Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The most important disease affecting the heart is atherosclerotic cardiovascular disease (ASCVD), caused by plaque in the coronary arteries. ASCVD can lead to thrombotic occlusion of coronary blood flow, causing an acute coronary syndrome (ACS). ACS with frank necrosis of any amount of myocardium is known as myocardial infarction ( MI ).
The primary tests for diagnosing and evaluating ACS are electrocardiography (ECG) and laboratory measurement of cardiac troponin (cTn).
Troponin is a complex of three proteins, part of the contractile apparatus of all muscle cells but with two isoforms that are specific to myocardium: cTnI and cTnT. These two proteins have different properties, but their clinical applications are similar. Clinically, MI is now essentially defined as an ACS that causes release of cTn.
The latest generation of cTn tests, referred to as high-sensitivity cTn (hs-cTn), can measure levels of the protein down to the normal range. With hs-cTn, elevated levels can typically be detected within an hour after the onset of MI. Plasma cTn then peaks in about 24 hours and declines to baseline over the next several days.
Patients presenting to an emergency department with symptoms suggesting ACS must be processed rapidly and precisely to provide lifesaving interventions as needed yet avoid the cost and morbidity of unnecessary procedures. Rapid measurement of cTn, and possibly secondary markers such as copeptin, plays a critical role.
The clinical laboratory also measures risk factors associated with the development and progression of ASCVD. Significant laboratory markers of risk include lipids (cholesterol, triglycerides, and specific lipoprotein fractions—see Chapter 18 ), homocysteine (Hcy), and C-reactive protein (CRP). Hcy is an amino acid that exacerbates thrombosis. CRP is an inflammatory marker that appears to reflect the severity of ASCVD and may contribute to its pathogenesis.
ASCVD and other heart diseases can impair the heart’s ability to pump blood, causing the clinical syndrome of heart failure (HF). B-type natriuretic peptide (BNP), a 32 amino acid peptide secreted by the cardiac ventricles in response to wall-stretch stimuli, is a marker of the presence and severity of HF. BNP testing is particularly useful for aiding the differential diagnosis of patients who present to an emergency department with shortness of breath.
Atherosclerosis affecting the carotid arteries or brain vasculature can lead to ischemic or hemorrhagic stroke. Some biomarkers show promise for the diagnosis or evaluation of stroke, but none are sufficiently discriminatory for routine use at present.
Measurement of d -dimer, a degradation product of fibrin, has excellent sensitivity but poor specificity for the diagnosis of pulmonary embolism and venous thrombotic disease in general.
Advances in methodology, standardization, and decision-point assignment for hs-cTn, BNP, d -dimer, and other cardiac-related tests are ongoing and essential for optimizing clinical outcomes.
Heart disease is an affliction intimately tied to high technology. Technology has had a causal role, in part by allowing people to live longer and in part by enabling a sedentary and overly consumptive lifestyle. During the twentieth century, heart disease rose from obscurity to become the leading cause of morbidity and mortality in developed nations ( Box 19.1 ). Diagnosis and treatment of heart disease also depend heavily on advanced technology, including electrophysiologic, imaging, catheterization, surgical, and clinical laboratory modalities. The following major laboratory applications will be the subject of this chapter.
Measurement of proteins present in cardiac myocytes indicates recent damage to cardiac muscle. These tests are used mainly for the diagnosis and management of ischemic events (acute coronary syndromes [ACSs]). Although many different markers of ischemic damage have been used in the past, at present the most important marker by far is cardiac troponin (cTn).
Measurement of substances that are damaging to the coronary arteries, or at least have proven association with atherosclerotic cardiovascular disease (ASCVD), is used to assess risk and select appropriate preventive measures. The most important laboratory risk factors are lipids, which are discussed in Chapter 18 . Two others will be discussed in this chapter: homocysteine (Hcy) and C-reactive protein (CRP).
Measurement of natriuretic peptides released from myocardium, particularly B-type natriuretic peptide (BNP) and the related inactive fragment, N-BNP or NT-pro-BNP , reflects the presence and severity of heart failure.
Laboratory testing has been less applicable to cerebrovascular disease, but some new biomarkers show promise. Measurement of d -dimer is useful in ruling out pulmonary embolus.
