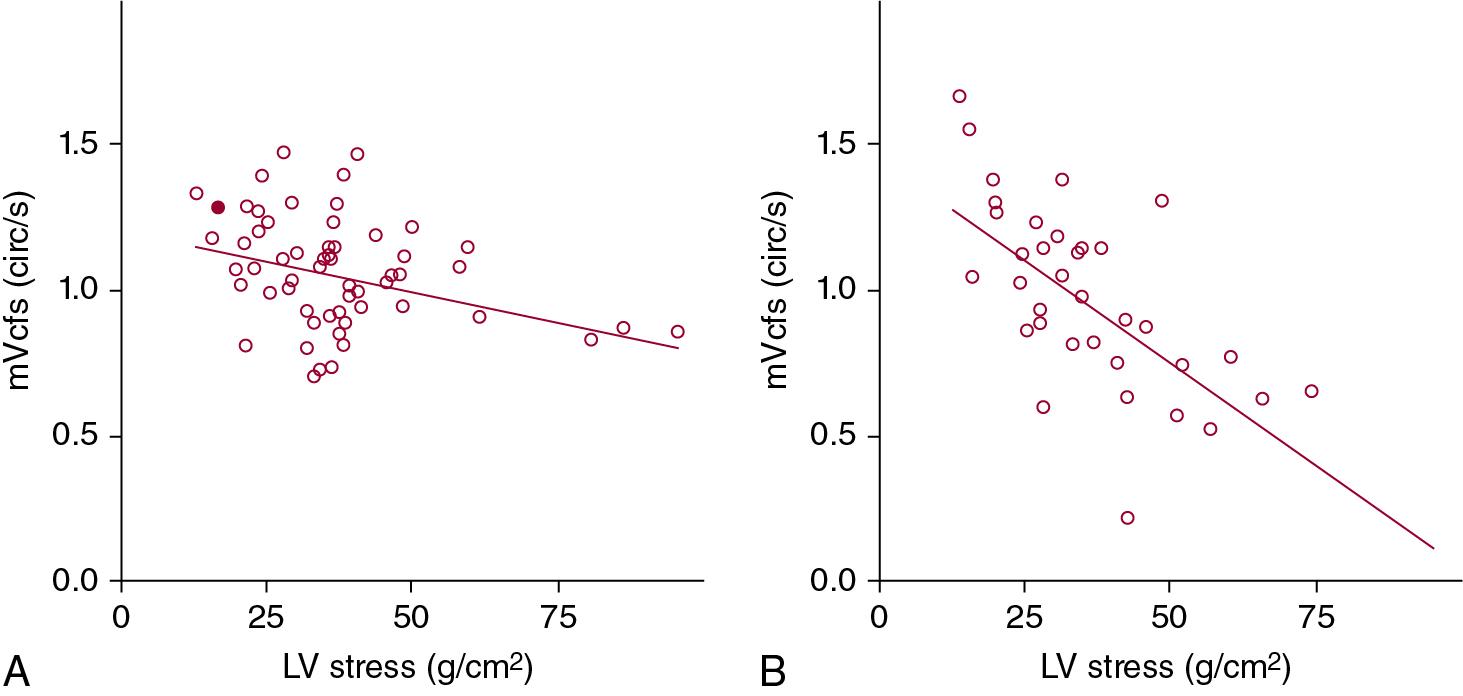
In the absence of simple techniques to measure cardiac output and systemic vascular resistance ( Chapter 14 ), clinicians have tended to rely on blood pressure as the sole assessment of circulatory compromise. However, in the VLBW neonate with a closed ductus arteriosus during the first 24–48 hours, there is only a weak relationship between mean blood pressure and cardiac output ( Figure 20.3 ). Relying on measurements of blood pressure alone can lead the clinician to make assumptions about the underlying physiology of the cardiovascular system that may be incorrect, especially during the period of early transition with the fetal channels open ( Chapter 3 ). Indeed, many hypotensive preterm infants potentially have a normal or high left ventricular output. , , One of the reasons for this apparent paradox relates to the presence of a hemodynamically significant ductus arteriosus, which causes an increase in left ventricular output while also causing a reduction in the overall systemic vascular resistance. In addition, variations in the peripheral vascular resistance may cause a change in the underlying cardiac output that does not affect the blood pressure. This phenomenon makes it possible for two infants with the same blood pressure to have markedly different cardiac outputs. Thus the physiologic determinants of blood pressure may affect the blood pressure in multiple ways – acting via an effect on cardiac performance and thus cardiac output, altering the vascular resistance, or sometimes altering both. The site where blood pressure is measured can also cause variation with a discordance between blood pressure measured via the commonly used post-ductal umbilical artery and the left ventricular output.
