Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Burns can be a devastating injury that is nondiscriminatory, affecting individuals of all ages, races, and socioeconomic backgrounds. In the United States the incidence of burn injuries continues to rise, with approximately half a million individuals requiring medical therapy, 40,000 hospital admissions, and more than 3000 deaths annually. The sequelae of burn injuries place a heavy financial burden on the national economy. As critical care management continues to improve, an increasing number of individuals with severe burns are surviving their initial injuries. Thus, there is an increasing need for plastic surgeons to provide appropriate acute burn care as well as reconstruction to help these individuals regain as much of their preinjury appearance and function as possible. This chapter will focus on the acute management of burn injuries, timing for surgical reconstruction, reconstructive options and anatomical considerations one must consider when managing patients with complex burn reconstruction needs.
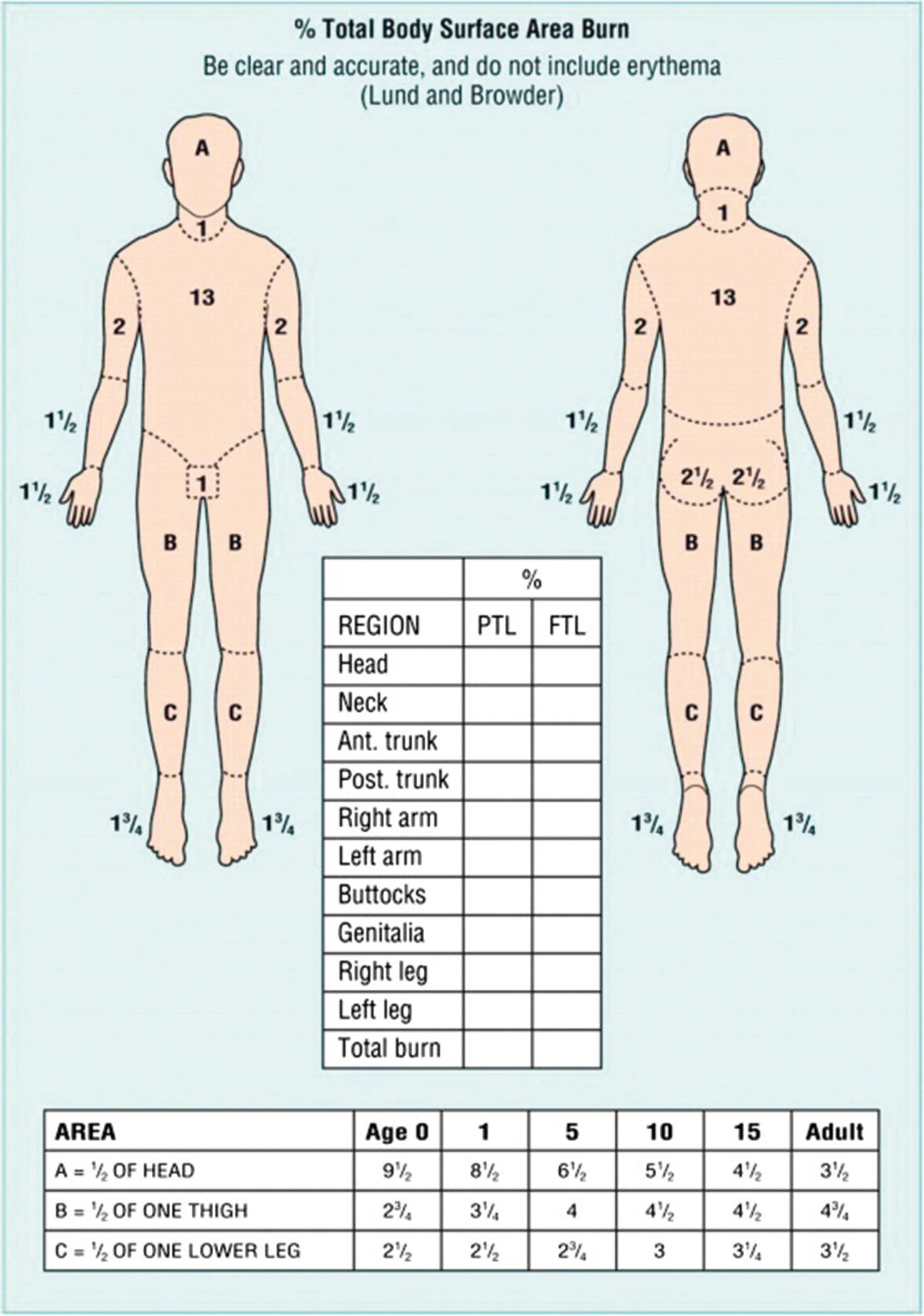
When evaluating a new burn patient, it is important to initially manage the patient by the guidelines outlined in Advance Trauma Life Support (ATLS). Once this assessment is completed attention can be turned to determining the extent and depth of the burn. The extent of a burn injury is quantified by total burn surface area (TBSA). To calculate TBSA, one can use a Lund and Browder chart, which employs the rule of 9s ( Fig. 13.1 ). There are slight variations between adults and children due to children’s heads being larger and extremities smaller when compared to adults. TBSA can also be estimated by equating the area of a patient’s hand to approximately 1% TBSA. The depth of burns can be broken down into superficial, partial thickness and full thickness. Superficial burns involve only the epidermis and do not result in blistering of the skin but are painful, with blanchable hyperemia. Partial-thickness burns are deeper burns that involve varying layers of the dermis and are classified as either superficial or deep depending on the extent of involvement. Superficial partial-thickness burns effect the papillary dermis and spare other skin appendages. This degree of burn presents with blistering of the skin and increased hyperemia but it is still blanchable. Deep partial-thickness burns involve all layers of the dermis (papillary and reticular) and physical examination will reveal decreased sensation and tissue that is pale in color. Full-thickness burns extend through the epidermis and dermis and down to subcutaneous tissue, muscle, and bone. These burns are insensate due to destruction of the nerves, nonblanchable, and typically have a leathery appearance. Additional ways to determine burn depth include examining tissue moisture, as superficial burns often appear moist whereas deeper burns appear dry. The surgeon can also look for the presence of hair follicle as deep burns will not have remnants of the hair follicle and/or the hair follicle can be removed easily upon attempt.
