Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
THE CARDIOVASCULAR SYSTEM plays a dominant role within the human body: a centrally located “powerhouse” provides oxygen and nutrients via an extensive network of vessels and capillaries throughout the body. All other organ systems depend on its normal development and function. At birth, and especially in the first few hours of life, the heart and the vascular system have to adapt to the extrauterine conditions. Prematurity, congenital defects, complications during labor and delivery, and many other factors can prevent or delay the necessary changes and cause significant morbidity.
A thorough understanding of the fetal circulation, the changes at birth, and the age-specific characteristics is important for the safe management of neonates, infants, and especially the growing number of preterm and small-for-gestational-age (SGA) infants who come to our diagnostic suites and operating rooms. Given the complex embryology and difficult transition from fetal to extrauterine life, it is amazing that more than 90% of neonates are delivered without any special interventions and that congenital heart defects occur in only 7 to 10 of every 1000 live births. (A detailed discussion of the embryologic development is beyond the scope of this chapter; the interested reader is referred to the excellent review by Van Praagh or Langman's classic embryology textbook. )
Congenital heart defects are among the most common birth defects. In the United States, approximately 32,000 infants are born every year with congenital heart disease (CHD); a significant number require urgent interventions in the catheterization laboratory or surgical procedures during the neonatal period. In addition, CHD is often associated with other, noncardiac anomalies, and many of these children will present for procedures outside the cardiac operating room. Pediatric anesthesiologists have to be able to classify and recognize the pathophysiologic effects of CHD on the cardiovascular system of the neonate or infant and the potential impact of anesthesia and surgical manipulations.
With recent advances in surgical techniques, critical care, and anesthesia management, 85% of all infants with CHD are now expected to reach adulthood. Anesthesiologists will increasingly encounter children with “repaired” or “palliated” CHD presenting for noncardiac procedures. Chapter 23 addresses specific long-term problems and anesthetic considerations for various repaired heart defects, but a few conditions deserve additional discussion: the basic changes in the exercise physiology of repaired heart defects, the characteristics of the Fontan physiology after single ventricle palliation, and the altered physiologic responses in the transplanted heart.
Many conditions require pharmacologic support with cardiovascular drugs, some of which can have significant age-specific effects. Well-controlled drug studies in infants and children are rare; dosing is often based on long-standing experience or extrapolation from adult data. Understanding the basic pharmacology of the most commonly used cardiovascular drugs and the special considerations for infants and children is essential for successful perioperative care. This chapter will help the pediatric anesthesiologist to understand the complexity of the neonatal cardiovascular system, the implications of CHD, basic pharmacologic considerations, and will provide the necessary tools to develop a safe management plan.
In utero, the placental gas exchange provides the fetus with poorly oxygenated blood; the partial pressure of oxygen (P o 2 ) in the umbilical vein is approximately 30 mm Hg, and in the umbilical arteries it is approximately 16 mm Hg. The fetal lungs are fluid filled and only minimally perfused (10%–15% of the cardiac output). The normal postnatal circulation can be described as a serial circuit: two pumps, the right ventricle (RV) and left ventricle (LV), support two different resistance systems, the pulmonary and systemic vasculatures, one after the other. In contrast, the fetal circulation is better explained by the concept of a parallel circuit: both ventricles provide systemic blood flow and a variety of fetal shortcuts or connections allow for mixing of oxygenated and deoxygenated blood ( Fig. 18.1 ). Oxygenated blood from the placenta returns via the umbilical vein to the portal venous system, where 30% to 50% of the blood flow is shunted across the ductus venosus to the inferior vena cava (IVC), bypassing the liver and thereby maintaining higher oxygenation and velocity. The rest of the umbilical venous blood passes through the hepatic microcirculation into the suprahepatic IVC.
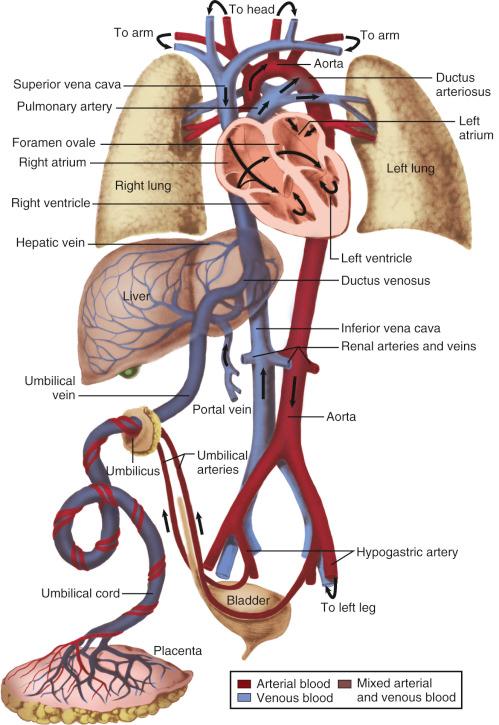
The IVC blood entering the right atrium (RA) is a mixture of bloodstreams with different velocities and saturations: the low-velocity, deoxygenated venous return from the lower body and hepatic veins and the high-velocity, oxygenated umbilical venous blood from the ductus venosus. Valve-like tissue in the RA (eustachian valve) and the Chiari network preferentially direct the high-velocity bloodstream from the IVC across the foramen ovale into the left atrium (LA), bypassing the RV and pulmonary vessels. In the LA, the oxygenated blood mixes with the minimal amount of venous return from the pulmonary circulation and is then ejected by the LV into the ascending aorta and the major vessels of the aortic arch. This blood, with a saturation of 65% to 70%, provides the oxygenation for the growing heart and brain.
Most of the venous return from the superior vena cava (SVC) and about 20% of the IVC blood flow (mainly the low-velocity, deoxygenated part) reach the RV and are pumped into the pulmonary artery (PA), where the high pulmonary resistance in the nonexpanded lung redirects 90% of the blood flow into the descending aorta via the ductus arteriosus . The bulk of the blood flow in the descending aorta is generated by the RV, with minor contributions from the LV. The blood has a saturation of only 55% to 60%; two-thirds of it returns to the placenta for oxygenation, and the rest is distributed to the intestines, the kidneys, and the lower part of the body ( Fig. 18.2 ).
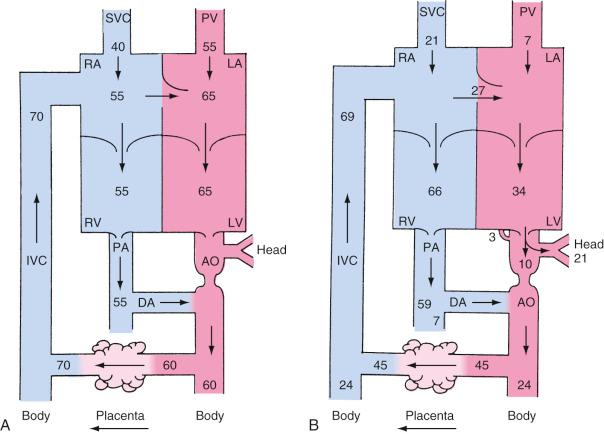
The fetal circulation has to support a growing fetus in a relatively cyanotic atmosphere (highest oxygen saturation, 65% to 70%). This difficult task is further complicated by the parallel circuit, which creates increased workload for the RV, and the limitations of the fetal shortcuts, which add additional volume load by incomplete shunting of oxygenated and deoxygenated blood. Initially, our understanding of the fetal circulation was based mainly on experimental animal data, but recent advances in ultrasound technology have facilitated assessment and monitoring of fetal cardiovascular parameters, especially stroke volume and cardiac output, under various conditions throughout the gestational period. RV stroke volume has been found to increase from about 0.7 mL at 20 weeks to 7.6 mL at 40 weeks, and LV stroke volume increases from 0.7 mL to 5.2 mL. The combined fetal cardiac output of both ventricles is estimated to be 400 to 425 mL/kg per minute, with an RV dominance because of the increased volume load. At 38 weeks, the RV provides approximately 60% of the combined cardiac output ( E-Table 18.1 ). Intrauterine growth restriction and placental compromise are associated with redistribution of cardiac output and relative changes in the size of the foramen ovale. A functional placenta, the fetal cardiovascular high-output state, greater hemoglobin concentrations, and additional alterations in oxygen binding and release (hemoglobin F, increased 2,3-diphosphoglycerate [2,3-DPG]) are all necessary to provide adequate tissue oxygenation for the developing fetus.
| PERCENTAGE OF COMBINED CO AT GESTATIONAL AGE | |||
|---|---|---|---|
| 20 Weeks | 30 Weeks | 38 Weeks | |
| Combined CO | 210 mL/minute | 960 mL/minute | 1900 mL/minute |
| Left ventricle | 47% | 43% | 40% |
| Right ventricle | 53% | 57% | 60% |
| Foramen ovale | 34% | 18% | 19% |
| Lungs | 13% | 25% | 21% |
| Ductus arteriosus | 40% | 32% | 39% |
Until recently, CHD was thought to be relatively well tolerated in utero, but growing evidence suggests that fetal cardiovascular defects can induce intrinsic autoregulatory changes in cerebral perfusion and thereby compromise brain development. Ultrasound and magnetic resonance imaging demonstrate that 25% to 40% of neonates with CHD have neurologic abnormalities before any surgical intervention.
At birth, a variety of humoral, biochemical, and physiologic changes occur abruptly. First, the placental circulation is eliminated shortly after the lungs expand. Second, expansion of the lungs to a normal functional residual capacity (FRC) results in an optimal geometric relationship of the pulmonary microvasculature. Third, air entering the lungs causes the alveolar P co 2 to decrease and the alveolar P o 2 to increase. These three factors act in concert to markedly reduce pulmonary vascular resistance (PVR). The net effect is a considerable increase in pulmonary blood flow, which augments pulmonary venous return to the left heart. Along with elimination of the placenta and the low-resistance umbilical circulation, the LV is suddenly subjected to increased volume and afterload ( Table 18.1 ). Typically, LV end-diastolic pressure, and thus LA pressure, increases enough to exert hydrostatic pressure on the septum primum, resulting in functional closure of the foramen ovale. In contrast to the increased stress for the LV, the RV is relatively unloaded by the transition to extrauterine life.
| Right Ventricle | Left Ventricle |
|---|---|
| Decreased Afterload: | Increased Afterload: |
| Decreased pulmonary vascular resistance | Placenta eliminated |
| Ductal closure | Ductal closure |
| Decreased Volume Load: | Increased Volume Load: |
| Eliminated umbilical vein return | Increased pulmonary venous return |
| Output diminished 25% | Output increased almost 50% |
| Transient left-to-right shunt at ductus |
The three fetal connections (ductus arteriosus, ductus venosus, and foramen ovale) close over a variable period after birth. The ductus arteriosus functionally (but not anatomically) closes in 58% of normal full-term infants by day 2 after birth and in 98% by day 4. Although many substances (such as eicosanoids) have been implicated in initiating constriction of the ductus, initial constriction probably occurs primarily in response to the increased arterial oxygen tension and the reduction in circulating prostaglandins that follow separation of the placenta. The response to oxygen is age dependent: term neonates usually demonstrate effective constriction of the smooth muscles in the ductal tissue when exposed to oxygen, whereas preterm infants respond poorly and often require medical (prostaglandin inhibitor) or even surgical therapy. Additional catecholamine-induced changes in PVR and systemic vascular resistance (SVR) and other substances such as acetylcholine contribute to ductal closure. Within 2 to 3 weeks, functional constriction is followed by a process of ductal fibrosis, leaving a band-like structure, the ligamentum arteriosum. With ligation of the umbilical vein, the portal pressure falls, triggering functional closure of the ductus venosus. This process rarely requires more than 1 to 2 weeks; by 3 months only fibrous tissue, the ligamentum venosum, is left.