An estimated 48% of the adult population (≥20 years of age), has some form of cardiovascular disease (including ASCVD, HF, stroke, and hypertension). Excluding hypertension, that reduces to 9.0% of the population, or about 24.3 million adults.
805,000 new or recurrent MIs occur each year, and 18.2 million Americans have experienced an MI or have other history of ASCVD.
795,000 new or recurrent strokes occur each year, and 7.0 million Americans have experienced a stroke.
6.2 million Americans have HF.
The economic cost of cardiovascular disease, including indirect costs such as loss of work productivity, etc., is estimated at about $555 billion for 2015, and to increase substantially in subsequent years, particularly for the elderly.
Favorable trends in ASCVD include less cigarette smoking and effective pharmacotherapy for hypertension and hyperlipidemia. However, the epidemic is now growing due to an alarming increase in the prevalence of obesity and type 2 diabetes.
It is ironic that for the heart, an organ that pumps several liters of blood each minute, the most important disease process is ischemia—the lack of an adequate blood supply. This is so because heart muscle depends on constant nutrition through a system of coronary arteries, which are highly vulnerable to the process of atherosclerosis. Atherosclerosis is a chronic process involving damage to endothelium and the buildup of vessel-occluding lesions called plaque . In the early stages of atherosclerosis, as coronary blood flow is gradually reduced, there are typically no symptoms or laboratory evidence of cardiac injury. Once the diameter of a coronary artery is reduced to less than 10% to 20% of its original size, chest pain (angina pectoris) often develops when demand for oxygen increases, particularly during exercise (exertional angina). More rapid reduction in blood flow can occur when plaque stimulates formation of a thrombus in a coronary artery, leading to an ACS. When a thrombus completely cuts off blood flow, the supplied muscle will develop irreversible ischemic damage, and the syndrome is a myocardial infarction (MI). When the blockage is not complete, irreversible muscle damage may be avoided, but the patient will experience severe angina, even at rest, and this syndrome is known as unstable angina (UA). The broad spectrum of heart disease resulting from impaired coronary blood flow has been referred to by many names and acronyms over the years: for example, atherosclerotic heart disease (ASHD), coronary heart disease (CHD), ischemic heart disease (IHD), etc. Organizations that publish consensus guidelines, such as the American Heart Association, have recently adopted the term atherosclerotic cardiovascular disease (ASCVD), which we will employ henceforth. Common acronyms used to describe heart disease are listed in Table 19.1 .
| ACS | Acute coronary syndrome |
| ASCVD | Atherosclerotic cardiovascular disease (also referred to as coronary heart disease [CHD], ischemic heart disease [IHD], and others) |
| CVD | Cardiovascular disease (comprising ASCVD, HF, stroke, and hypertension) |
| HF | (Congestive) heart failure |
| MI | (Acute) myocardial infarction |
| NSTEMI | Non–ST-elevation myocardial infarction |
| STEMI | ST-elevation myocardial infarction |
| UA | Unstable angina |
MIs can be categorized by whether they are accompanied by characteristic changes on the electrocardiogram (ECG), a recording of electrical activity in the heart. Only requiring simple voltage recordings between electrodes attached to the skin surface, the ECG was introduced in the late nineteenth century and has played a central role in cardiac diagnosis ever since. Portions of the waveform associated with each heartbeat are assigned the letters P through U. Severe MIs, generally involving transmural damage to the myocardium, disrupt electrical flow and typically cause the rapid appearance of readily identified changes known as ST-segment elevations and the later appearance of Q-waves. These are categorized as ST-elevation MIs (STEMIs). Lesser amounts of damage may cause ECG alterations that are not sufficiently specific for diagnosis or may cause no ECG change whatsoever. These smaller MIs are known as non-ST elevation MIs (NSTEMIs). Even with only a small amount of damage to myocardium, any ACS event carries risk for possibly lethal arrhythmias and for future events. Damage to a sizable quantity of cardiac muscle carries the additional risk of compromising the heart’s ability to pump blood, leading to the clinical syndrome of heart failure (HF), which is discussed later.