In our practice we typically wait approximately 3 days to allow burns to fully declare themselves as some burns may progress over time unless the patient has obvious large regions of full-thickness eschar. This thought process is in part due to the Jackson burn model, which describes the distinct zones within the burned area. The central aspect of the burn is classified as the zone of coagulation, which represents the most severely damaged portion of the wound. This area of the burn is nonsalvageable and should be treated with early excision and grafting. The tissue surrounding the zone of coagulation is the zone of stasis. This region represents damaged tissue and underlying vasculature and may be salvageable with appropriate fluid resuscitation. The outer region is the zone of hyperemia and represents tissue that should recover. The hyperemia is due to increased blood flow secondary to vasodilation. Similar to the zone of stasis, appropriate resuscitation is key to this tissue surviving. Once the extent of the patient’s burns is evaluated, if the patient is not at a designated burn center it is important to determine whether transfer to a burn center is required.
Burns result in damage to varying degrees of the skin and subcutaneous layers as already mentioned. The depth of the burn depends on multiple factors such as the thickness of the tissue in that area, the mechanism of injury (thermal, electrical or chemical) and the duration of the resulting injury. Thermal injury results in damage to the blood vessels in the skin and subsequent thrombus formation in the vessels. There is also damage to the surrounding tissue leading to local release of inflammatory mediators such as tumor necrosis factor-alpha, interferon-gamma, and numerous interleukins with the development of subsequent edema. The loss of proteins can lead to fluid shifts and fluid resuscitation is a key component of the acute resuscitative management. This will be discussed in detail later in this chapter. Loss of the skin, which is a protective barrier to the body, can also result in problems with thermoregulation.
The mechanism of burns can typically be classified as thermal, chemical or electrical in nature. The majority of burns are thermal burns. These typically can be classified as either wet burns secondary to hot liquids or dry burns as a result of fire injury. Electrical burns are typically associated with work injuries, result in deeper tissue injury, and represent a small percentage of all burns that present to burn centers. Unlike thermal burns, TBSA does not always represent the extent of the patient’s injury in electrical burns. When evaluating electrical burns, it is important to know the voltage involved, resistance, and type of current as well as determine the entrance and exit wounds. The extent of the injury depends on the resistance of the tissue involved. Electricity will pass through tissues with low resistance (i.e., nerves, vessels, muscle) resulting in less heating of the adjacent tissue but can lead to cell rupture due to electrical current driving water into cell membranes leading to electroporation. Bone, on the other hand, has high resistance and as a result electricity will not pass through it. Instead the electricity travels along the bone, which heats to a higher temperature then surrounding tissue causing tissue/muscle injury. Electrical burns do not only damage tissues but they can lead to cardiac arrhythmias, injury to solid organs and the gastrointestinal tract, and lead to possible compartment syndrome in extremities due to the deeper tissue/muscle injury as a result of the electricity traveling along the bone. Electrocardiogram monitoring is therefore important following electrical burns.
