Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiovascular disease continues to be the global leader when it comes to cause of death, despite a steadily declining mortality rate. In 2016, the global age-adjusted death rate had decreased by 14.5% compared with 2006. Cardiovascular disease often results in irreversible damage to the myocardium that contributes to cardiomyopathies and ultimately results in heart failure (HF). HF is the leading cause of death in the United States, with an increasing global prevalence. , Between 2013 and 2016, 6.2 million Americans age 20 years or older had HF compared with only 5.7 million between 2009 and 2012. Current therapies aim to treat symptoms of HF and slow its progression, but there has been no success at regenerating cardiomyocytes that have been lost. This chapter provides an overview of the pathophysiologic process of cardiac remodeling that leads to HF and approaches to regenerating the myocardium.
Cardiac remodeling refers to structural and functional changes to the heart. It can be an adaptive response to nonpathologic stimuli, such as exercise, pregnancy, or postnatal growth, but it becomes maladaptive after injury or disease. Although there is an association between right ventricular (RV) function and HF, remodeling generally refers to the left ventricle (LV) and will be the focus of this chapter. Maladaptive cardiac remodeling occurs after damage to the myocardium, with major causes including myocardial infarction (MI), cardiomyopathies (dilated and hypertrophic), valve diseases, and hypertension, among several others. As expected, more remodeling often results in worse outcomes for the patient. Remodeling is a multifaceted process that involves changes to cardiomyocytes, vasculature, fibrosis, inflammation, and electrophysiologic properties. Therefore diagnosis is based on several factors, including morphologic changes in cavity diameter, mass, geometry, scar formation, fibrosis, and inflammatory infiltrate. With the complex network and interrelatedness of these parameters, several diseases can cause cardiac remodeling and the progression to HF. Interestingly, different types of injury or disease can result in different mechanisms of remodeling, subsequently leading to different types of HF.
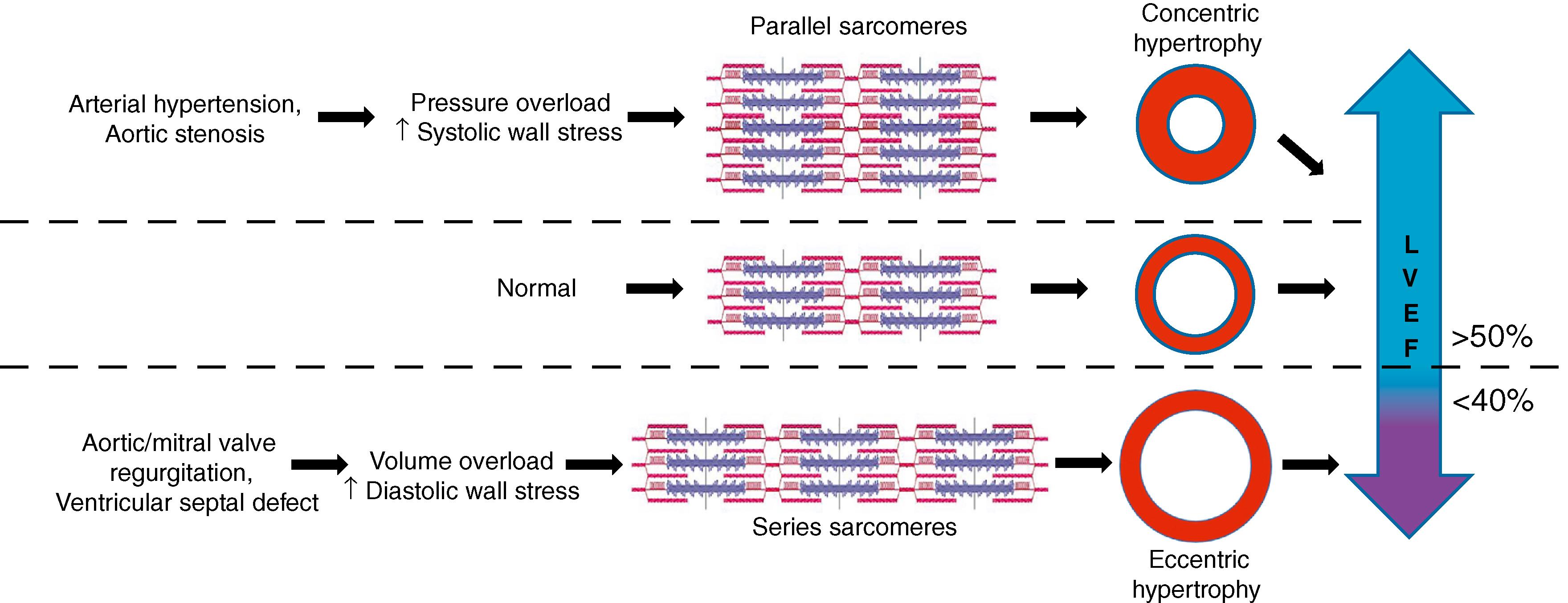
HF is categorized into two groups: HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF). The left ventricular ejection fraction (LVEF) measures the contractility of the heart muscle, with normal values ranging from 50% to 70% of the blood being pumped out of the LV on each contraction. HFrEF is determined by a cut-off level of less than 40% LVEF, whereas HFpEF is determined by HF with at least 50% LVEF. The ongoing effort to better characterize HF and its underlying mechanism to provide accurate diagnoses and course of treatment has introduced a borderline or “mid-range” (HFmEF) area where LVEF is at least 40% but less than 50%. , Nevertheless, LVEF is influenced by the loading and geometry of the ventricles that are affected by cardiac remodeling, making LVEF an imprecise measurement of contraction force and function for the accurate characterization of HFrEF and HFpEF.