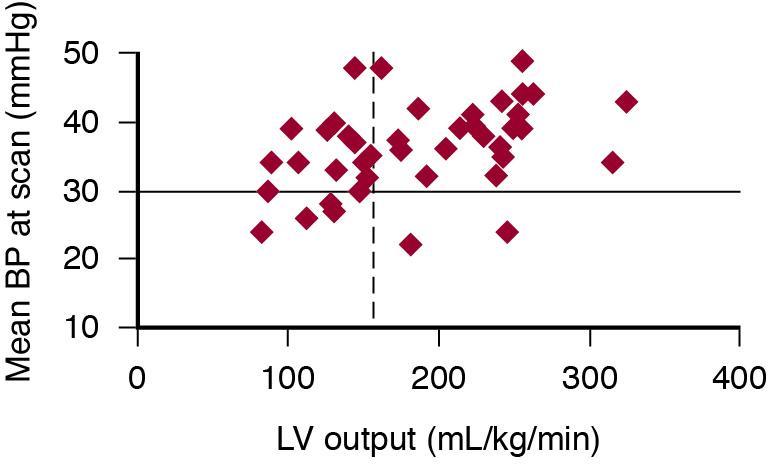
Both GA and postnatal age are major determinants of the systemic blood pressure, as can be seen by examining nomograms and tables of normal blood pressure data (see Chapter 3 ). Generally, blood pressure is higher in more mature infants and progressively increases with advancing postnatal age. The reasons why blood pressure increases with postnatal age are unclear but are probably related to changes in the underlying vascular tone mediated by various humoral regulators and possibly up-regulation of receptors involved in myocardial responses. Simultaneously, there are temporal physical changes in the transitional circulation, such as closure of the ductus arteriosus, which will affect both blood pressure and blood flow.
There is evidence that sick VLBW infants have relative adrenal insufficiency and that this condition may be one of the underlying causes of cardiovascular dysfunction, with the propensity to inflammation in these patients contributing to the pathogenesis of clinical conditions such as bronchopulmonary dysplasia. Low cortisol levels have been documented in hypotensive infants requiring cardiovascular support. The use of antenatal glucocorticoids to assist in fetal lung maturation may therefore have an additional effect of improving neonatal blood pressure. Likely mechanisms for this effect include acceleration of adrenergic receptor expression and maturation of myocardial structure and function. The enhanced adrenergic receptor expression also increases the sensitivity of the myocardium and peripheral vasculature to endogenous catecholamines. Randomized controlled trials of the use of antenatal glucocorticoids have shown variable effects on the neonatal blood pressure. In some trials there was an increase in mean blood pressure of VLBW infants in the treated group with a decreased need for cardiovascular support, while others have shown little difference between the mean blood pressures of infants whose mothers did and did not receive antenatal steroids.
Acute blood loss in the VLBW infant is unusual and can result from prenatal events (such as feto-maternal hemorrhage, antepartum hemorrhage or twin-twin transfusion syndrome) or intrapartum events (such as a tight nuchal cord resulting in an imbalance between blood flow to and from the fetus or postnatally from a large subgaleal hematoma or hemorrhage into an organ such as the liver or brain). Acute blood loss can result in significant hypotension, but due to the immediate compensatory mechanisms of the cardiovascular system, this effect may be delayed. Similarly, a drop in the infant’s hemoglobin level can also be delayed following significant acute hemorrhage.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here