Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cardiac arrhythmias are common and result from an ectopic focus or a reentry circuit.
Surgical and catheter-based ablative therapies can abolish the origins of arrhythmias by interposition of scar tissue along the reentrant pathway or by isolating an ectopic area.
Supraventricular arrhythmias can be hemodynamically unstable, especially in the setting of structural heart disease. In some cases, persistent tachycardia can lead to tachycardia-induced cardiomyopathy.
Surgical treatment of atrial fibrillation (ie, maze procedure) has been employed with good success and has been modified to avoid the sinus node in an effort to minimize occurrences of chronotropic incompetence.
In adults, most episodes of sudden cardiac death are the result of ventricular tachyarrhythmias due to ischemic and nonischemic cardiomyopathy.
Preoperatively, identify the type of cardiac implantable electronic device (CIED) (eg, transvenous implantable pacemaker, intracardiac pacemaker, transvenous implantable cardioverter-defibrillator, subcutaneous implantable cardioverter-defibrillator) and the manufacturer of the generator.
Establish contact with the patient's CIED physician or clinic to obtain records and perioperative prescription (Heart Rhythm Society [HRS]). Have the CIED interrogated by a competent authority shortly before the procedure (American Society of Anesthesiologists [ASA]).
Determine the patient's underlying rate, rhythm, and pacing dependency to determine the need for asynchronous or external backup pacing support.
If magnet use is planned, then ensure that magnet behavior (pacing mode, rate, atrioventricular delay, shock therapy suspension) is appropriate for the patient.
If electromagnetic interference is likely or if a central venous catheter guidewire will be placed into the chest, then consider asynchronous pacing for the pacing-dependent patient and suspension of antitachycardia therapy for any implantable cardioverter-defibrillator (ICD) patient. Magnet application might be effective, although magnet use has been associated with inappropriate ICD discharge. Magnet application will never create asynchronous pacing in any type of ICD.
Monitor cardiac rhythm/peripheral pulse with pulse oximeter (plethysmography) or arterial waveform.
Ask the surgeon to avoid the use of the monopolar electrosurgical unit (ESU) or limit ESU bursts to less than 4 seconds separated by at least 2 seconds. Use the bipolar ESU if possible; if not possible, then pure cut (monopolar ESU) is better than “blend” or “coag.”
Place the ESU dispersive electrode in such a way as to prevent electricity from crossing the generator-heart circuit, even if the electrode must be placed on the distal forearm and the wire covered with sterile drape.
Temporary pacing might be needed, and consideration should be given to the possibility of CIED failure.
Have the CIED interrogated by a competent authority postoperatively. Some rate enhancements can be reinitiated, and optimum heart rate and pacing parameters should be determined. The ICD patient must be monitored until the antitachycardia therapy is restored.
Cardiac rhythm disturbances are common and an important source of morbidity and mortality. Atrial fibrillation is the most common sustained cardiac arrhythmia in the general population. Prevalence is strongly associated with age, occurring in less than 1% of individuals younger than 55 years old but in almost 10% of those older than 80 years.
The treatment of cardiac arrhythmias has shifted over the past two decades to catheter-based and surgical ablation from pharmacologic therapy because of the drugs' limited efficacy and increased risk of death owing to their negative inotropic and proarrhythmic effects. Data from prospective, randomized trials showing improved survival for patients with implantable cardioverter-defibrillators (ICDs) compared with those given antiarrhythmic drugs bolstered the shift to nonpharmacologic treatments.
Current management options for cardiac arrhythmias include surgical and catheter ablative techniques using various energy sources. The principle in all cases is identification of the electrophysiologic mechanism of the arrhythmia followed by ablation of the involved myocardium using surgical incisions, cryothermy, or radiofrequency (RF) current. As the techniques have become more complex and time intensive, the need for anesthesia support has grown. Anesthesiologists caring for patients undergoing these procedures must be familiar with the anatomy of the normal cardiac conduction system, the electrophysiologic basis of common cardiac rhythm disorders, and the various approaches to ablative treatment.
The sinoatrial (SA) node ( Fig. 3.1 ) is a spindle-shaped structure composed of highly specialized cells located in the right atrial sulcus terminalis, which is lateral to the junction of the superior vena cava (SVC) and the right atrium. Box 3.1 summarizes the anatomy of the cardiac pacemaker and conduction system.