Significant risk factors for HFpEF include age, hypertension, gender (female), and the onset of atrial fibrillation (AF). The leading cause of HFrEF, however, is coronary artery disease (CAD). In particular, the associated ischemia and hypoxia from occlusion of the microcirculation can trigger the remodeling process that progresses to HFrEF. The hypoxic environment leads to cell death of cardiomyocytes by apoptosis and necrosis within a day of acute MI, causing an inflammatory reaction. Collagenases and other proteinases are also activated to remove necrotic tissue, and in the process they can alter the structure of the connective tissue. Within a week of the MI, the collagen structure is replaced by myofibroblasts that reconstitute the structural network of scar formation. Thus contractile cardiomyocytes are replaced by noncontractile fibrosis. This contributes to diastolic dysfunction with abnormalities in the relaxation of ventricles that result in ventricular stiffness and increased diastolic pressures, which are characteristic of HFpEF. Systemic inflammation is a notable feature of HFpEF that impairs the endothelium and vasculature, leading to fibrosis and further exacerbating stiffening of the ventricle and diastolic dysfunction. The different factors associated with remodeling will be discussed in greater detail in this chapter.
Mechanotransduction is the cellular response to mechanical forces and is an underlying mechanism to cardiac remodeling under pathophysiologic conditions. The beating heart is a highly dynamic mechanical environment, and its ability to adapt to mechanical forces is essential for proper cardiac function. Patients with eccentric hypertrophy are associated with HFrEF, whereas those with concentric hypertrophy are more likely to develop HFpEF ( Fig. 56.1 ). One explanation is that different types of biomechanical forces (e.g., stress, strain, shear, tensile, and compression forces) trigger different responses in the heart. Volume overload is a result of increased chamber size with normal wall thickness that induces strain on cardiomyocytes, whereas pressure overload is caused by normal chamber size but increased wall thickness, resulting in fiber stress. Ischemia and dilative cardiomyopathy are common causes of volume overload that exert both strain and shear force on the heart muscle and walls of the chambers. This strain causes cardiomyocytes to increase in length, resulting in eccentric hypertrophy with increased mass of the LV and chamber volume but still a normal mass to volume ratio (M/V ratio). , By contrast, arterial hypertension and aortic stenosis are examples of pressure overload that can cause fiber stress and progression to HFpEF. Fiber stress increases cardiomyocyte width, resulting in concentric hypertrophy, as indicated by increased wall thickness with an overall increase in the M/V ratio. The law of Laplace also applies to the heart, which states that the ventricular wall stress is determined by the pressure, radius, and wall thickness. An increase in cardiac pressure, such as in CAD, will increase tension to the ventricular wall. The heart adapts to this increased tension by increasing thickness and radius, a process that unfortunately becomes maladaptive over time and leads to eccentric hypertrophy.