The foramen ovale is functionally closed when the LA pressure exceeds the RA pressure, but it remains anatomically patent in most infants, in 50% of children younger than 5 years of age, and in 25% to 30% of adults. Echocardiographic studies have confirmed right-to-left shunting via the foramen ovale in healthy infants emerging from general anesthesia, and this can be a significant cause of persistent arterial desaturation at that time despite ventilation with 100% oxygen.
Compared with the adult, the neonatal myocardium is immature and incompletely developed ( Table 18.2 ). Differences in cytoarchitecture and metabolism account for many of the functional limitations. The neonatal heart contains fewer muscle cells and more connective tissue than the adult myocardium. Contractile elements represent only 30% of the total cardiac mass, in contrast to 60% in the adult. The ratio of surface area to mass and water to collagen content are greater in neonates than older children. There are fewer myofibrils within the muscle cells, and they tend to be less organized (i.e., not parallel to the long axis of the cell). The sarcoplasmic reticulum and the T-tubule network, both important components of rapid and effective calcium regulation, are incompletely developed, and the immature myocardium relies substantially on the calcium flux through the sarcolemma to initiate and terminate contraction. One practical consequence of this disorganized and immature myocardium is a greater degree of contractile dysfunction in the infant exposed to substances that decrease extracellular ionized calcium, such as citrate (blood products) and albumin; there is also increased sensitivity to inhalational anesthetics and calcium channel blockers.
| Immature Myocardium | Adult Myocardium | |
|---|---|---|
| Cytoarchitecture |
|
|
| Metabolism |
|
|
| Function |
|
|
Reduced numbers of underdeveloped mitochondria and maturational differences in various signaling pathways and related messenger systems are also characteristic of the neonatal myocardium. Immature mitochondrial enzyme activity for fatty acid transport may explain the primary use of carbohydrates and lactates as energy sources and might be a reason for the greater anaerobic tolerance and faster recovery after periods of ischemia. A variety of developmental changes in contractile proteins occur from fetal through early postnatal life, including changes in pH, calcium sensitivity, and adenosine triphosphate (ATP) hydrolyzing activity. The key features of the immature cardiac function are summarized in Table 18.2 .
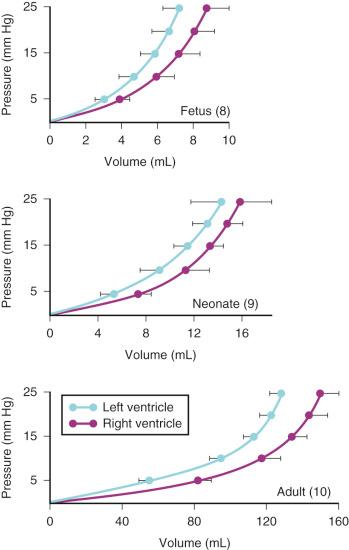
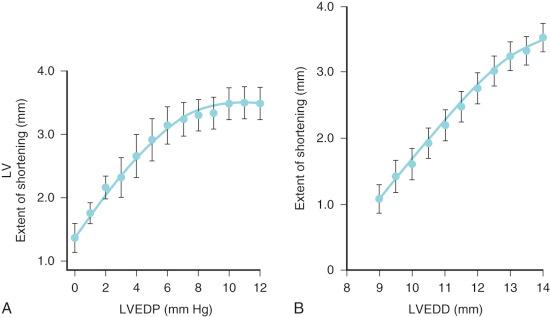
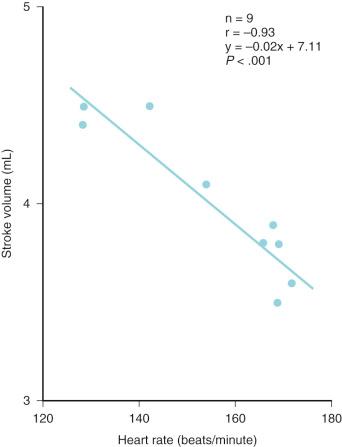
The increased amount of noncontractile tissue in the neonate decreases ventricular compliance and limits the response to an increased preload. Compliance of both ventricles progressively increases during fetal life and the postnatal period, so that maximal stroke volume occurs at a significantly reduced atrial pressure in the neonate compared with the fetus ( Figs. 18.3 and 18.4 ). The high metabolic rate of the neonate (oxygen consumption, 6-8 mL/kg per minute, compared with 2-3 mL/kg per minute in the adult) requires a proportional increase in cardiac output. The neonatal heart meets this demand, in part, by a greater heart rate (HR). The cardiac output is commonly described as depending primarily on HR owing to a fixed stroke volume, but echocardiographic studies in human fetuses and neonates have demonstrated the capacity to increase stroke volume ( Fig. 18.5 ) In fact, the neonate uses both tachycardia and stroke volume adjustments to meet metabolic demand. On the other hand, neonates exhibit exquisite sensitivity to pharmacologic agents that produce negative inotropic or chronotropic effects. At birth, both ventricles are equal in mass and connected via a common septum. Increased pressures in one ventricle shifts the septum, decreasing compliance of the other ventricle. The net effect is a reduction in cardiac output. Neonates and infants often present with biventricular failure as a result of this interventricular dependence.
Immature autonomic regulation of cardiac function persists throughout the neonatal period. Both sympathetic and parasympathetic innervation of the heart can be demonstrated at birth. However, evidence suggests that development of the sympathetic nervous system is incomplete at both the postganglionic nerve-receptor level and the receptor-effector level. The sympathetic system reaches maturity by early infancy, whereas the parasympathetic system reaches maturity within a few days after birth. The relative imbalance of these two components of the autonomic nervous system at birth may account for the clinical observation that neonates are predisposed to marked vagal responses to a variety of stimuli.
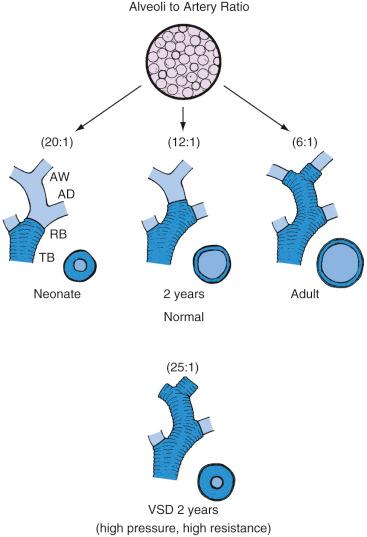
At birth, pulmonary vascular development is incomplete. Lung sections demonstrate a diminished number of arterioles, and the arterioles exhibit thick medial muscularization ( Fig. 18.6 ). The pulmonary vasculature matures during the first few years of life. During this period, arterioles proliferate faster than alveoli, and the medial smooth muscle thins and extends more distally in the vascular tree. PVR continues to decrease as long as pulmonary mechanics and alveolar gas composition remain favorable, with a significant decrease occurring immediately after birth as the result of lung expansion and oxygenation. Progressive remodeling of the pulmonary vasculature facilitates further decreases in PVR (assuming normal physiology) during the first 2 to 3 months of life; by 6 months of age, the PVR has almost reached adult levels.
The fetal pulmonary vasculature is extremely reactive to a number of stimuli. Hypoxia, acidosis, increased levels of leukotrienes, and mechanical stimulation (e.g., coughing on an endotracheal tube) can cause significant and prolonged increases in PVR (e.g., reactive pulmonary hypertension). On the other hand, acetylcholine, histamine, bradykinin, prostaglandins, β-adrenergic catecholamines, and nitric oxide (NO) are strong vasodilators. In the first days after birth, many pathophysiologic conditions can trigger severe and sustained increases in PVR and prevent the normal adjustment to extrauterine life ( E-Table 18.2 ). The acute load imposed on the RV can induce diastolic dysfunction and promote right-to-left shunting via the foramen ovale. Once PVR exceeds the SVR, a right-to-left shunt develops through both the ductus arteriosus and the foramen ovale. This situation is called persistent fetal circulation , and it can result in a life-threatening hypoxemia that may require inhaled NO, sildenafil, or extracorporeal support (i.e., extracorporeal membrane oxygenation) (see Chapter 21 ) to provide oxygenation and sustain life.
| Prematurity | Sepsis |
| Pulmonary disease | Acidosis |
| Hypoxemia | Hypothermia |
| Hypercarbia | High altitude |
| Congenital heart disease | Prolonged stress |
Pulmonary vascular occlusive disease (PVOD) describes the structural changes in the pulmonary vasculature after long-standing exposure to abnormal pressures and flow patterns in utero and after birth. Lung biopsies demonstrate thickened muscle layers in the small pulmonary arteries, intimal hyperplasia, scarring, and thrombosis as well as a decreased number of distal (intraacinar) arteries. Over time, these changes lead to a progressive and ultimately irreversible obstruction to pulmonary blood flow with increases in PVR and PA pressures. The very muscularized pulmonary arteries are also extremely sensitive to pulmonary vasoconstrictors, which can easily trigger a pulmonary hypertensive crisis.
Many cardiac defects are associated with abnormal pulmonary flow patterns and can be categorized into three basic groups:
Exposure of the pulmonary vasculature to systemic arterial pressures and high flow: The classic example is a large, nonrestrictive ventricular septal defect (VSD) with rapid progression of PVOD.
Exposure of the pulmonary vasculature to high flow without increased pressure: Large atrial septal defects (ASDs) and small, restrictive patent ductus arteriosus (PDA) defects fall into this category. PVOD develops much more slowly in this setting.
Obstruction of pulmonary venous drainage resulting in increased PA pressures: Pulmonary vein stenosis (e.g., total anomalous pulmonary venous return [TAPVR], cor triatrium) or increased LA pressures (e.g., mitral atresia, congenital aortic stenosis, severe coarctation) can cause backpressure in the pulmonary vasculature and induce PVOD.
The muscle tone in the pulmonary arteries is regulated by numerous factors, and various therapeutic interventions can be used to manipulate the PVR ( Table 18.3 ) :
Arterial oxygen tension (Pa o 2 ): Alveolar as well as arterial hypoxia increases PVR. A Pa o 2 value less than 50 mm Hg, especially when associated with an acidic pH (<7.4), leads to significant pulmonary vasoconstriction. On the other hand, increased inspired oxygen can lead to pulmonary vasodilation and overcirculation.
Arterial carbon dioxide tension (Pa co 2 ): Hypercapnia increases PVR, independent of the blood pH. In contrast, hypocapnia induces alkalosis and thereby decreases PVR. Reliable pulmonary vasodilation can be achieved with a Pa co 2 of 20 to 33 mm Hg and a pH of 7.5 to 7.6.
pH: Respiratory and metabolic acidosis increase PVR; alkalosis reduces PVR.
Lung volumes: PVR is optimized at a lung volume close to the FRC; larger volumes compress small intraalveolar vessels, and smaller volumes can cause atelectasis and vascular collapse.
Stimulation of the sympathetic nervous system: Catecholamine surges from stress, pain, or light anesthesia can trigger significant increases in PVR.