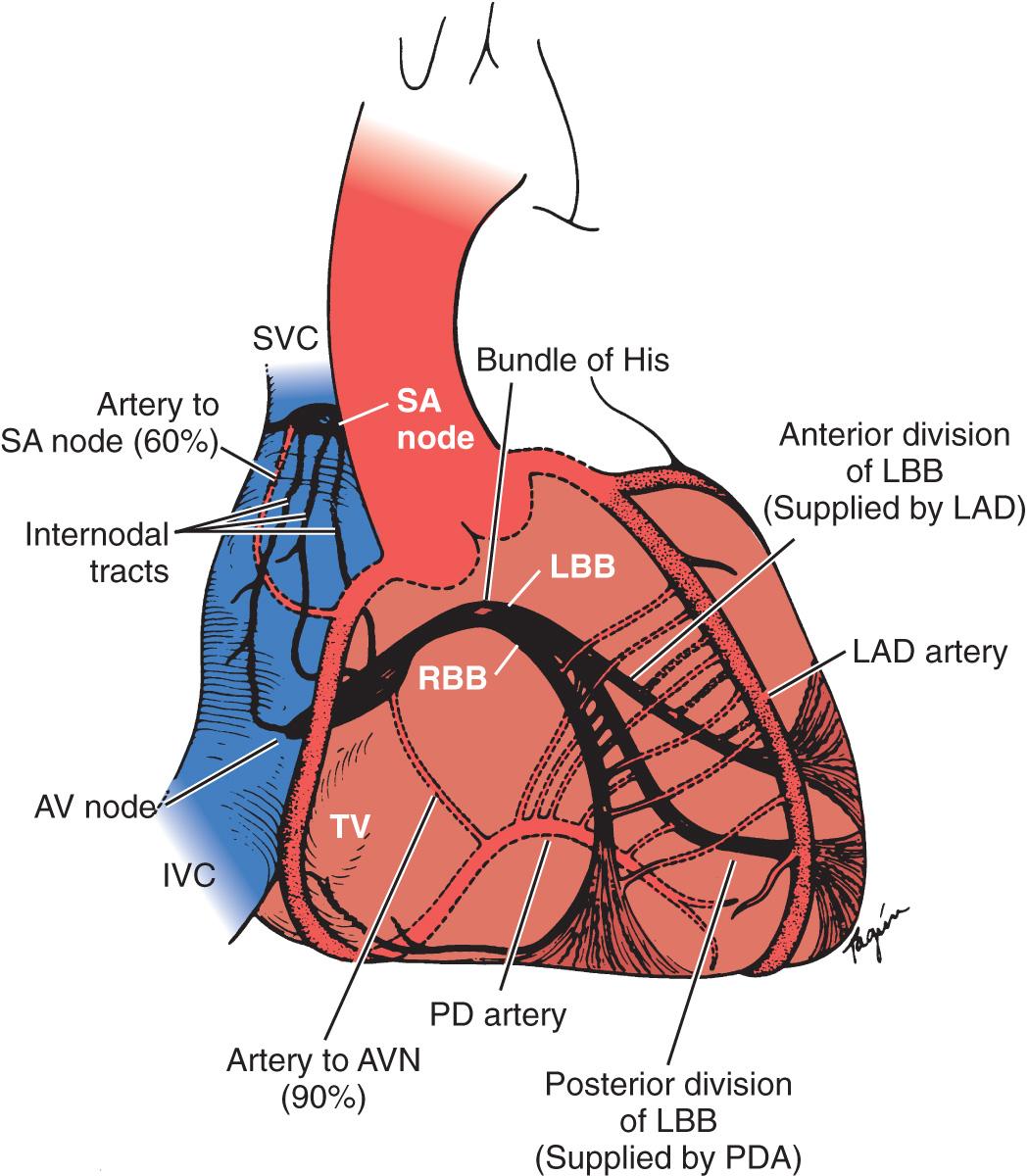
Sinus node
Internodal conduction
Atrioventricular junction
Intraventricular conduction system
Left bundle branch
Anterior fascicle
Posterior fascicle
Right bundle branch
Purkinje fibers
Despite previous controversy about the existence of specialized conduction pathways connecting the SA node to the atrioventricular (AV) node, electrophysiologists agree that preferential conduction unequivocally exists and that spread of activation from the SA node to the AV node follows distinct routes by necessity because of the peculiar geometry of the right atrium. The orifices of the superior and inferior cava, fossa ovalis, and ostium of the coronary sinus divide the right atrium into muscle bands, limiting the number of routes available for internodal conduction. These routes, however, do not represent discrete bundles of histologically specialized internodal tracts comparable to the ventricular bundle branches.
The AV junction corresponds anatomically to a group of discrete, specialized cells that are morphologically distinct from working myocardium and divided into a transitional cell zone, compact portion, and penetrating AV bundle (ie, His bundle).
The mechanisms of cardiac arrhythmias are broadly classified as focal mechanisms that include automatic or triggered arrhythmias or as reentrant arrhythmias ( Box 3.2 ). Cells that display automaticity lack a true resting membrane potential and instead undergo slow depolarization during diastole. Diastolic depolarization results in the transmembrane potential becoming more positive between successive action potentials until the threshold potential is reached, producing cellular excitation. Cells possessing normal automaticity can be found in the SA node, subsidiary atrial foci, AV node, and His-Purkinje system.