Due to the fact that TBSA may underrepresent the extent of damage sustained in an electrical burn, the Parkland formula may not provide adequate resuscitation. The Parkland formula can be used as a starting point but urine output (1 mL/kg per hour) should be used to evaluate resuscitation. When examining the current involved in an electrical burn this can typically be classified as either low voltage (<1000 V) or high voltage (>1000 V). Low voltage electrical burns typically occur within buildings and have more localized damage. High voltage injuries typically involve greater tissue damage and organ damage.
Due to the extensive thermal injury that can occur as the electrical current travels along the bone it is important to evaluate for compartment syndrome in affected extremities. Compartment syndrome is typically recognized by the 6 Ps: paresthesia, pain out of proportion, poikilothermia, pallor, pulselessness, and paralysis. Compartment pressures should be measured in limbs with concern and if the pressure is >30 mmHg, compartment release should be considered. Additionally, the extent of injury often progresses beyond the initial 3 days and thus we typically wait closer to 1 week before performing definitive closure with skin grafts to minimize graft loss following compartment release. Electrical injury frequently effects muscles and can lead to rhabdomyolysis. In patients with myoglobinuria it is important to check creatine kinase. The treatment for rhabdomyolysis is fluid resuscitation with a goal urine output of 2 mL/kg per hour. Increased excretion of myoglobin through the urine can also be done with mannitol or bicarbonate to alkalize the urine.
Chemical burns are the least frequent of burn injuries that present to burn centers. A majority of chemical burns occur at the workplace. The type of chemical determines the mechanism of injury as well as the treatment options. Most chemical injuries can be classified as acid, alkali, or organic compounds. When a patient presents to the emergency room with concern for a chemical burn it is important to remove any clothing on the patient that has been contaminated by the compound in order to prevent further injury. It is also very important to protect yourself from injury from the chemical so personal protective equipment is necessary. For the majority of chemical burns, once all clothing is removed it is important to thoroughly irrigate the burn to remove the inciting agent. This is true for most but not all agents. Potassium, sodium, and lithium can result in an explosion when these compounds come in contact with water and thus irrigation should be avoided.
Acid burns are typically limited to the area that was exposed and the resultant injury is caused by coagulation necrosis. Hydrofluoric acid, commonly used for glass etching, is a unique example as it is a weak acid but the fluoride ion is a noxious element. Hypocalcemia can occur due to the fluoride ions chelating calcium ions with serious consequences for the patient. Treatment of hydrofluoric acid burn is topical calcium gel to neutralize the fluoride ion. Fertilizer, drain cleaners, and wet cement are examples of alkali compounds. Unlike acid burns which are usually self-limiting, alkali burns can effect deeper tissues as they will continue to denature proteins and cause liquefaction necrosis until the inciting agent is completely removed. Finally, organic compounds, such as petroleum or phenol, can affect cell membranes as well as have toxic effects on organs. Phenol should be wiped off with polyethylene glycol or ethyl alcohol soaked sponges as the agent is water-insoluble. Phenol is associated with liver toxicity and can lead to cardiac arrhythmias.
During the initial evaluation of a burn patient it is important to determine the mechanism of injury. In patients who have sustained thermal injury, specifically as a result of fires in closed spaces, evaluation for inhalation injury is very important. Studies have shown that inhalation injury can increase the mortality associated with burn injuries by 20%. Signs of inhalation injury typically include facial burns, singed nasal hairs, ashy sputum, and hoarseness. In patients where there is concern for possible inhalation injury it is important to perform early bronchoscopy to evaluate the patient’s airway and to suction out any particulate matter. We often send quantitative cultures from each lung at the time of admission for intubated patients given the high incidence of community acquired pneumonia. Pneumonia in the setting of inhalation injury has been shown to increase mortality by 60%. If there is evidence of airway injury, which can present as edema or erythema caused by superheated air or the presence of soot, it is important to intubate early to protect the airway. Multiple bronchoscopies with suction should be performed if large amounts of particulate matter remain. Intubation at the time of respiratory collapse can be extremely challenging.