Vasodilators: Most intravenous (IV) agents used for pulmonary vasodilation also affect the systemic circulation and induce hypotension. Alternatively, inhaled substances such as NO or prostacyclin can provide a more selective pulmonary vasodilation (see “ Cardiovascular Pharmacology ”).
| Increasing PVR | Decreasing PVR |
|---|---|
| PEEP | No PEEP |
| High airway pressures | Low airway pressures |
| Atelectasis | Lung expansion to FRC |
| Low F io 2 | High F io 2 |
| Respiratory and metabolic acidosis | Respiratory and metabolic alkalosis |
| Increased hematocrit | Low hematocrit |
| Sympathetic stimulation | Blunted stress response (deep anesthesia) |
| Direct surgical manipulation | Nitric oxide |
| Vasoconstrictors: phenylephrine | Vasodilators: milrinone, prostacyclin, others |
In summary, the pulmonary vasculature undergoes a complex maturation process that can be influenced by a multitude of external factors and congenital heart defects. Persistent fetal circulation and PVOD are examples of inadequate adaptation and development. In cases of increased PVR, ventilator strategies using greater inspired oxygen concentrations, lung volumes close to the FRC, and interventions aiming for a Pa o 2 greater than 60 mm Hg, a Pa co 2 of 30 to 35 mm Hg, and a pH of 7.5 to 7.6 can improve pulmonary blood flow.
CHD can be defined as “a gross structural abnormality of the heart or intrathoracic great vessels that is actually or potentially of functional significance.” This definition covers a wide array of defects, which are among the most common congenital malformations. However, the precise incidence of CHD, both collectively and by individual anatomic subset, varies depending on definition, method of case identification, and epoch ( E-Table 18.3 ). Including all categories of CHD, large epidemiologic surveys place the prevalence between 4 and 50 cases per 1000 live births. When stratified according to trivial, moderate, and severe forms, the incidence for moderate and severe forms of CHD has been relatively consistent, at about 6 per 1000 live births.
| Lesion | No. of Studies | Mean | SD | Lower Quartile | Median | Upper Quartile | NERICP 1975-77 |
| Ventricular septal defect | 43 | 3570 | 2878 | 1757 | 2829 | 4482 | 645 |
| Persistent ductus arteriosus | 40 | 799 | 1399 | 324 | 567 | 782 | 135 |
| Arterial septal defect | 43 | 941 | 1043 | 372 | 564 | 1059 | 65 |
| AV septal defect | 40 | 348 | 165 | 242 | 340 | 396 | 110 |
| Pulmonary stenosis | 39 | 729 | 731 | 355 | 532 | 836 | 73 |
| Aortic stenosis | 37 | 401 | 543 | 161 | 256 | 388 | 41 |
| Coarctation of the aorta | 39 | 409 | 246 | 289 | 356 | 492 | 165 |
| Tetralogy of Fallot | 41 | 421 | 188 | 291 | 356 | 577 | 196 |
| d -TGA | 41 | 315 | 115 | 231 | 303 | 388 | 218 |
| Hypoplastic right heart | 32 | 222 | 199 | 105 | 160 | 224 | — |
| Tricuspid atresia | 11 | 79 | 52 | 24 | 92 | 118 | 56 |
| Ebstein anomaly | 5 | 114 | 138 | 38 | 40 | 161 | 12 |
| Pulmonary atresia | 11 | 132 | 125 | 76 | 83 | 147 | 69 |
| Hypoplastic left heart | 36 | 266 | 216 | 154 | 226 | 279 | 163 |
| Truncus arteriosus | 30 | 107 | 71 | 61 | 94 | 136 | 30 |
| DORV | 16 | 157 | 103 | 82 | 127 | 245 | 32 |
| Single ventricle | 23 | 106 | 70 | 54 | 85 | 136 | 54 |
| TAPVR | 25 | 94 | 46 | 60 | 91 | 120 | 58 |
| All cyanotic CHD | 37 | 1391 | 590 | 1078 | 1270 | 1533 | 888 |
| All types of CHD a | 43 | 9596 | 7484 | 6020 | 7669 | 10,567 | 2033 |
| Bicuspid aortic valve | 10 | 13,556 | 13,049 | 5336 | 9244 | 13,817 | — |
a Excluding bicuspid valves, isolated partial anomalous venous connection, and silent ductus arteriosus.
Anatomic diagnoses within the population of infants with CHD vary according to the method used to identify cases. In 2002, Hoffman and Kaplan compiled 62 epidemiologic studies published after 1955 and investigated the potential causes for the wide variability in the reported incidence of CHD. More recent studies based mainly on prenatal and postnatal echocardiographic screening data often include a large number of trivial lesions (e.g., tiny VSDs, nonstenotic bicuspid aortic valve, “silent” PDA) for which no interventions may be required; other data collections, such as the New England Regional Infant Cardiac Program (NERICP), a registry of children with CHD who died or required catheterization or surgery during the first year of life, are clearly biased toward more severe forms of CHD.
The increasing availability of prenatal diagnostic methods may influence the relative prevalence of reported lesions as well as their outcome. When fetal echocardiography is used, the apparent shift toward more complex lesions may reflect technical limitations in identifying simple defects. In addition, evaluation in utero skews the results because it includes fatally malformed fetuses that will not survive to term. The prevalence of CHD among spontaneous abortions reaches 20% and remains as large as 10% among stillborn infants. In one study, 50% of women whose children were given a prenatal diagnosis of CHD elected to terminate the pregnancy, particularly when presented with complex heart lesions.
On the other hand, female infants with severe CHD have a mortality rate that is 5% less than similarly affected male infants, and with increased survival rates more females will reach childbearing age, where they continue to have reduced mortality. The recurrence risk of CHD for their offspring is about 3% to 4%.
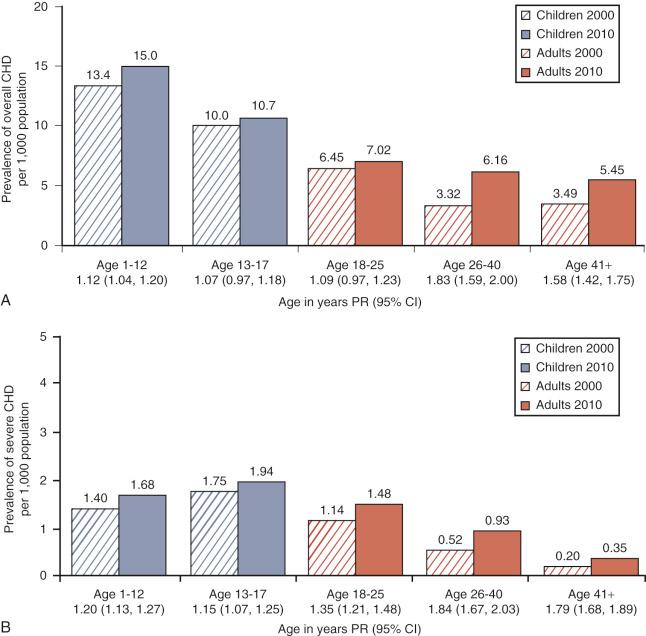
A study from Canada examined the changing epidemiology of CHD with respect to prevalence and age distribution in the general population between 1983 and 2010. The prevalence of all categories of CHD in 2010 was ~13 per 1000 in children (<18 years of age) and ~6 per 1000 in adults. For the subcategory of severe CHD, the prevalence was ~1.8 per 1000 in children and ~0.62 per 1000 in adults. In 2010, 60% of all patients with severe CHD were adults, compared with 49% in 2000 and 35% in 1985. Between 1983 and 2010 the prevalence of CHD has been steadily increasing for both children and adults but at a different pace: from 1985 to 2000 the increase for severe CHD was 85% in adults and 22% in children compared with 57% for adults and 11% for children from 2000 to 2010. The median age of all patients with severe CHD was 11 years in 1985, 17 years in 2000 and 25 years in 2010, reflecting the fact that more children with CHD were surviving to adulthood ( E-Fig. 18.1 ). Improved survival may be attributed to improved prenatal care, early diagnostic imaging, and major advances in pediatric cardiac care, particularly for those with severe CHD; these improved outcomes will continue to influence the future demographic profile. The growing number of adolescents and adults with CHD will require long-term follow-up with experienced cardiologists and access to specialized care facilities; this will require a thorough understanding of their underlying pathophysiology by all members of the adult care team, including anesthesiologists.
CHD consists of an almost endless array of anatomic and functional variants. Many different classification systems have been introduced, some using a segmental approach to anatomic features, others by examining the amount of pulmonary blood flow (cyanotic versus acyanotic) or the common physiologic characteristics (e.g., volume versus pressure overload). Several of these classifications are discussed in Chapter 16 . However, certain defects are better described using the concepts of shunting (physiologic, anatomic, simple or complex), intercirculatory mixing, and single ventricle physiology, which are presented in the following sections.
Shunting occurs when blood return from one circulatory system (systemic or pulmonary) is recirculated to the same system, completely bypassing the other circulation. For example, if deoxygenated blood from the systemic veins flows directly to the aorta, the result is a right-to- left shunt with recirculation of deoxygenated blood in the systemic circulation. In contrast, redirection of oxygenated blood from the pulmonary veins to the PA causes a left-to-right shunt with recirculation of oxygenated blood within the pulmonary circulation. The terms physiologic and anatomic are often used to describe shunting. Basically, any kind of recirculation of blood within one circulatory system is called physiologic shunting . In most cases, physiologic shunting is caused by an anatomic shunt (i.e., a communication between the cardiac chambers or the great vessels), but physiologic shunting can also exist by itself, as in the classic transposition physiology.
To really understand the pathophysiology of shunting and its implications, it is important to introduce the concepts of effective and total systemic/pulmonary blood flows. Effective blood flow is the quantity of venous blood from one circulatory system that reaches the arterial system of the other circulatory system. Effective pulmonary blood flow is the volume of systemic venous blood reaching the pulmonary circulation, whereas effective systemic blood flow is the volume of pulmonary venous blood reaching the systemic circulation. Effective pulmonary blood flow and effective systemic blood flow are always equal, no matter how complex the lesions. Total blood flow , on the other hand, is the sum of recirculated and effective blood flow and a measure of the workload of the circulatory system. Total systemic and pulmonary blood flows are not equal. Even in healthy patients there is a small amount of normal physiologic shunting (e.g., thebesian cardiac veins, bronchial vessels), but with CHD the difference can be quite substantial. Physiologic shunting or recirculation should be viewed as a noneffective, superfluous load added to the essential nutritive blood flow (effective blood flow).