Focal mechanisms
Automatic
Triggered
Reentrant arrhythmias
Normal automaticity
Sinoatrial node
Subsidiary atrial foci
Atrioventricular node
His–Purkinje system
Triggered mechanisms occur from repetitive delayed or early afterdepolarizations
Reentry
Unidirectional block is necessary
Slowed conduction in the alternate pathway exceeds the refractory period of cells at the site of unidirectional block
Diagnosis of the underlying mechanisms of the arrhythmia may require invasive electrophysiologic testing. Studies involve percutaneous introduction of catheters that provide electrical stimulation and record electrograms from various intracardiac sites. Initial recording sites often include the high right atrium, bundle of His, coronary sinus, and the right ventricle. The catheters are most often introduced through the femoral vessels under local anesthesia. Systemic heparinization is required, particularly when catheters are introduced into the left atrium or left ventricle. The most common complications from electrophysiologic testing are those associated with vascular catheterization. Other complications include hypotension (1% of patients), hemorrhage, deep venous thrombosis (0.4%), embolic phenomena (0.4%), infection (0.2%), and cardiac perforation (0.1%). Proper application of adhesive cardioversion electrodes before the procedure facilitates rapid cardioversion-defibrillation in the event of persistent or hemodynamically unstable tachyarrhythmia resulting from stimulation protocols.
The approach to the care of patients undergoing percutaneous therapies for supraventricular arrhythmias involves similar principles ( Box 3.3 ). Anesthesiologists must be familiar with preoperative electrophysiology study (EPS) results and the characteristics of associated supraventricular arrhythmias (eg, rate, associated hemodynamic disturbances, syncope), including treatments. Tachyarrhythmias may recur at any time during surgical and percutaneous treatments. Transcutaneous cardioversion-defibrillation adhesive pads are placed before anesthesia induction and connected to a defibrillator-cardioverter. Development of periprocedural tachyarrhythmias is unrelated to any single anesthetic or adjuvant drug.
Familiarity with electrophysiologic study results and associated treatments
Transcutaneous cardioversion-defibrillation pads placed before induction
Hemodynamically tolerated tachyarrhythmias treated by slowing conduction across accessory pathway rather than the atrioventricular node
Hemodynamically significant tachyarrhythmias treated with cardioversion
Avoidance of sympathetic stimulation
Treatment of hemodynamically tolerated tachyarrhythmias is aimed at slowing conduction across the accessory pathway rather than the AV node. Therapy directed at slowing conduction across the AV node (eg, β-adrenergic–blocking drugs, verapamil, digoxin) may enhance conduction across accessory pathways and should be used only if proved safe by a prior EPS. Recommended drugs include amiodarone and procainamide. One consideration is that antiarrhythmic drugs may interfere with electrophysiologic mapping. Hemodynamically significant tachyarrhythmias developing before mapping are usually treated with cardioversion.
Accessory pathway ablation is typically performed under conscious sedation, and general anesthesia is reserved for selected patients such as those unable to tolerate the supine position.
Droperidol depresses accessory pathway conduction, but the clinical significance of small antiemetic doses is likely minimal. Opioids and barbiturates have no proven electrophysiologic effect on accessory pathways and are safe in patients with Wolff-Parkinson-White (WPW) syndrome. Normal AV conduction is depressed by halothane, isoflurane, and enflurane, and preliminary evidence suggests that these volatile anesthetics may also depress accessory pathway conduction. The major goal of managing supraventricular ablative procedures is to avoid sympathetic stimulation and the development of tachyarrhythmias. An opioid-based anesthetic technique with supplemental volatile anesthetics is typically used.
Anesthesiology teams are increasingly asked to care for patients undergoing catheter-based ablative procedures for atrial fibrillation. Monitored anesthesia care may be possible in some situations, but general anesthesia is typically chosen because of the duration of the procedure and the demand for no patient movement during critical lesion placement.
The choice of anesthesia depends on the patient's physical status, including comorbid conditions and ventricular dysfunction. General anesthesia with high-frequency jet ventilation (HFJV) can minimize thoracic excursion during respirations, which increases catheter-tissue contact. HFJV necessitates the use of intravenous anesthesia, which usually consists of a propofol infusion combined with a short-acting narcotic infusion such as remifentanil. HFJV risks include pneumothorax, barotrauma, inadequate ventilation or oxygenation, respiratory acidosis, pneumomediastinum, gastric distension, and aspiration.
Left atrial appendage (LAA) thrombus must be excluded with transesophageal echocardiography (TEE) before proceeding with catheter-based ablation. During catheter-based ablation of atrial fibrillation, patients undergo direct arterial pressure and esophageal temperature monitoring. Acute increases in esophageal temperature of only 0.1°C are communicated to the electrophysiologist. Immediately terminating RF energy and cooling the catheter tip with intraprobe saline at room temperature limit the spread of myocardial heating.
Because heparin is administered during the procedure, the activated clotting time is monitored. Constant vigilance is mandated for pericardial tamponade, and immediate transthoracic echocardiography should be performed when abrupt hypotension develops. Percutaneous pericardial drainage is emergently performed, which typically restores blood pressure. Continued collection of pericardial blood after protamine reversal of heparin anticoagulation may necessitate transfer of the patient to the operating room for a sternotomy and repair of the atrial defect.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here