Changes in volume and pressure can activate neuroendocrine pathways, which can be maladaptive and exacerbate disease progression, particularly in HFrEF. , Normally, baroreceptors in the aorta and carotid sinus provide inhibitory signals to the central nervous system that repress the sympathetic output to the heart and peripheral circulation. If the baroreceptor activity decreases, the reflex can increase sympathetic tone to result in increased heart rate, contractility, and peripheral vasoconstriction. The activation of the sympathetic system activates β1-adrenergic receptors on the juxtaglomerular apparatus, which, when combined with peripheral vasoconstriction of the afferent renal artery, will cause the juxtaglomerular apparatus to release renin into the afferent arteriole. Renin induces production of angiotensin II, which then leads to the release of aldosterone that increases salt and water retention through multiple mechanisms. This renin-angiotensin-aldosterone system (RAAS) is highly active in patients with HF. Atrial natriuretic peptides are released to counterbalance increased RAAS activity, but patients with HF have peripheral resistance to natriuretic peptides, therefore causing RAAS to remain unopposed and resulting in chronic vasoconstriction and development of volume overload.
Thus, for patients with HFrEF, angiotensin-converting enzyme (ACE) inhibitors, beta blockers, and aldosterone antagonists are used to target the neuroendocrine effects and decrease remodeling. , More recently, the combination drug LCZ696, which consists of an angiotensin II receptor antagonist (valsartan) and an antihypertensive agent (sacubitril), has become a leading therapeutic option after results showed it to be more effective in lowering rates of cardiovascular death and hospitalization compared with ACE inhibitors in patients with HFrEF. , Most successful therapies for HFrEF continue to modulate the neurohormonal activation that drives HFrEF and its progression. HFpEF, however, does not respond to the modulation of neuroendocrine activation, resulting in a rapidly increasing incidence rate that now surpasses 50% of all HF patients today and continues to grow in prevalence.
Additionally, the activation of RAAS increases the production of reactive oxygen species (ROS). ROS interacts with pathways that uncouple nitric oxide synthase and activates mitochondrial oxidative stress, thereby increasing vascular stiffness and endothelial dysfunction. Aside from the activation of RAAS, there are other sources of ROS that contribute to the cardiac dysfunction related to remodeling. A change occurs in the metabolic process during cardiac remodeling that reduces fatty acid oxidation and increases glucose oxidation. This leads to the accumulation of triglycerides and lipotoxicity, in addition to mitochondrial atrophy and dysfunction. This oxidative stress also causes protein oxidation, lipid peroxidation, DNA damage, proliferation of fibroblasts, and cellular dysfunction, among other adverse effects, which could induce apoptosis during the process of remodeling.
Inflammatory signaling pathways are also involved as a response to biomechanical and neuroendocrine factors in the interrelated mechanism of pathogenic cardiac remodeling. Inflammation is found in all HF patients and is a hallmark of ventricular hypertrophy. , Regardless of the cause of HF, a significant increase in macrophages occurs because of the expansion of local resident macrophages or by recruitment and differentiation of circulating monocytes. , Inflammation is an important feature of disease emergence and progression in HFpEF, just as neuroendocrine activation is a significant component of HFrEF. Inflammation in HFrEF mostly occurs as a consequence of a primary heart affliction, whereas inflammation in HFpEF is often systemic and induces disease emergence. In HFpEF, circulating inflammatory biomarkers, such as interleukin 1 receptor-like 1 (IL1RL1) and cystatin-C, are more elevated than in HFrEF, and there is a higher rate of inflammatory-related morphologic changes in other organs, such as the kidneys, lungs, and skeletal muscles. , Higher levels of high-sensitivity C-reactive proteins (a measurement of inflammation) are associated with greater HFpEF disease burden, supporting the role and comorbidity of inflammation with HFpEF.