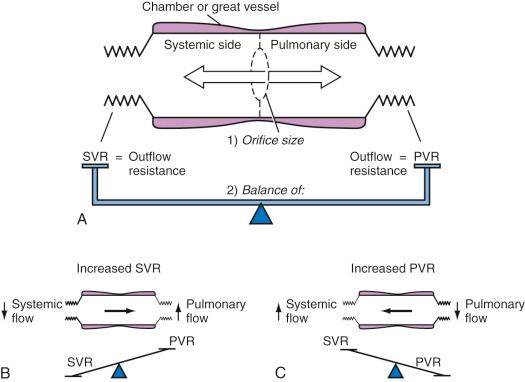
Anatomic shunts are communications between the two circulatory systems, either within the heart or at the level of the great vessels. They can be divided into simple and complex shunts, depending on the presence of additional outflow obstructions. In simple shunts without any additional outflow obstruction, the size of the communication (the so-called shunt orifice) determines the flow characteristics. For small orifices (restrictive shunts) with large pressure gradients across the communication, the size of the opening essentially regulates the amount of shunting. Changes in SVR or PVR have little influence. In contrast, for large orifices or nonrestrictive shunts (also classified as dependent shunts ), the quantity and direction of blood flow are controlled by the respective outflow resistances (i.e., the ratio of SVR to PVR) ( Table 18.4 and Fig. 18.7 ).
| Restrictive (Small Shunt Orifice) | Nonrestrictive (Large Shunt Orifice) | |
|---|---|---|
| Examples | Small ASD, VSD, or PDA; modified Blalock-Taussig shunt | Large VSD, PDA, CAVC |
| Pressure gradient across shunt | Large | Small or none |
| Direction and magnitude of shunt | Independent of PVR/SVR | PVR/SVR dependent |
| Influence of pharmacologic and ventilatory interventions | Minimal | Large |
Complex shunts are defined by an additional outflow obstruction, which can be at various levels within the ventricle, valves, or great vessels and is often described as subvalvular, valvular, or supravalvular. These obstructions can be fixed (e.g., valvular stenosis) or variable (e.g., dynamic infundibular obstruction by muscle bundles) . Shunt flow and direction are determined by the combined resistance across the outflow obstruction and the pulmonary/systemic vascular beds. For severe obstructions downstream, SVR or PVR will have little influence on the shunt. Tetralogy of Fallot (TOF) is a good example of a complex shunt lesion. The amount of right-to-left shunt and therefore the amount of cyanosis are influenced by the degree and type of right ventricular outflow tract obstruction (RVOTO). This is especially evident in the setting of a dynamic infundibular obstruction, where changes in preload, contractility, and HR can lead to significant decreases in pulmonary blood flow and increased shunting ( Table 18.5 ).
| Partial Outflow Obstruction | Complete Outflow Obstruction | |
|---|---|---|
| Examples | TOF, VSD/PS, VSD/coarctation | Tricuspid or mitral atresia, Pulmonary or aortic atresia |
| Shunt magnitude and direction | Relatively fixed | Totally fixed |
| Dependence on PVR/SVR ratio | Inversely related to obstruction | Independent |
| Pressure gradient across shunt | Dependent on shunt orifice and degree of obstruction | Dependent only on shunt orifice |
The concept of intercirculatory mixing is often used to explain the unique physiology in children with transposition of the great arteries (TGA). In this cardiac defect, the aorta arises from the RV, transporting deoxygenated blood back to the right heart, and the PA originates from the LV, returning oxygenated blood to the pulmonary circulation (see Fig. 17.6 ). Unless there is some mixing of blood via an ASD, VSD, or PDA, this defect will result in a complete separation of the two systems, a parallel circulation with 100% physiologic shunting, or recirculation of oxygenated and deoxygenated blood that is incompatible with life once the fetal ductus arteriosus has closed. Effective pulmonary blood flow (i.e., deoxygenated blood reaching the pulmonary vascular bed for oxygenation) has to be provided by some form of right-to-left shunt; effective systemic blood flow (i.e., oxygenated blood returning to the systemic circulation) must be achieved by a left-to-right shunt. Intercirculatory mixing is the combined systemic and pulmonary effective blood flow and is only a small portion of the total blood flow. The bulk of the respective systemic and pulmonary total blood flows consists of recirculated blood ( Fig. 18.8 ). Usually the total blood flow and the volume in the pulmonary system are two to three times greater than in the systemic circulation.
![FIGURE 18.8, Saturations, pressures, and blood flows in transposition of the great arteries with a nonrestrictive atrial septal defect and a small left ventricular (LV) outflow tract gradient. Intercirculatory mixing occurs at the atrial level. Effective pulmonary and effective systemic blood flows are equal (1.1 L/minute per m 2 ) and are the result of a bidirectional anatomic shunt at the atrial level. The physiologic left-to-right shunt is 9.0 L/minute per m 2 ; this represents blood recirculated from the pulmonary veins to the pulmonary artery (PA). The physiologic right-to-left shunt is 1.2 L/minute per m 2 ; this represents blood recirculated from the systemic veins to the aorta (Ao). Total pulmonary blood flow (Q̇ P = 10.1 L/minute per m 2 ) is almost five times greater than the total systemic blood flow (Q̇ S = 2.3 L/minute per m 2 ). The bulk of pulmonary blood flow is recirculated pulmonary venous blood. In this depiction, pulmonary vascular resistance (PVR) is low (approximately 1/35 of systemic vascular resistance [ SVR ]) and there is a small (17 mm Hg peak to peak) gradient from the LV to the PA. These findings are compatible with the high pulmonary blood flow depicted. LA, left atrium; RA , right atrium; RV , right ventricle. FIGURE 18.8, Saturations, pressures, and blood flows in transposition of the great arteries with a nonrestrictive atrial septal defect and a small left ventricular (LV) outflow tract gradient. Intercirculatory mixing occurs at the atrial level. Effective pulmonary and effective systemic blood flows are equal (1.1 L/minute per m 2 ) and are the result of a bidirectional anatomic shunt at the atrial level. The physiologic left-to-right shunt is 9.0 L/minute per m 2 ; this represents blood recirculated from the pulmonary veins to the pulmonary artery (PA). The physiologic right-to-left shunt is 1.2 L/minute per m 2 ; this represents blood recirculated from the systemic veins to the aorta (Ao). Total pulmonary blood flow (Q̇ P = 10.1 L/minute per m 2 ) is almost five times greater than the total systemic blood flow (Q̇ S = 2.3 L/minute per m 2 ). The bulk of pulmonary blood flow is recirculated pulmonary venous blood. In this depiction, pulmonary vascular resistance (PVR) is low (approximately 1/35 of systemic vascular resistance [ SVR ]) and there is a small (17 mm Hg peak to peak) gradient from the LV to the PA. These findings are compatible with the high pulmonary blood flow depicted. LA, left atrium; RA , right atrium; RV , right ventricle.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/CardiacPhysiologyandPharmacology/8_3s20B9780323429740000185.jpg)
The arterial saturation (Sa o 2 ) is influenced by the volumes and saturations of recirculating and effective systemic blood flows and can be calculated with the use of the following equation:
Increasing the intercirculatory mixing will improve the arterial saturations, and in severely cyanotic neonates with TGA, intact ventricular septum, and inadequate atrial communication, a balloon atrial septostomy (balloon dilation of an existing patent foramen ovale or small ASD, either echo-guided at the bedside or under fluoroscopy in the catheterization laboratory) can be lifesaving. Additional measures to improve systemic and pulmonary venous saturations (e.g., blood transfusion, inotropic support, ventilatory strategies) can help to stabilize the arterial saturation.
Single ventricle physiology defines the circulation present in a wide variety of complex cardiac defects. It is characterized by complete mixing of systemic and pulmonary venous blood return at either the atrial or the ventricular level; the mixed blood is then distributed to both systemic and pulmonary circulations in parallel. The defects can consist of one anatomic single ventricle with severe hypoplasia and inflow or outflow obstruction of the other one (hypoplastic left heart syndrome [HLHS] or pulmonary atresia with intact ventricular septum) or even two well-developed ventricles with atresia of the outflow tract or severe obstruction (TOF with pulmonary atresia, interrupted aortic arch). In some lesions, a PDA is the only source of systemic or pulmonary blood flow; these are called duct-dependent circulations. In others, intracardiac communications provide adequate blood flow to both circulations ( Table 18.6 ).
| Congenital Heart Defect | Aortic Blood Flow From | Pulmonary Blood Flow From |
|---|---|---|
| Hypoplastic left heart syndrome | PDA | RV |
| Neonatal critical aortic stenosis | PDA | RV |
| Interrupted aortic arch | Proximal LV, distal PDA | RV |
| Tetralogy of Fallot with pulmonary atresia | LV | PDA, MAPCAs |
| Pulmonary atresia with intact septum | LV | PDA |
| Tricuspid atresia 1B (VSD and PS) | LV | LV through VSD to RV |
| Truncus arteriosus | LV and RV | Aorta |
| Double inlet left ventricle, no TGA | LV | LV through VSD to bulboventricular foramen |
Irrespective of the anatomic features, in single ventricle physiology the ventricular output (delivered by one or two ventricles) is the sum of the pulmonary and systemic blood flows. The distribution of the respective flows depends on the relative outflow resistances into the two parallel circulations. Oxygen saturations in the aorta and PA are equal. The severity and location of anatomic obstructions and the ratio of PVR to SVR determine the balance of flows to the two circulations
The following equation illustrates the various factors that influence the arterial saturation (Sa o 2 ) in a single ventricle physiology:
Accordingly, three major variables determine arterial saturation and the initial management options for patients with single ventricle physiology:
The ratio of pulmonary to systemic blood flow ( Q̇ pulm /Q̇ sys ). With high Q̇ pulm /Q̇ sys , a greater percentage of the blood in the ventricle (or ventricles) is oxygenated because more fully saturated pulmonary venous blood is entering the heart to mix with desaturated systemic venous return. Saturations greater than 85% can be achieved only by significant pulmonary overcirculation. Q̇ pulm /Q̇ sys can be influenced by careful manipulations of the PVR/SVR ratio.
Systemic venous saturation (S sys v o 2 ): For a given Q̇ pulm /Q̇ sys and pulmonary venous saturation (S pulm v o 2 ), any decrease in S sys v o 2 causes a decrease in arterial saturation. Oxygen delivery and consumption are the basic determinants for Sv o 2 . Adequate oxygen delivery depends on cardiac output and arterial oxygen content and thus on hemoglobin levels and arterial saturation. All measures that increase oxygen delivery (e.g., transfusion to increase the hematocrit to 0.45-0.50 or decrease oxygen consumption (e.g., adequate analgesia and sedation during painful procedures) improve arterial saturations.
Pulmonary venous saturation (S pulm v o 2 ): Normally the blood in the pulmonary veins should be fully saturated (S pulm v o 2 = 100%) on room air, but lung disease, V̇/Q̇ mismatch, or large intrapulmonary shunts can cause pulmonary venous desaturation. V̇/Q̇ mismatch usually responds to therapy with increased inspired oxygen, whereas intrapulmonary shunts are refractory to oxygen therapy. Pulmonary venous desaturation will decrease arterial saturations.
Children with CHD, including those with lesions considered repaired, exhibit an array of abnormalities elicited during exercise testing consistent with reduced exercise capacity ( E-Table 18.4 ). It is worthwhile to review the various exercise testing abnormalities to gain insight into the limitations imposed by the presence of congenital heart lesions.
| Defect | ↑ Peak V̇ o 2 | ↓ Peak HR | ↓ Peak O 2 Pulse | ↑ V̇ E /V̇ co 2 | ↓ VAT |
|---|---|---|---|---|---|
| Repaired TOF/truncus arteriosus | +++ | ++ | +++ | +++ | ++ |
| Fontan | ++++ | +++ | ++++ | ++++ | +++ |
| PVOD | ++++ | + | ++++ | ++++ | ++++ |
| Ebstein anomaly | +++ | ++ | +++ | ++ | ++ |
| Status post atrial switch | +++ | ++ | +++ | ++ | ++ |
| Aortic valve disease | ++ | + | ++ | + | ++ |
| Coarctation | ++ | + | ++ | + | +++ |
| Dilated cardiomyopathy | ++++ | + | ++++ | ++ | ++++ |
| Hypertrophic cardiomyopathy | ++ | + | ++ | + | ++ |
| Isolated PR | + | + | + | + | + |
a This table assumes that the patient is not receiving β-blocker or other antiarrhythmic therapy that might impair the chronotropic response to exercise.