Cardiac muscle is relatively resistant to ischemia compared with other cells, such as neurons and renal tubular epithelial cells, in which even short duration of ischemia can lead to cell death. In experimental animals, complete blockage of blood flow to an area of the heart does not cause cell death (MI) until after at least 20 to 30 minutes of ischemia; if blood flow had been previously restricted, cell death often is delayed for up to an hour. With complete coronary artery occlusion, there is typically a gradient of ischemia, with oxygen deprivation worst in areas receiving blood flow last (the subendocardial portions of the ventricular wall). Cells near the border between ischemic myocardium and normally perfused myocardium may receive some oxygen supply and thus may remain viable for several hours. The longer the duration of ischemia, the higher is the percentage of cells at risk that will die; 3 hours of ischemia increases cell death to 80% of cells at risk, and 6 hours of ischemia causes the death of almost 100% of cells at risk. For this reason, early recognition of persistent ischemia and intervention to restore blood flow are needed to minimize cell death. Blood flow is most commonly restored by manipulating the plaque (usually by inflating a balloon) via a catheter inserted into a peripheral vein and then threaded into the coronary circulation (percutaneous coronary intervention [PCI]). A mesh tube, or stent, may be inserted at the same time to maintain the patency of the coronary artery. When flow is blocked at multiple sites, it may be necessary to perform a coronary artery bypass graft (CABG).
Despite the central role of atherosclerosis and thrombosis in ACS, infarction can be the result of other processes causing an imbalance between oxygen supply and demand in the myocardium. The classical “heart attack” caused by coronary artery occlusion is now referred to as a type 1 MI . A type 2 MI is a similar clinical syndrome, not involving thrombotic occlusion of a coronary artery but rather caused by vasospasm, anemia, or other situations, often related to severe systemic illness, leading to insufficient oxygen delivery to myocardium. Extending this classification system, a type 3 MI occurs by definition when there is strong clinical evidence for MI but the patient succumbs before it can be confirmed by ECG and biochemical evidence. Types 4a, 4b, 4c, and 5 MI are related to cardiac interventional procedures ( ).
The pathobiology of atherosclerosis and ACS remains incompletely understood ( ; ). Formation of obstructive plaques probably begins with nonobstructive lesions known as fatty streaks, which have been observed in the coronary arteries of young individuals dying in combat or in accidents. The lesions are likely triggered by uptake of oxidized low-density lipoprotein (LDL) particles by macrophages, which then invade the coronary endothelium. Inflammatory cells and mediators play a role in the evolution of the lesion, which eventually becomes a structure containing a lipid core (mainly cholesterol esters) surrounded by numerous macrophages and other inflammatory cells and covered with a cap of endothelialized connective tissue. Advanced lesions also contain new blood vessels and calcium deposits. Plaques in coronary arteries were formerly regarded as passive, physiologically irreversible barriers to blood flow. In recent years, their more dynamic role in ACS has been appreciated. A balance of inflammatory mediators, shear forces, and other factors can cause the fibrous cap of the plaque to strengthen or weaken. Erosion of the cap can expose thrombogenic material, leading to deposition of platelets and eventually enlargement of the lesion. More ominous is actual rupture of the plaque, causing thrombosis with enough occlusion to result in ACS ( ; ). A major contributor to this concept of plaque vulnerability has been the finding that cholesterol-lowering statin drugs can diminish the risk of ACS substantially without causing appreciable diminution of the degree of stenosis caused by atherosclerotic lesions (see Chapter 18 ). The severity of coronary atherosclerotic disease has traditionally been evaluated by coronary angiography, which uses a radiopaque “dye” to image blood flow through the coronary arteries. Besides being invasive, this test has the limitation that it can identify restrictions only to coronary blood flow; it says nothing about the biology of the plaque lesions. Computed tomography can noninvasively image the accumulation of calcium in atherosclerotic lesions (the “calcium score”), but ability to predict the future behavior of lesions is still limited ( ). Blood tests that directly reflect plaque vulnerability and the extent of atherosclerosis may be available in the future, but at present a patient’s propensity to an ASCVD event is assessed indirectly through the measurement of risk factors, each of which appears to play a contributory but not necessarily definitive role. Several clinical parameters, summarized in Table 19.2 , have been established as important risk factors. Additionally, hundreds of laboratory tests have been studied in relation to ASCVD risk. Of these, several lipid tests, as discussed in Chapter 18 , have the best-established role in risk assessment. Two newer markers, Hcy and CRP, are discussed later.