In addition to physical injury due to inhalation of smoke and superheated air, there is also a risk of carbon monoxide poisoning. Patients will present with altered mental status and/or agitation and typically have hyperemic mucous membranes. This is due to carbon monoxide’s higher affinity for hemoglobin than oxygen. More specifically, carbon monoxide has an approximately 200 times higher affinity for hemoglobin. It is important to check the patient’s carboxyhemoglobin level to rule in or out carbon monoxide poisoning, the patient should be given 100% oxygen to help outcompete the carbon monoxide for binding sites on the hemoglobin as 100% oxygen substantially decreases the half-life of carboxyhemoglobin.
Cyanide poisoning is another complication of smoke inhalation. Hydrogen cyanide can be found in paints, plastics, and other household materials that can be combusted during a fire. Cyanide inhibits oxygen utilization in the electron transport chain due to it binding to the terminal cytochrome thus preventing cytochrome c oxidase function. Presentation is very similar to carbon monoxide poisoning, with patients having altered mental status in addition to alterations of the central nervous and circulatory system. There is no specific test for cyanide poisoning but decreased oxygen uptake can be calculated by measuring venous blood oxygen saturation compared to arterial blood oxygen saturation. A difference of greater than 10% suggests inadequate oxygen extraction. Treatment of cyanide poisoning is hydroxycobalamin, which chelates cyanide to cyanocobalamin. Empirical treatment of suspected cyanide poisoning is a reasonable option given the effectiveness and low toxicity of treatment. Clinicians, however, should note that this treatment causes bodily fluids, including urine and wound exudate, to turn red.
The key to the acute management of burn injuries is appropriate fluid resuscitation. Due to burn injuries damaging the protective epidermal layer, patients have extensive loss of proteins and fluid. As mentioned earlier in this chapter, during the initial phase of injury there is a substantial inflammatory response which can lead to increased capillary permeability during this period. It is important to restore interstitial volume to allow for proper organ perfusion and thus oxygenation. Fluid resuscitation is based on the Parkland formula (4 mL × %TBSA × weight (kg)), which calculates the amount of fluid that should be given in the first 24 hours. In elderly patients or patients with congestive heart failure, this formula should start closer to 2 mL × %TBSA × weight (kg). Half of this volume is given in the first 8 hours while the rest is given over the next 16 hours. When calculating TBSA, this should apply only to partial- or full-thickness burns. In adult patients the resuscitative fluid of preference is typically lactated Ringer’s solution, but in children D5 ½ lactated Ringer’s is used due to the risk of hypoglycemia given their small stores of glycogen. During the resuscitative effort, the patient’s urine output is monitored to make sure that adequate resuscitation is occurring. In adult patients an optimal volume of urine output is typically 0.5 mL/kg per hour and in children it is higher, at 1 mL/kg per hour. This should be managed on an hourly basis to avoid under- or over-resuscitation as this can have negative effects on the injured tissues. Additionally, normalization of laboratory values can also be followed, for example lactate and pH. During difficult to resuscitate scenarios, monitoring of cardiac output is recommended. Additionally, newer protocols will advise switching from crystalloid to colloid resuscitation if, during the resuscitation, fluid has been increased above the protocol several times and it is estimated that the patient will receive 6 mL × kg × TBSA.
Although the majority of morbidity associated with burn injuries is due to damage to the skin, there are significant effects to other organ systems as well. Due to the loss of a protective barrier there can be alterations in core body regulation. It is important to closely monitor patient’s temperature to maintain normothermia. Additionally, this loss of barrier function can also lead to bacterial and fungal infection. It is important to closely monitor for signs of infection and take quantitative cultures to help guide antibiotic therapy if indicated. Immediately after a burn injury there is an initial decrease in cardiac output and metabolism. This changes in the first 24–48 hours and switches to increased cardiac output and a hypermetabolic state. This produces an overall catabolic state, which necessitates increasing nutrition intake. In patients with TBSA totaling >40% it has been shown that their metabolic rates are doubled. This can remain elevated for up to 12 months following their initial injury. In order to maintain body weight following substantial burns, calorie intake should be 40 kcal per % TBSA plus 25 kcal/kg of body weight. In children suggested intake is 1800 kcal/m 2 in addition to 2200 kcal/m 2 for the area of burn. Not only is calorie intake important, patients need a high protein diet in order to properly heal their wounds. It is suggested that in order to maintain their body mass patients must increase their protein intake to 1.5–2 g/kg of body weight. There is also damage to the gastrointestinal tract, which can result in translocation of gut bacteria.