Oxygen consumption (V̇ o 2 ) is equal to the product of cardiac output and O 2 extraction. O 2 extraction is equal to the arterial-venous oxygen content difference. Peak V̇ o 2 is the greatest measure of V̇ o 2 obtained during a progressively more difficult exercise test. V̇ o 2 at rest is defined as 1 metabolic equivalent energy expenditure unit or 1 MET (approximately 3.5 mL O 2 /kg per minute). A typical elite endurance athlete can reach 20 to 22 METs, or 70 to 77 mL O 2 /kg per minute, at peak exercise. Activities of daily living require at least 4 METs or 14 mL O 2 /kg per minute. Peak V̇ o 2 is the best overall assessment of the capabilities of the cardiovascular system, but determination of normal values is difficult owing to the effects of age, gender, effort, and body composition (e.g., adipose tissue) on peak V̇ o 2 . Nonetheless, peak V̇ o 2 has been demonstrated to be a reliable predictor of hospitalization and mortality in patients with a wide variety of congenital heart lesions.
During exercise, the HR normally increases linearly with increases in V̇ o 2 . Normal peak HR is generally defined (in beats per minute [beats/minute]) as 220 minus age in years. In children with chronotropic incompetence, which is defined as the inability to increase HR to greater than 80% of the predicted value at peak exercise, the relationship between HR and V̇ o 2 is depressed. Chronotropic incompetence is an indicator of poor prognosis and is most commonly the result of sinus node dysfunction. By comparison, well-trained endurance athletes have a normal peak HR and a depressed HR/V̇ o 2 relationship, because they can generate a larger-than-normal stroke volume increase as exercise progresses. The inability to increase stroke volume (discussed later) during exercise results in an increased HR/V̇ o 2 relationship as a compensatory mechanism.
The oxygen pulse is the quantity of oxygen delivered per heartbeat. The peak O 2 pulse is calculated by dividing the peak V̇ o 2 by the peak HR. Because peak V̇ o 2 = cardiac output × O 2 extraction and because O 2 extraction remains remarkably constant over a wide range of exercise, O 2 pulse is proportional to stroke volume. Determination of normal peak O 2 pulse is hampered by the same factors that confound determination of normal peakV̇ o 2 . In addition, O 2 pulse overestimates stroke volume in the presence of erythrocytosis and underestimates it in the presence of anemia or reduced arterial O 2 saturation. O 2 pulse is reduced in patients with impaired ventricular function, severe valvular regurgitation, or pulmonary vascular disease. It is also uniformly reduced in those with Fontan physiology as a consequence of the inability of this circulation to augment systemic ventricular preload during exercise.
The respiratory exchange ratio (RER) is defined as the ratio V̇ co 2 /V̇ o 2 (ratio of the volume of CO 2 produced per minute to the volume of oxygen consumed per minute). A normal resting RER is between 0.67 and 1.0, depending on the precise composition of protein, carbohydrates, and fat in the diet. As exercise intensifies, anaerobic metabolism commences and the lactate threshold is reached; buffering of lactic acid with bicarbonate causes the carbon dioxide production (V̇ co 2 ) to increase out of proportion to oxygen consumption (V̇ o 2 ), resulting in an increased RER. An RER of 1.09 or greater is thought to indicate the onset of anaerobic metabolism and to be consistent with a good effort. Because RER increases only if anaerobic metabolism occurs, exercise limitation and low V̇ o 2 owing to musculoskeletal problems or poor effort are associated with an RER less than this threshold.
The ventilatory anaerobic threshold (VAT) is used to identify the onset of anaerobic metabolism that occurs before V̇ o 2 peaks and is relatively effort and motivation independent. As aerobic exercise progresses, minute ventilation (V̇ E ) increases in direct proportion to V̇ co 2 and V̇ o 2 . When anaerobic metabolism commences and CO 2 production increases as lactic acid is buffered, V̇ E increases accordingly. VAT is the point at which V̇ E /V̇ o 2 and V̇ E /V̇ co 2 diverge, with V̇ E increasing in proportion to V̇ co 2 but out of proportion to V̇ o 2 . An important characteristic of successful endurance athletes is the ability to reach and sustain effort at an anaerobic threshold that is a large percentage (80%–85%) of peak V̇ o 2 .
Ventilation efficiency can be assessed with the use of the V̇ E /V̇ co 2 slope. This relationship is defined as 863 • V̇ co 2 /[Pa co 2 • (1 − V D /V T )], where V D /V T is the ratio of physiologic dead space to tidal volume. The V̇ E /V̇ co 2 slope can be thought of as the number of liters of ventilation required to eliminate 1 L of CO 2 . Normal children have a V̇ E /V̇ co 2 slope of less than 28. To maintain a normal Pa co 2 during exercise, children with increased V D /V T and reduced ventilatory efficiency have a greater than normal increase in V̇ E and therefore a steeper V̇ E /V̇ co 2 slope. Increased V D /V T is the consequence of either reduced V T in the setting of a normal V D or pulmonary flow maldistribution and subsequent V̇/Q̇ mismatch that increases V D . The latter is the major source of inefficient ventilation and steepening of the V̇ E /V̇ co 2 slope in children with cardiac disease.
In children with PA stenosis, (e.g., repaired TOF), pulmonary hypertension, or increased LA pressure from any cause (e.g., LV systolic or diastolic dysfunction, mitral valve disease), an increase in the V̇ E /V̇ co 2 slope is associated with increased mortality. When pulmonary stenosis is corrected in children with TOF, the V̇ E /V̇ co 2 slope and peak V̇ o 2 improve.
Children with Fontan physiology also exhibit an increase in V̇ E /V̇ co 2 slope. These children have inherent nonhomogeneous pulmonary perfusion at rest owing to the lack of pulsatile pulmonary blood flow. In addition, there is poor recruitment of the distal pulmonary vasculature during exercise. The presence of a Fontan fenestration further contributes to this increase in the V̇ E /V̇ co 2 slope by allowing mixed venous blood high in CO 2 to be shunted into the systemic circulation. This produces, via central chemoreceptor stimulation, an increase in V̇ E out of proportion to V̇ co 2 . Fontan fenestration closure eliminates this right-to-left shunt and reduces the V̇ E /V̇ co 2 slope but does not improve the peak V̇ o 2 . The reason is that the primary limitation to increases in V̇ o 2 during exercise in Fontan patients is the inherent inability of the pulmonary vascular bed to substantially increase surface area, flow, and preload delivery to the systemic ventricle.
Francis Fontan, a French cardiac surgeon, described a new treatment for complex cardiac malformations with only one ventricle in 1971. To decrease the chronic volume overload for the single ventricle and normalize oxygenation, he separated the systemic and pulmonary circulations by directly connecting the systemic venous return (SVC and IVC) to the PA, without a pumping chamber. This created a circulation wherein pulmonary blood was driven solely by a nonpulsatile pressure gradient across the pulmonary vascular bed, with the single ventricle being the sole source of kinetic energy. All other shunt connections were interrupted. The original indication was tricuspid atresia, but over the years the classic Fontan technique has been modified in many ways and is now used for various complex cardiac lesions with single ventricle physiology, such as HLHS, double-inlet RV, and pulmonary atresia with intact septum (see also Chapters 17 and 23 ).
It is impossible to create a Fontan circulation at birth; high PVR and small vessel sizes prevent adequate pulmonary blood flow. In the neonatal period, palliative procedures such as stage I Norwood operation with aortic arch reconstruction, atrial septostomy, and aortopulmonary shunts (modified Blalock-Taussig shunt) or the Sano modification of the Norwood procedure (RV-to-PA conduit) aim for balanced systemic and pulmonary blood flows, allowing the infant to grow for several months despite cyanosis and volume load on the ventricle. At the age of 3 to 6 months, an intermediate procedure called the bidirectional Glenn operation or superior cavopulmonary anastomosis, is performed. The SVC is connected directly to the PA, providing nonpulsatile pulmonary blood flow, whereas the IVC remains connected to the heart. As a result, the volume load on the ventricle is significantly reduced, but oxygenated and deoxygenated blood still mix and the saturations remain in the low 80% range. By the age of 1 to 5 years, most of these children are ready for the Fontan circulation. With adequate growth and maturation of the pulmonary vascular bed, the resistance should be small enough to allow the complete separation of the systemic and pulmonary flows. The IVC is now also connected to the PA, most often via a lateral tunnel in the atrium or an extracardiac conduit, with or without a small fenestration (small opening in the baffle or conduit connecting the systemic venous return with the common atrium of the single ventricle). The fenestration can provide a residual right-to-left shunt in case of sudden increases in PVR, maintaining ventricular preload and function. This seems to facilitate the adaptation to the new loading conditions, shorten the recovery time, and decrease the incidence of early complications. The fenestration often occludes spontaneously, or it is closed during a cardiac catheterization and hemodynamic evaluation with a special device ( Fig. 18.9 ; see also Fig. 17.11, Fig. 17.12, Fig. 17.13 ).
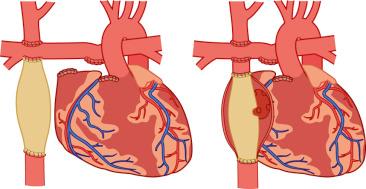
The Fontan operation has dramatically improved the mortality rates for children with single ventricles, but the success comes at a price: chronic systemic venous hypertension and congestion have been implicated in a multitude of potential early and long-term complications, including arrhythmias, residual right-to-left shunts, coagulopathies with increased risk for thrombosis and stroke, lymphatic dysfunction with pleural effusions, and protein-losing enteropathy. Late cardiac failure and poor functional outcome remain risks for patients with Fontan circulations. The anatomy of the single ventricle and the type of Fontan connection influence the duration of freedom from complications. Children with systemic RVs and the classic atriopulmonary Fontan procedure (RA directly anastomosed to the PA) tend to have a shorter duration of freedom from complications than those with systemic LVs and newer Fontan modifications ( E-Figs. 18.2 and 18.3 ).
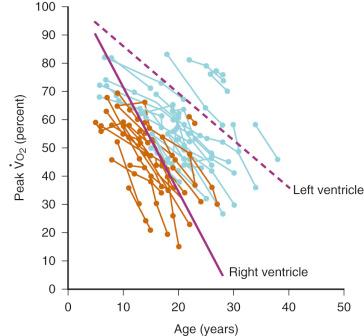
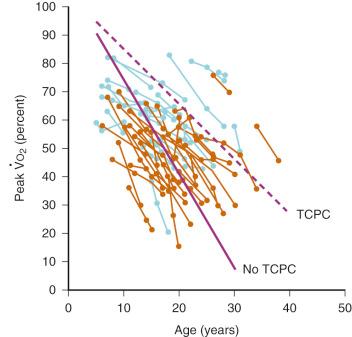
Inherent limitations of the Fontan circulation, such as altered control of cardiac output with decreased hemodynamic response to stress and reduced exercise tolerance, have been documented ( Fig. 18.10 ). Even at rest, cardiac output is usually only 70% (range, 50%- 80%) of normal for body surface area. Cardiac output is classically determined by four factors: preload, contractility, HR, and afterload. Over a physiologic range, cardiac output improves with increased preload, contractility, and HR and with decreased afterload. For the Fontan circulation, the determinants of cardiac output are more complex ( Fig. 18.11 ). The classic determinants of cardiac output are less effective, while other factors, such as transpulmonary gradient and PVR, must be considered. The following mechanisms regulate cardiac output in children with Fontan physiology.