| Risk Factor | Recommendations |
|---|---|
| Age | In earlier guidelines, arbitrary age cutoffs, e.g., male >45 years, female >55 years, were used as indicators of ASCVD risk. Current guidelines recommend a continuous risk calculation based on the “pooled cohort equations” that incorporates age, gender, and race along with clinical and laboratory data. Risk assessment at regular healthcare visits is recommended for adults age 40–75 years who do not have a history of ASCVD. |
| Smoking | All adults should be assessed at every healthcare visit for tobacco use; those who use tobacco should be assisted and strongly advised to quit. |
| Poor diet | Diet should emphasize intake of vegetables, fruits, nuts, whole grains, lean vegetable or animal protein, and fish and minimize the intake of trans fats, processed meats, refined carbohydrates, and sweetened beverages. |
| Obesity | For adults with overweight and obesity, counseling and caloric restriction are recommended for achieving and maintaining weight loss. |
| Sedentary Lifestyle | Adults should engage in at least 150 minutes per week of accumulated moderate-intensity physical activity or 75 minutes per week of vigorous-intensity physical activity. |
| Hypertension | Nonpharmacologic interventions are recommended for all adults with elevated blood pressure or hypertension. For those requiring pharmacologic therapy, the target blood pressure should generally be <130/80 mm Hg. |
| Diabetes | For adults with type 2 diabetes mellitus, lifestyle changes, such as improving dietary habits and achieving exercise recommendations, are crucial. If medication is indicated, metformin is first-line therapy, followed by consideration of a sodium-glucose cotransporter 2 inhibitor or a glucagon-like peptide-1 receptor agonist. |
| Family history | Premature ASCVD in primary relatives (males, age <55 years; females, age <65 years) is a risk-enhancing factor for patient-clinician discussion. |
| Other | Chronic inflammatory disease, chronic kidney diseases, and some other conditions are risk-enhancing factors for patient-clinician discussion. Aspirin has little or no net benefit for primary prevention. |
Next to ASCVD, and often as a direct consequence of it, the most important heart disease is HF. HF is a clinical syndrome with prominent symptoms—including fatigue, shortness of breath, and pulmonary edema—resulting from impairment in the heart’s pumping ability. It is most commonly caused by damage to the myocardium, as results from ASCVD. Because of the high incidence of ASCVD and the improving survival of people who suffer from it, HF is a rapidly increasing problem, especially in the elderly. HF can also be caused by mechanical problems, such as valvular disease, which interfere with the pump function of the heart. When the problem is related to filling of the left ventricle during diastole, it is often referred to as diastolic HF.
Diagnosing HF and monitoring its progression can be difficult. A defining parameter for systolic HF is the left ventricular ejection fraction (LVEF), which is the fraction of the left ventricle’s blood volume that is ejected during systole. It can be measured by echocardiography or by radionuclide ventriculography. Symptomatic HF is usually associated with LVEF <40%, but correlation between LVEF and subjective symptoms is poor. Laboratory testing can contribute to the evaluation and management of HF via measurement of natriuretic peptides and other biomarkers, as discussed later.
Of the myriad other diseases that can affect the heart, brief mention should be made of genetic diseases that, although relatively uncommon, have disproportionate importance because of their potentially life-threatening consequences. The advent of molecular diagnostics—sometimes referred to as “personalized cardiology” in this context—has created a major role for the clinical laboratory in diagnosis and management of these disorders. The syndrome of hypertrophic cardiomyopathy, described more than a century ago, occurs in 1 in 500 individuals and is the most common cause of sudden death in the young. At this date, thousands of variants in at least 15 genes have been related to this disorder; they affect various proteins of the contractile apparatus, including the troponins, actin, and myosin, and generally are inherited in autosomal-dominant fashion ( ). The long-QT syndrome, an abnormality in ventricular repolarization that can cause sudden death, occurs in about 1 in 2000 individuals. It has several distinct variants caused by both autosomal-dominant and -recessive mutations in ion channels and other proteins ( ). Identifying the affected gene can guide therapy in some cases as well as provide diagnostic confirmation ( ).