An important cytokine released by macrophages is interleukin 6 (IL-6), which reduces cardiomyocyte hypertrophy and protects cardiomyocytes from oxidative damage. Recent work has shown that microRNA-132 inhibits cardiomyocyte apoptosis and myocardial remodeling after MI by targeting interleukin-1β (IL-1β). Furthermore, investigations into small molecule compounds have resulted in compound VCP979, which inhibits p38-MAPK. VCP979 was found to have antiinflammatory and antifibrotic activities that mitigate remodeling after ischemic and reperfusion injury in mice. These examples partially illustrate the complex network of inflammatory factors involved in cardiac remodeling, but many of its mechanisms remain unknown.
Cardiac fibrosis often follows hypertrophy as a part of the remodeling process. After MI, mechanical stress, combined with specific hormones, growth factors, and cytokines, induces fibroblast proliferation and migration to the injured area, followed by transdifferentiation into myofibroblasts. Mild fibrosis in HF is generally beneficial because it reduces cardiomyocyte workload and resists chamber deformation. Nevertheless, higher levels of fibrotic remodeling are associated with higher mortality, increased hospitalization rates, and increased incidence of adverse cardiac events. , The extent to which fibrosis becomes pathologic is a topic of debate, but it is widely accepted that gene expression and signaling pathways are altered by cardiac fibrosis. Models of cardiac pressure overload have demonstrated that inflammatory cytokines, such as tumor necrosis factor-α and IL-6, along with transforming growth factor (TGF)-β-Smad2/3, are the underlying mechanisms of interstitial fibrosis. Furthermore, mechanical stress can directly lead to cardiac fibrosis through the focal adhesion-integrin complex, which has mechanosensory properties in fibroblasts to promote the formation of the extracellular matrix and myofibroblast conversion. The focal adhesion kinase (FAK) promotes the activity of the adhesion-integrin complex, and studies have demonstrated that activating FAK induces myofibroblast conversion, whereas FAK knockdown reduces fibrosis. ,
The calcium transport system regulates the supply of calcium to contractile proteins during contraction. During cardiac remodeling, the calcium transport system is disrupted, decreasing L-type calcium channels, ryanodine receptors, and calsequestrin and calmodulin kinase activity, as well as reducing phospholamban phosphorylation. , This downregulation decreases the supply of calcium during systole and increases calcium supply in diastole, contributing to dysfunction in HF. Sodium and potassium channels are also affected during ischemic or dilated cardiomyopathy-related HF. In both cases, significant decreases in sodium channel SCN5A, calcium channels CACNA1C and CACNA1H, and potassium channels KCNJ11 and KCNJ12 can occur, in addition to significant increases in KvLQT1 and KCNJ4 potassium channel–related genes. Changes to these ion channels can lead to arrhythmias and fibrillation, which are also capable of inducing and exacerbating cardiac remodeling. Furthermore, atrial arrhythmias have distinct contributions to atrial remodeling compared with remodeling associated with ventricular arrhythmias. One study that examined the effects of tachypacing the atria versus the ventricles found that connexin43 (Cx43) gene expression was downregulated and genes for collagen were upregulated only in atrial tachypacing, whereas ventricular tachypacing resulted in greater fibrosis and natriuretic-peptide activation. Finally, MI can lead to arrhythmogenic ventricular remodeling through the activation of the cardiac sympathetic afferent reflex (CSAR) involving the transient receptor potential cation channel subfamily V member 1 (TRPV1) channel. TRPV1-afferent signaling promotes many aspects of cardiac remodeling, including fibrosis and electrical stability of the ventricular myocardium.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here