With an increasing number of individuals surviving their initial burn injuries and thus living longer, burn injuries can become a lifelong ailment. Determining the correct procedure and timing for burn reconstruction is important in order to provide these patients with the best possible outcome. Whenever an injury is sustained to the skin, the body’s natural response is to heal through scar formation and contracture. This is determined in part by the depth of injury and the amount of tension sensed across the wound. The greater the penetration of burn into the dermis, the more contraction that occurs due to higher populations of myofibroblasts as the wound heals. Recent studies have also identified the fibroblasts involved in scar formation to be located in the deeper dermis. Scar maturation can take at least 12 months if not longer to complete and the timing of reconstruction should take this into account. During the healing process, some patients will develop keloid or hypertrophic scars. In one study looking at military personnel who sustained burn injuries, the authors found that 80% of patients developed some extent of hypertrophic scarring during their healing.
When planning reconstruction for a patient, the general mantra is to consider the reconstructive ladder: choosing the simplest option to produce an acceptable reconstruction while limiting donor site morbidity. Due to the complexity of burn reconstruction, this is not always the case. In many instances the extent and location of the burn will limit your reconstructive options and more complex operations may be indicated. Most superficial and superficial partial-thickness burns can be treated with local wound care and will heal without scar. Deep partial-thickness and full-thickness burns, however, will always form a scar regardless of the treatments: surgical or healing by secondary intention.
In the acute setting, attention should first be directed to the resuscitative effort. During this period of time, burns of intermediate depth can progress to full thickness or remain at a more partial thickness injury level. Typically, burns are allowed to declare their depth over the first 3 days. An exception to this is in patients with large areas of full-thickness burn or eschar where early excision or escharotomies should be performed to prevent burn wound sepsis or compartment syndrome in circumferential burns of the extremities, chest or digits. Escharotomies can be performed at the bedside or in the operating room. Once the patient is stable, the next goal should be for excision of any dead tissue and coverage with autologous or homologous tissue. The first step is to tangentially excise dead and necrotic tissue to reveal healthy underlying tissue to create a wound bed that can successfully accept a graft. In order for a graft to survive, it requires underlying tissue with an adequate blood supply to support the graft. Excision of unhealthy damaged tissue can be performed using a Weck or Watson blade. The Versajet is an additional tool that can be used, though it is typically reserved for difficult areas such as the eyelids or hands. Once debridement is performed, meticulous hemostasis should be obtained. This can be done with thrombin spray and/or epinephrine-soaked non-stick gauze followed by compression.