Preload: The RV usually provides the kinetic energy to distend the pulmonary vasculature and create a preload reservoir for the LV, thereby enabling an increase in cardiac output up to fivefold or greater with exercise. The lack of a pre-pulmonary pump leads to a significant decrease in available pulmonary blood volume and, consequently, reduced or absent LV preload reserve.
Contractility: During the staged palliation, the single ventricle typically develops from a volume-overloaded and dilated ventricle to a hypertrophied, underfilled ventricle. Although the contractile response to β-adrenergic stimulation seems to be preserved, the resulting increase in cardiac output is diminished, most likely owing to limited preload reserve.
Heart rate and rhythm: Within the physiologic range, atrial pacing at different HRs does not alter cardiac output because there is a simultaneous decrease in stroke volume. Normalization of HR increases the reduced cardiac output associated with severe bradycardia or tachycardia. During exercise testing, Fontan patients demonstrate chronotropic incompetence, a blunted HR response to exercise. This is likely the result of autonomic dysfunction or abnormal reflex control. In contrast to the HR, cardiac rhythm is of utmost importance. Ectopy or loss of atrioventricular (AV) synchronization compromises ventricular filling and decreases the transpulmonary gradient.
Afterload: The Fontan circulation is characterized by increased afterload, which is a physiologic response to decreased cardiac output and occurs because a single ventricle is ejecting into two large resistance beds (systemic and pulmonary vascular) arranged in series. Autonomic regulation and activation of various endocrine systems increase the systemic venous resistance and help to maintain adequate perfusion pressures and venous tone. Because of the limited preload reserve, attempts at afterload reduction often result in significant hypotension. On the other hand, excessive afterload, such as that which occurs with residual aortic arch obstruction, is poorly tolerated.
Transpulmonary flow: Transpulmonary flow is directly proportional to the gradient between the systemic venous pressure (usually between 10 and 15 mm Hg, rarely >20 mm Hg) and the preventricular atrial pressure, which is determined by the functional status of the AV valve, the ventricle, the rhythm, and the potential presence of outflow obstruction. Transpulmonary flow is inversely proportional to the resistance over the Fontan circuit. This resistance is largely determined by PVR, but mechanical obstruction such as stenosis or thrombosis may also play a role. The geometry of the cavopulmonary connections is also important in that turbulent flow produces energy loss and a reduction in effective driving pressure. It has been suggested that PVR is the key determinant of transpulmonary flow, delivery of pulmonary venous flow to the systemic ventricle, and, consequently, cardiac output ( Fig. 18.12 ).
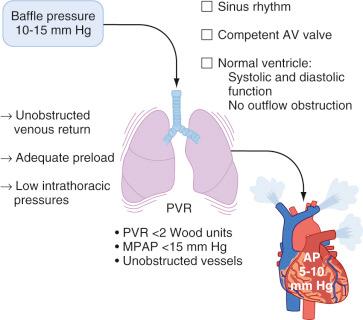
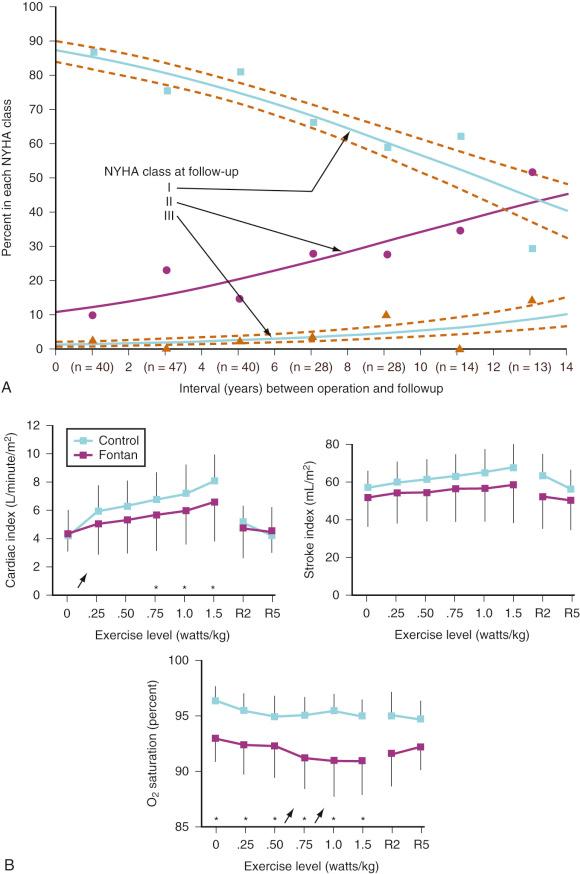
In conclusion, the Fontan circulation can be described as a serial circulation with a single kinetic energy pump. Increased systemic venous pressures are necessary to create the transpulmonary pressure gradient that drives flow across the pulmonary vascular bed; however, these increased pressures simultaneously increase the ventricular afterload. Cardiac output depends on an adequate preload and low PVR. Decreased cardiac output at rest and limited exercise tolerance are characteristics of the Fontan circulation.
According to the International Society for Heart and Lung Transplantation (ISHLT), children younger than 18 years of age account for about 13% of all heart transplantations. Every year, approximately 450 cardiac transplants in children are reported to this voluntary registry, mainly from centers in Europe and North America. Major indications are cardiomyopathies, CHD, and a growing number of retransplantations, especially in older children. The median survival time (the time at which 50% of recipients are still alive) has improved over the years, mainly because of reduced early posttransplant mortality. Survival time is currently 20.6 years for infants, 17.2 years for children aged 1 to 5 years, 13.9 years for children aged 6 to 10 years, and 12.4 years for teenagers. Ninety-four percent of transplant recipients describe a normal functional status with no limitations during physical activity.
As survival continues to improve, more and more children with transplanted hearts will present to operating rooms and sedation suites for diagnostic studies and general procedures. A basic understanding of the physiologic changes in the transplanted heart and the implications of current immunosuppressive therapy are important for safe management.
After transplantation, the function of the surgically denervated heart depends primarily on an intact Frank-Starling mechanism and stimulation from circulating catecholamines. The classic Frank-Starling mechanism describes the ability of the cardiac muscle to increase contractility in response to stretch or tension (e.g., increasing cardiac output with increases in venous return). Afferent and efferent denervation has multiple effects on circulatory control mechanisms and leads to physiologic changes, including an increase in the resting HR and a blunted response to stress and exercise. Despite excellent physical activity, exercise testing easily demonstrates that heart transplant recipients can usually achieve only 60% to 70% of normal capacity. In the transplanted heart, exercise-induced increase in cardiac output is initially caused by an increase in stroke volume, a highly preload-dependent process. Tachycardia occurs only later, in response to circulating catecholamines. Further details of the altered physiology are summarized in Table 18.7 . The incidence, timing, and extent of sympathetic reinnervation are still being investigated, but the positive effects on cardiac performance have been clearly demonstrated. During standardized exercise testing, transplant recipients with evidence of reinnervation show improved endurance with greater peak HRs and better contractile function.
| Increased filling pressures (LVEDP 12 mm Hg 4–8 weeks after transplantation) |
| Low-normal left ventricular ejection fraction |
| Restrictive physiology (stiff heart) |
| Increased afterload |
| Afferent denervation |
| No angina during ischemia |
| Altered cardiac baroreceptors and mechanoreceptors |
| Less stress-induced increase in systemic vascular resistance |
| Increased blood volume due to decreased natriuresis and diuresis |
| Efferent denervation |
| Resting tachycardia (loss of baseline vagal tone) |
| Impaired chronotropic response to stress (dependent on circulating catecholamines) |
| Altered response to medications |
| No heart rate response to atropine, glycopyrrolate, and digitalis |
| Possible severe bradycardia or cardiac arrest with neostigmine |
| Exacerbated response to calcium channel blockers, β-blockers, adenosine |
| Exacerbated response to direct acting sympathetic agents |
| Decreased response to indirect-acting agents such as dopamine and ephedrine |
| Electrophysiology |
| High incidence of sinus node dysfunction in immediate postoperative period, normal AV node |
| Shift to β 2 -receptors |
| Possible sympathetic reinnervation: timing and extent variable |
| Enhanced contractile response and exercise tolerance |
| Higher peak heart rates during exercise |
Chronic denervation also causes an altered response to many medications. Atropine, glycopyrrolate, digoxin, and pancuronium have no chronotropic effect on the denervated heart. Sympathomimetics that act indirectly, such as ephedrine and dopamine, have a blunted response, whereas direct-acting adrenergic agents, such as epinephrine, isoproterenol, and dobutamine, can cause exaggerated effects and should be carefully titrated. A single-center retrospective study did not find any negative effects of neostigmine in patients with heart transplants, but several case reports have described profound bradycardia and even cardiac arrest after neostigmine was used for reversal of neuromuscular blockade. Neostigmine has been shown to produce an atropine-sensitive, dose-dependent bradycardia in both recent (<6 months) and remote (>6 months) cardiac transplants. Direct stimulation of postganglionic nicotinic cholinergic receptors with denervation hypersensitivity, a direct effect of the old sinoatrial node on the pacemaker cell of the new sinoatrial node, and parasympathetic reinnervation have been postulated as potential mechanisms. Avoidance of neuromuscular blockade, use of short-acting neuromuscular blocking agents without reversal, and use of edrophonium for reversal have all been suggested. Edrophonium seems to have less effect on the HR in this population than neostigmine.
The denervated heart is also extremely sensitive to adenosine. The magnitude and duration of the effects on the AV node are three to five times greater, and the initial and subsequent doses should be reduced by 50%. Calcium channel blockers and β-blockers are associated with exaggerated bradycardia and hypotension. The lack of reflex tachycardia can also lead to profound hypotension with the use of direct vasodilators such as nitroglycerine, nitroprusside, or hydralazine; therefore initial doses of these medications should also be reduced.
Children who have undergone cardiac transplantation continue to experience morbidity associated with immunosuppressive therapy. Rehospitalization for treatment of infections and episodes of rejection is common, especially during the first year (~42%). Acute rejection episodes are a major threat. With the latest immunosuppressive regimens, the incidence has decreased from almost 30% during the first year in 2008 to 15% in 2014. Rejection is thought to be associated with the development of cardiac allograft vasculopathy (CAV) or coronary artery disease, which is a major cause of morbidity and graft failure. Indeed, 10 years after transplantation, ~16% of infants, ~27% of children 1 to 10 years of age, and ~37% of adolescents have CAV. Most centers include annual coronary angiography or intravascular echocardiography as a part of regular rejection surveillance. Children with CAV present the same anesthetic challenges as adults with severe coronary artery disease and ischemic heart disease. Aggressive immunosuppressive therapy with induction and maintenance regimens carries its own risks. A detailed discussion is beyond the scope of this chapter but, in general, monoclonal or polyclonal T-cell antibodies (OKT3, ATG) and specific interleukin-2 receptor antagonists (basiliximab, daclizumab) are used for the induction phase and various combinations of corticosteroids, calcineurin inhibitors (cyclosporine, tacrolimus, FK506), mechanistic target of rapamycin inhibitors (mTOR inhibitors: sirolimus, everolimus) and antiproliferative agents (azathtioprine, mycophenolate mofetil) are used for maintenance. Adverse effects are common and include neurotoxicity with seizures, hypertension, liver and renal dysfunction, hyperlipidemia, diabetes, gingival hypertrophy, hypertrichosis, bone marrow suppression, and posttransplantation lymphoproliferative disease.