Biochemical markers of myocardial damage were essentially a serendipitous discovery in the early 1950s, when Ladue and coworkers were investigating the enzymes now known as aspartate and alanine transaminase ( ). Surveying patients on the hospital wards, which at that time had many patients with suspected or confirmed MI, these investigators noted that serum transaminase levels rose sharply after an MI. They also put forward a rapid and practical spectrophotometric assay for serum transaminase ( ) and thus was born the era of “cardiac enzymes.” The simple underlying principle, as with other organ markers, is that cell death will cause release of cellular proteins into the circulation. (A more difficult question, still not fully resolved, is the extent to which reversible cell damage can cause protein leakage ( ; ).
It was immediately recognized that transaminases were abundant in liver muscle, skeletal muscle, and other tissues and therefore offered poor sensitivity and specificity for cardiac diagnosis. Superior enzymic markers were sought. Over the next few decades, testing for CK-MB (the MB isoenzyme of creatine kinase, also known as CK2) and, to a lesser extent, the LD1 isoenzyme of lactate dehydrogenase became popular. However, these required cumbersome isoenzyme separations and still offered limited cardiac specificity. It was only with the introduction of monoclonal antibody technology that a systematic search could be made for proteins specific to myocardium; that search led to the discovery of cardiac troponins ( ). For further discussion of transaminases and the classical “cardiac enzymes,” see Chapter 21 .
Troponin (Tn) is a regulatory complex of three proteins that resides at regular intervals in the thin filament of striated muscle ( ; ). The three individual proteins are tropomyosin-binding subunit (TnT, 36 kDa), inhibitory subunit (TnI, 24 kDa), and calcium-binding subunit (TnC, 18 kDa). The Ca ++ trigger for muscle contraction is transmitted via the Tn complex, which causes a conformational change in another thin-filament component, tropomyosin, allowing interaction between actin and myosin to proceed. In contrast to nearly all other muscle proteins, TnT and TnI both have distinct forms, coded by different genes, in cardiac muscle (myocardium), as well as in type 1, or “slow-twitch” and type 2, or “fast twitch,” skeletal muscle fibers. Note that in a clinical context, “troponin” nearly always refers to cardiac-specific troponin because measurement of the noncardiac form has no clinical application. Here, we will employ the terms cTnI and cTnT for the respective cardiac proteins.
cTnI is always produced in the heart but never (or extraordinarily rarely) at any other site. Hence, cTnI is a tissue marker with extremely high sensitivity and specificity. With cTnT, the situation is a little more complicated because it has been detected in fetal skeletal muscle and diseased skeletal muscle. Posttranslational modifications may cause detectable differences between cTnT produced in myocardium and cTnT produced in diseased skeletal muscle that can improve their distinction by immunoassay ( ). However, in rare cases. patients may have neuromuscular disease, without cardiac involvement, associated with increases in cTnT but not cTnI ( ; ).
The cTnT and cTnI within cardiac myocytes are predominantly bound to the muscle fibers and released slowly over the course of 1 to 2 weeks following myocardial injury. Thus, although cTnI and cTnT are relatively small proteins that are rapidly cleared, their plasma levels fall slowly after MI. A small fraction of cTn in the myocardial cell is free within the cytoplasm (or more loosely bound): 6% to 8% for cTnT and slightly less (2%–4%) for cTnI ( ). This fraction is released rapidly from injured cardiac myocytes. An increase in cTn is generally detectable using the most sensitive assays within 1 hour of an MI. cTn then reaches a peak at about 24 hours postevent, which may be thousands-fold greater than the baseline level ( Fig. 19.1 ). Due to brisk renal clearance, a rapid decline in serum cTn follows, but because of the slow release of fiber-bound cTn, the rate of decline slows and there may even be a small secondary increase. It is important that such an increase not be interpreted as evidence of reinfarction. Circulating cTn declines to baseline levels in about 5 to 10 days, depending on infarct size.