Several excellent review articles describe the anesthetic management of children with heart transplants (see also Chapter 23 ). A thorough preoperative evaluation with attention to episodes of rejection, presence of coronary artery disease, and organ dysfunction; a detailed medication history with investigation of major side effects; consideration of the denervated physiology; and appropriate choice of anesthetic drugs and other medications are essential components of a sensible anesthetic plan for those with a transplanted heart.
Many factors influence the selection of appropriate inotropic and vasopressor therapies, including the clinical situation, underlying cardiac abnormalities, and perfusion requirements of other organs. The major goal is to improve tissue oxygenation. Oxygen delivery is principally dependent on cardiac output and oxygen content. In addition to an increased cardiac output (i.e., optimal HR, preload, contractility, and afterload), adequate hemoglobin concentration and oxygen saturation values are important components. On the other hand, a careful balance of pulmonary and systemic blood flows can be crucial for certain congenital heart defects.
Catecholamines and catecholamine-like agents remain the most commonly used inotropic and vasoconstrictor drugs. It is likely that improvements in cardiac output in neonates in response to drugs such as dopamine or dobutamine are the result of increases in both HR and contractility. Some evidence exists in infants and young children after cardiac surgery that the increases in cardiac output produced by dopamine and dobutamine may be more related to a positive chronotropic effect than to an increase in the intrinsic contractile state. With few exceptions, drugs that primarily increase afterload, such as α-adrenergic agonists, have limited use in children. Large increases in afterload without corresponding improvements in contractile state are often poorly tolerated by infants and children, particularly in the context of significant underlying contractile dysfunction.
Commonly used drugs, their doses, and a summary of their effects on selected cardiac functions are presented in Table 18.8 ; most of this information has been empirically derived from studies in adults. Limited direct information regarding the effects of commonly used vasoactive drugs in children at different ages and in various pathophysiologic states is available. Neonates, infants, and small children demonstrate unique responses to inotropic and vasoactive drugs primarily because of age-specific pharmacokinetics; differences in receptor types, number, and function; and a variability in drug delivery. Important variations in the volume of distribution and measured plasma concentrations have been observed in children receiving inotropic agents. As much as a 10-fold range in plasma concentration has been reported for a given infusion rate.
| Agent | Intravenous Dose | Comments |
|---|---|---|
| Dopamine | 2–20 µg/kg per minute infusion | Primary effects at β 1 , β 2 , and dopamine receptors, somewhat related to dose; lower doses (2–5 µg/kg per minute) can increase contractility and can also have a direct dopaminergic receptor effect to increase splanchnic and renal perfusion; increasing doses increase contractility via β-effects and increase likelihood of α-mediated vasoconstriction; effects depend on endogenous catecholamine stores. |
| Dobutamine | 2–20 µg/kg per minute infusion | Relatively selective β 1 stimulation; also potential β 2 stimulation, tachycardia, and vasodilation, especially at higher doses (>10 µg/kg per minute); may be less potent than dopamine, especially in immature myocardium; no significant α-adrenergic effects; tachydysrhythmias perhaps more likely than with dopamine; effects are independent of endogenous catecholamine stores. |
| Epinephrine | 0.02–2.0 µg/kg per minute infusion | Primary β-effects to increase contractility and vasodilation at lower doses (0.02–0.10 µg/kg per minute); increasing doses (>0.1 µg/kg per minute) are accompanied by increased contractility and increased α-mediated vasoconstriction; may be best choice to augment contractility and perfusion, especially in situations of severely compromised ventricular function, shock, or anaphylaxis. |
| Isoproterenol | 0.05–2.0 µg/kg per minute infusion | Pure, nonselective β-agonist; significant inotropic, chronotropic (β 1 and β 2 ), and vasodilatory (β 2 ) effects; may be an effective pulmonary vasodilator in some children; tachycardia and increased myocardial oxygen consumption may be dose limiting; tachydysrhythmias may also occur; bronchodilator. |
| Norepinephrine | 0.05–2 µg/kg per minute infusion | Primary effects on α 1 -, α 2 -,and β 1 -receptors, no significant clinical effects on β 2. Increased systemic blood pressure and cardiac output (α 1 and β 1 ) as well as improved pulmonary blood flow (α 2 ). Less tachycardia. Mainly used for treatment of septic shock and pulmonary hypertension. |
| Phenylephrine | 1–10 µg/kg bolus, 0.1–0.5 µg/kg per minute infusion Preterm and term infants may require as much as a 30 µg/kg bolus (see Chapter 17 ) |
Pure α-mediated vasoconstriction; no increase in contractility. |
| Vasopressin | 0.0003–0.002 U/kg per minute | Induces intense vasoconstriction via V 1a receptors in the vascular endothelium. May cause vasodilation via V 2 receptors in specific tissues (release of nitric oxide and vasodilating prostaglandins). Used for the treatment of vasodilatory shock, refractory hypotension, and pulmonary arterial hypertension. Careful when programming syringe pumps: multiple dosing forms (U/minute, U/kg per hour, U/kg per minute, or mU/kg per minute). |
| Amrinone | 0.75–1 mg/kg repeated twice, maximum 3 mg/kg Neonates and infants may require loading doses of 2–4 mg/kg and infusions of 10 µg/kg per minute |
Increases cyclic adenosine monophosphate by phosphodiesterase inhibition; positive inotropy, positive lusitropy, and smooth muscle vasorelaxation; hypotension; reversible thrombocytopenia. |
| Milrinone | 50–75 µg/kg loading dose, 0.5–1.0 µg/kg per minute infusion | Similar to amrinone (antiplatelet effects may be less). |
| Calcium chloride Calcium gluconate |
10–20 mg/kg per dose (slowly) 30–60 mg/kg per dose (slowly) |
Positive inotropic and direct vasoconstricting effects; inotropy is significant only if ionized calcium is low and/or ventricular function is depressed by other agents; can slow sinus node; increases electrophysiologic abnormalities from hypokalemia and digoxin. |
| Digoxin | Total digitalizing dose (TDD): | TDD given in divided doses: |
| Premature: 20 µg/kg | ||
| Neonate (1 mo): 30 µg/kg | ||
| Infant (<2 yr): 40 µg/kg | ||
| Child (2–5 yr): 30 µg/kg | ||
| Child (>5 yr): 20 µg/kg | ||
| Maintenance: 2.5–5 µg/kg q12 hour |
Substantial pharmacodynamic variability (i.e., variability in the serum concentration required to produce the desired effect) can also be observed. Some of these differences are related to receptor maturation and function. For example, it appears that β-adrenergic receptors have a high density in the term neonate and young infant but their coupling to adenyl cyclase may be incomplete. In addition to developmental changes, which are to some extent controlled by thyroid hormone, β-receptor, and adenyl cyclase activities are diminished in response to sustained administration of exogenous β-agonists and also as a result of increased endogenous catecholamine concentrations, which are often seen as a complication of moderate to severe heart failure and other forms of severe stress (e.g., sepsis).
In the neonatal myocardium, chronic catecholamine exposure may upregulate adrenergic receptor number or function, or both, perhaps mimicking the normal developmental program of increasing sympathetic nervous system activity as term approaches. With further maturation in early postnatal life, β-adrenergic receptor density declines. The impacts of various pathophysiologic states on these processes have been incompletely identified. For example, congestive heart failure, cardiopulmonary bypass (CPB), and ischemic reperfusion all lead to decreased β-receptor and adenyl cyclase expression and activity. On the other hand, the myocardium of infants with TOF exhibits increased β-receptor density and greater receptor-stimulated adenyl cyclase activity with increased gene and protein expression.
One must pay particular attention to technical issues when administering vasoactive infusions to infants. Infusions are often specifically prepared as very concentrated, nonstandardized solutions to minimize the amount of volume infused; hence, the potential for dose or concentration error is substantial. One study at a tertiary care children's hospital demonstrated that the actual concentration of prepared solutions varied significantly. Because of the high concentration of these drugs relative to the child's size, small errors (either in calculation or in infusion pump flow rate) can have a large impact on the actual amount of drug delivered. The extremely small infusion rates can also lead to a delay in drug delivery and effect (see Chapters 8 and 52 ). Confirming that the pump drive mechanism is actually delivering drug at the distal end of the infusion tubing, connecting the infusion tubing as close to the child as possible, and using a carrier infusion to “push” the medication at a constant rate are important steps to ensure the safety and effectiveness of drug infusions. The rate of the carrier infusion is also crucial. Most standard infusion setups require rates in excess of 5 mL/hour to effect rapid (less than ~10 minutes) changes in the concentration of drug delivered to the infant and therefore preclude all attempts of fluid restriction.
Dopamine continues to be the most frequently used inotropic agent in neonates, infants, and children. It has activity at α-, β-, and dopaminergic receptors. Dopamine augments cardiac contractility through two mechanisms. First, it directly stimulates cardiac β 1 -receptors and provokes norepinephrine release from cardiac sympathetic nerve terminals. Second, circulating concentrations of endogenous epinephrine and norepinephrine increase during dopamine infusions, leading to the suggestion that at least some of the effects of a dopamine infusion are indirectly mediated via induced release of endogenous catecholamines. Because of its indirect effects, particularly the release of myocardial norepinephrine stores, the response to dopamine may be diminished in children with congestive heart failure or other relatively long-standing forms of hemodynamic stress.
Activity at dopaminergic receptors in the kidney and gastrointestinal tract can lead to improved perfusion of these organ systems. The evidence that dopamine specifically and selectively improves renal perfusion via stimulation of renal dopaminergic receptors (i.e., as opposed to a nonspecific and generalized improvement in cardiac output that might occur with any positive inotrope) is conflicting. Regardless of the mechanism, most evidence indicates that renal blood flow and perfusion are increased by dopamine, even at very large doses.
As in the case with other inotropes, pharmacokinetic studies of dopamine have shown wide variability in serum concentration in neonates and children. Because of variability in the plasma concentration for a given infusion rate, as well as the wide range of serum concentrations necessary to produce a given effect, doubling or halving a dopamine infusion rate may be a logical approach to bracketing the optimal dose. The frequent practice of changing the infusion rate by small proportions (i.e., 5% to 10%) may be inconsistent with our current understanding of the pharmacokinetics and pharmacodynamics of most inotropes.
Neonates have classically been considered to have a greater dependence on HR, a reduced myocardial compliance, and a relative resistance to inotropic effects of exogenous catecholamines. Nonetheless, there is substantial echocardiographic evidence that small dopamine infusion rates (≤5 µ/kg per minute) increase myocardial contractility before significant increases in HR. Evidence regarding the effects of dopamine in sick preterm infants is also somewhat controversial. There may be a relative dissociation between its effects on the renal and mesenteric beds in these infants, such that a portion of the increase in arterial blood pressure during a dopamine infusion may result from mesenteric vasoconstriction and an actual decrease in mesenteric blood flow.
Although it is generally accepted that large infusion rates (>10 to 15 µg/kg per minute) of dopamine cause substantive vasoconstriction, studies have shown that both cardiac output and renal blood flow improve, even at very large doses (≥20 µg/kg per minute) in neonates and infants.
The effects of dopamine on PVR are variable. Both minimal effect and increased PVR have been observed. The effects of dopamine on PVR most likely depend on the dose as well as the underlying state of the vascular endothelium and smooth muscle. Vasoconstriction may be more likely after ischemia-reperfusion and in the presence of hypoxia. Conversely, the presence of vasodilators, such as nitroprusside, or α-adrenergic blockers, such as phenoxybenzamine, can prevent increased PVR in response to dopamine. Overall, dopamine remains the drug of choice in most infants and children, owing to its beneficial effects on mesenteric and renal blood flow, lesser chronotropic effects than some other agents, and a somewhat reduced arrhythmogenic potential.