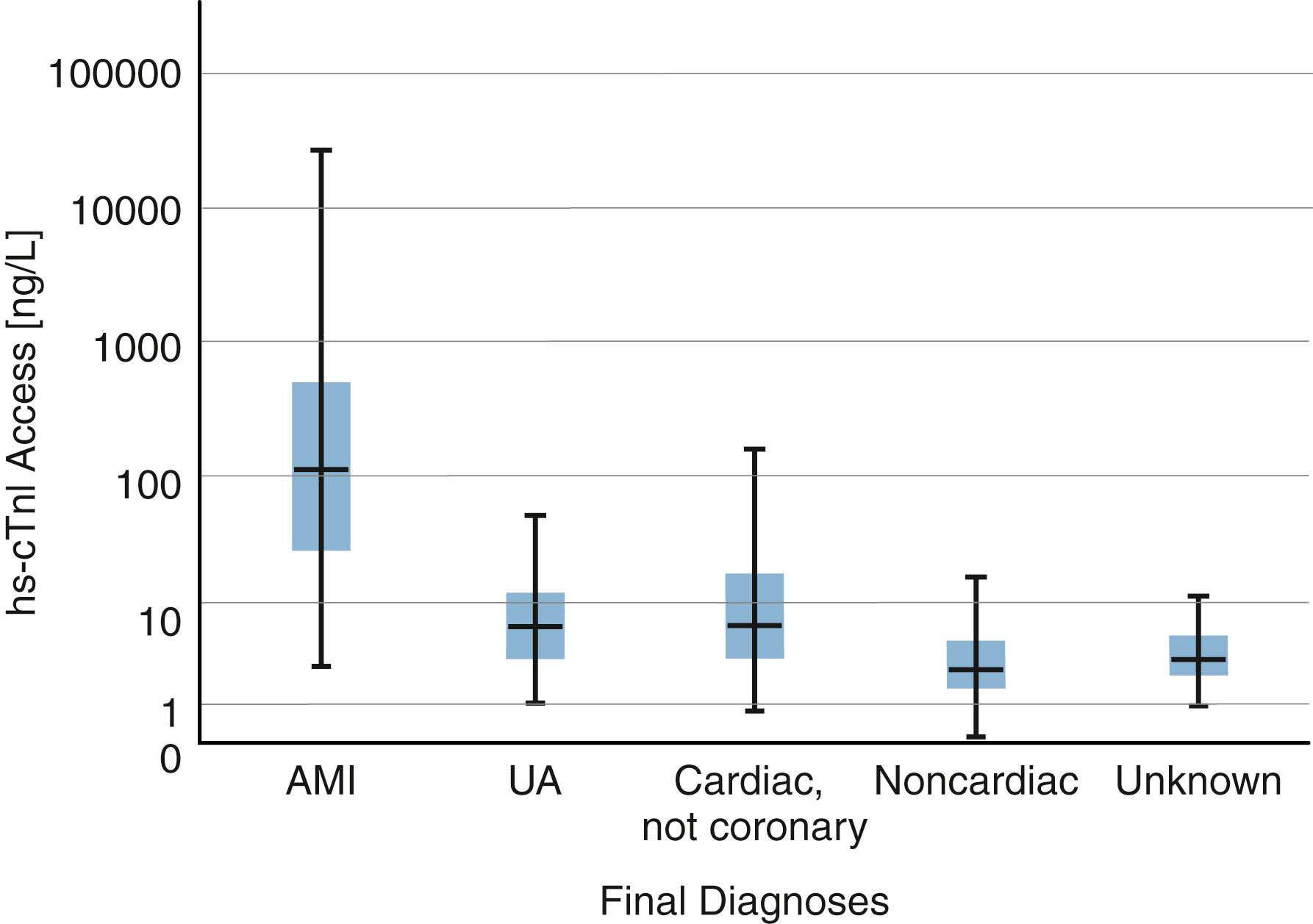
cTnT and cTnI are present at very low concentrations in normal serum. Assays introduced in the 1990s, which we may refer to as “traditional” assays, had detection limits around 10 to 50 ng/L and could not reliably measure cTn in healthy individuals. More modern high-sensitivity (hs) assays have detection thresholds around 1 to 2 ng/L and an upper 99th percentile, commonly used as the reference range limit, of around 10 to 30 ng/L depending on patient characteristics (age, gender) and the particular assay. Levels above this threshold indicate myocardial damage, but it is important to recognize that slight elevations can reflect a very small amount of myocyte damage having questionable clinical significance. Even substantial elevations have many causes unrelated to MI or ASCVD. cTn elevations have been observed in pericarditis, myocarditis, pulmonary embolism, renal failure, sepsis, other critical illness, and even after intense exercise ( ; ). Pediatric data are limited, but it is clear that both cTnI and cTnT are increased in the neonatal period ( ; ). The range of cTnI concentrations measured in patients presenting to an emergency department (ED) with suspicion of ACS is illustrated in Figure 19.1 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here