Dobutamine is a structural analog of isoproterenol. It was developed to provide relatively selective β-adrenergic receptor stimulation. Its inotropic and α-adrenergic effects are somewhat less potent than those of dopamine. Dobutamine does possess significant β 2 -adrenergic receptor agonist properties, accounting for its peripheral vasodilatory properties. Substantial vasodilation and tachycardia occur at larger infusion rates (≥10 µg/kg per minute). The tendency toward tachycardia and tachyarrhythmia may be greater in neonates than in older children or adults. There is some evidence from immature animal models that the efficacy of dobutamine is reduced, perhaps because of greater circulating catecholamine concentrations and alterations in β-receptor expression and function.
Because the actions of dobutamine do not depend on endogenous catecholamine stores, the drug may be more effective in increasing cardiac output in patients with severe congestive heart failure or cardiogenic shock. In children with normal LV function, dobutamine increases LV relaxation. It also improves diastolic relaxation by decreasing end-systolic wall stress. Evidence indicates that dobutamine improves LV contractility in neonates with LV dysfunction, dispelling the notion that there is relative resistance in neonates. Dobutamine does not selectively improve renal or mesenteric blood flow independently of its effect on increasing cardiac output. The improvement in cardiac output with dobutamine is related to both an increased contractility and decreased SVR via vasodilation. Pulmonary vasodilation in the presence of an increased PVR may also occur.
As is the case with dopamine, exponential increases in serum concentrations are required to produce linear improvements in cardiac index. There is also substantial pharmacokinetic variability in the plasma concentrations of dobutamine. Tolerance may occasionally develop. In one animal study, high-dose dobutamine infusion was associated with significant dysfunction of platelet aggregation after hypoxia and reoxygenation.
Isoproterenol is a pure, nonselective β-adrenergic agonist. It increases HR and contractility and vasodilates mesenteric and renal vessels and skeletal muscle. Isoproterenol is also a fairly effective vasodilator of the pulmonary circulation. The tachycardia, which almost always accompanies its use, and greater contractility cause an increase in myocardial oxygen consumption, which is usually well tolerated. However, these changes may be limiting in compromised hearts. The pulmonary vasodilation produced by isoproterenol may be useful in settings in which tachycardia is either unimportant or somewhat beneficial. Systemic vasodilation induced by isoproterenol can be sufficiently profound as to cause systemic hypotension. The positive chronotropic effects of isoproterenol may be useful in children with bradycardia. The drug is increasingly used in electrophysiology suites to facilitate the detection of abnormal conduction pathways in infants and children under general anesthesia. Isoproterenol is also a potent bronchodilator. Prolonged use or large doses of isoproterenol and other catecholamines may be associated with the development of myocardial fibrosis.
Epinephrine has α-, β 1 -, and β 2 -adrenergic agonist effects. Data derived mainly from studies in adults indicate that lower doses of 0.02 to 0.1 µg/kg per minute are associated with predominantly β-adrenergic effects. In this range, increases in HR and systolic blood pressure and reduced diastolic blood pressure owing to skeletal muscle vasodilation predominate. Doses between 0.1 and 0.2 µg/kg per minute have mixed α- and β-effects. At larger doses, α-adrenergic–induced vasoconstriction is significant, and hence there is reduced skin, muscle, renal, and mesenteric blood flow. Compared with pure α-agonists, epinephrine provides significant inotropic effect. The effects of epinephrine do not depend on endogenous tissue catecholamine stores. Based on experience, epinephrine seems to be effective in children who do not respond to dopamine or dobutamine, particularly those with significant dysfunction of the systemic ventricle in the immediate postoperative period. The addition of moderate vasoconstriction to increased contractility may be advantageous to maintain myocardial perfusion and may also increase both systemic and pulmonary blood flow in children with shunt-dependent circulations. Important adverse effects include dysrhythmias (usually ventricular) and, at larger doses, regional ischemia and hypoperfusion as the result of vasoconstriction.
Pharmacokinetic studies have shown a linear relationship between serum concentration and infusion rate, but again there is significant variability in the individual response to a specific concentration. In infants and children with postoperative low cardiac output syndrome, increased concentrations of glucose and of lactate have been reported after 1 to 2 hours of an epinephrine infusion of 0.1 µg/kg per minute.
Norepinephrine is often indicated as a first- or second-line treatment for severe hypotension associated with septic shock, but it has also been used in the treatment of persistent pulmonary hypertension of the newborn and other forms of pulmonary hypertension. This endogenous adrenergic agent activates both α- and β-receptors. Compared with epinephrine, it seems to be equally effective on β 1 -receptors, slightly less on α 1 -receptors, and has no clinically significant effects on β 2 -receptors. Norepinephrine has been shown to increase systemic blood pressure as well as cardiac output, oxygen delivery, and splanchnic perfusion in animal models and clinical studies. It causes less tachycardia than epinephrine. Through activation of α 2 -receptors and release of NO, norepinephrine reduces PVR and can improve pulmonary blood flow. Norepinephrine has a very rapid onset of action with a duration of effect of only 1-2 minutes. It is metabolized by catechol-O-methyltransferase and monoamine oxidase, the inactive metabolites are eliminated in the urine. Pharmacokinetic data are mainly derived from adult studies; the few available pediatric reports emphasize the wide interindividual variability and the need for careful titration. The initial dose of 0.05 to 0.1 µg/kg per minute has to be slowly increased to the desired effect, usually in a range of 0.1 to 2 µg/kg per minute. In a small prospective observational study in term neonates, the majority responded to a mean dose of 0.5 ± 0.4 µg/kg per minute, with a range of 0.2 to 7.1 µg/kg per minute. A retrospective study in children in septic shock reported mean initial doses of 0.5 ± 0.4 µg/kg per minute to 2.5 ± 2.2 µg/kg per minute, with a maximum individual dose of 10.5 µg/kg per minute. Norepinephrine may be administered via peripheral venous access (and is recommended to avoid delays in treatment) until central access can be established, but some institutions report IV infiltrates in up to 15% of children on vasoactive infusions especially during transport. Accordingly, ongoing vigilance is important. Other side effects include arrhythmias and hypertension, which usually respond to dose reductions.
Phenylephrine is a pure α-adrenergic agonist. As such, its major function is to cause peripheral vasoconstriction. It has no β-adrenergic or inotropic effect and therefore does not increase contractility. It may be temporarily used to improve afterload, systemic blood pressure, and, therefore, critical organ blood flow. But without concurrent inotropic support, an isolated acute increase in afterload is often poorly tolerated, particularly by a compromised ventricle. There are a at least three situations in which phenylephrine can be extremely useful. The first is to increase systemic afterload and decrease right-to-left shunting in children with TOF and dynamic RVOTO (tet spell). This pure α-adrenergic effect is particularly important in this situation, since any additional increase in contractility would worsen the outflow obstruction. Second, phenylephrine is also beneficial in cyanotic children who depend on a systemic-to-PA shunt for pulmonary blood flow and adequate oxygenation. The increased afterload may increase flow across the shunt and improve pulmonary blood flow. Third, acute hypotension in children with hypertrophic obstructive cardiomyopathy or critical aortic stenosis increases the outlet obstruction; phenylephrine increases afterload attenuating the severity of the obstruction.
Arginine vasopressin is a peptide secreted by the pituitary gland. Secretion is promoted by angiotensin II and increased stimulation from hypothalamic osmoreceptors; increased activity from cardiopulmonary baroreceptors and increased levels of natriuretic peptide inhibit the secretion of vasopressin. Vasopressin acts at the tissue level by binding to specific receptors. It causes vasoconstriction via vasopressin 1 (V 1a ) receptors and renal reabsorption of water, renal secretion of renin, and synthesis of renal prostaglandins via vasopressin 2 (V 2 ) receptors. In addition, vasopressin may mediate vasodilatation via V 2 receptors by increasing the release and synthesis of NO and vasodilating prostaglandins. Vasopressin also sensitizes baroreceptors and therefore may cause vasodilatation by decreasing sympathetic activity. Normally, vasopressin acts primarily via V 2 receptors in the kidney to promote water retention. However, during extreme hypotension, vasopressin may act via V 1a receptors in the vascular endothelium to induce intense vasoconstriction.
In adults, vasopressin has been shown to be beneficial in the treatment of vasodilatory shock and during cardiopulmonary resuscitation. A few pediatric case reports and small observational studies have demonstrated improved blood pressure and accelerated weaning of inotropic support with low-dose vasopressin. However, a multicenter, randomized, controlled trial in children with vasodilatory shock did not confirm these findings. Low-dose vasopressin (0.0005-0.002 U/kg per minute) had no beneficial effects compared with placebo; there was even a suggestion of increased mortality. Further studies are necessary to establish the effectiveness and safety of vasopressin in children. Currently its role as a “rescue” medication for the treatment of catecholamine-resistant vasodilation during or after congenital cardiac surgery and for refractory hypotension in extremely low–birth-weight (ELBW) infants is being investigated. In 2013, a Cochrane Review found no eligible studies to analyze the effects of vasopressin and its analogues on refractory hypotension in neonates. All available evidence for a role of vasopressin in children is based on case reports, small case series and three randomized controlled trials in older children. A small pilot study in 20 infants, published in 2015, compared vasopressin and dopamine as the primary treatment for hypotension in ELBW infants. Vasopressin appeared to be safe and effective, but this interpretation must be tempered by the small sample size. The prophylactic use of low dose vasopressin (0.0003 U/kg per minute) in the early postoperative phase after the Norwood or arterial switch procedure has been reported to decrease catecholamine and fluid requirements. Refractory pulmonary hypertension is another potential indication for vasopressin and its analogues. In animal studies and in vitro experiments with human tissue, vasopressin causes pulmonary vasodilation via endothelium-dependent release of NO or by direct activation of smooth muscle receptors. This vascular response seems to be age and disease specific and could explain the conflicting results in the literature. Several case reports and case series describe the successful use of vasopressin for this indication, but further studies are necessary to assess appropriate dosing and safety. Extrapolated from adult data, pediatric dosing algorithms currently range from 0.0003 to 0.002 U/kg per minute. Careful attention to the infusion rate is important, especially when programming syringe pumps. The literature and common reference tools often cite the doses in units per minute (U/minute), units per kilogram per hour (U/kg per hour), units per kilogram per minute (U/kg per minute), or even milliunits per kilogram per minute (mU/kg per minute), which can be quite confusing and readily lead to dosing errors.
Phosphodiesterase inhibitors, which include amrinone, milrinone, and enoximone, are the most commonly used non–catecholamine-mediated inotropic agents. Their mechanism of action is also relatively straightforward. Phosphodiesterases degrade cyclic adenosine monophosphate (cAMP) to 5′-AMP. Phosphodiesterase inhibitors prevent this degradation and therefore increase levels of cyclic nucleotides, primarily cAMP. The increased concentration of this secondary messenger leads to an increase in calcium availability and thus increased contractility. Because the response is related to an increase in cAMP and not purely to inhibition of phosphodiesterase, the greatest effect occurs if initial levels of cAMP exceed normal values. In this way, synergy exists with β-agonists. The absence of adrenergic stimulation minimizes effects on HR, rhythm, and dependency on endogenous tissue catecholamine stores. In addition to positive inotropic effects, these drugs also have significant lusitropic properties (i.e., diastolic relaxation) and promote peripheral vasodilation. Phosphodiesterase drugs may also have substantial antiinflammatory properties that are currently not well understood.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here