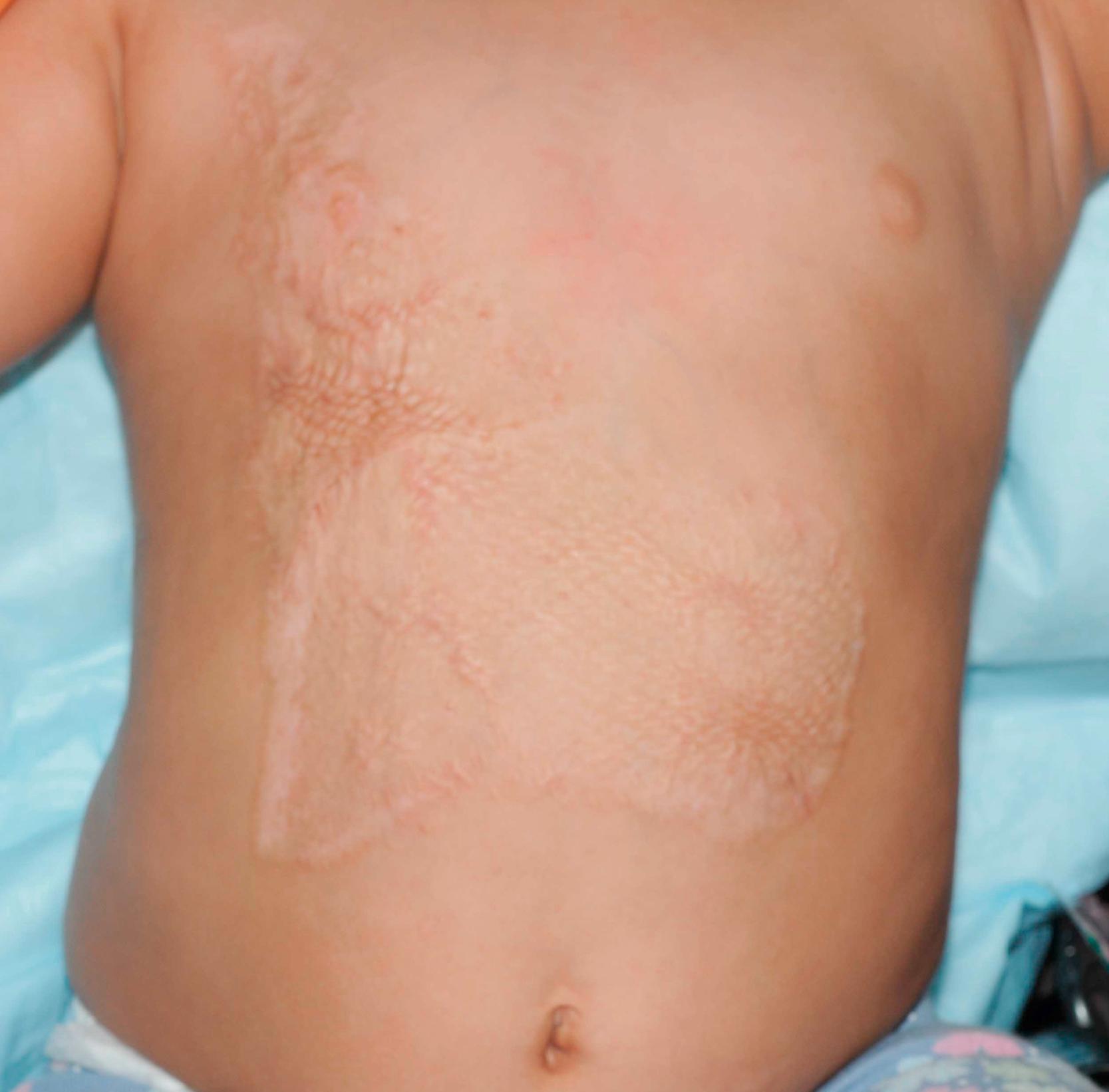
Options for grafting are variable and will be discussed in detail later in this chapter. The optimal graft choice greatly depends on the quality of the underlying wound bed to allow for the best opportunity for the graft to survive and thus a successful reconstruction. Early coverage done in the acute setting is performed to decrease the need for further reconstruction in the future. The thickness of the grafts harvested and how much they are stretched or meshed will have a long-term effect on the final outcome. For example, the mesh appearance of the graft is almost impossible to completely eliminate. Additionally, hypertrophic scars are often more prominent in those with widely meshed and Meek grafts due to the need of the interstices to heal in secondarily ( Fig. 13.2 ). We prefer sheet grafts for small areas or areas that are visible, especially in young healthy patients. In adults our practice is to typically harvest skin grafts that are 0.012 inches thick. If grafting to the face or hand, we use thicker grafts measuring 0.018 inches. In children, thinner grafts are used, measuring 0.008–0.010 inches.
When autografting is not a possibility either due to the quality of the recipient site or lack of donor sites there are several alternatives available to help bridge this period. Allografting/homografting is the use of human cadaveric skin which is a useful option when: (1) the burn surface area is too large to cover with autograft; (2) the wound bed is not ready for autograft as is the case in deep burns, elderly, diabetic, and debilitated patients. Allograft can serve as a test of the wound bed prior to harvesting a donor site and to allow for granulation tissue to grow in. For these patients, the allograft can be incorporated and then, after granulation tissue is established, the allograft can be removed and replaced with autograft with minimal additional debridement. This is especially useful in instances of malnourished or older, debilitated patients where debridement is down to adipose tissue. Xenografting is the use of graft from another species, usually porcine, which can be used in burns that are superficial partial thickness and would heal secondarily without needing an autograft. This additional layer can alleviate pain during dressing changes by serving as a layer between the painful skin and the wound dressing. Xenografts will begin to fall off 7 days after placement due to the lack of revascularization.
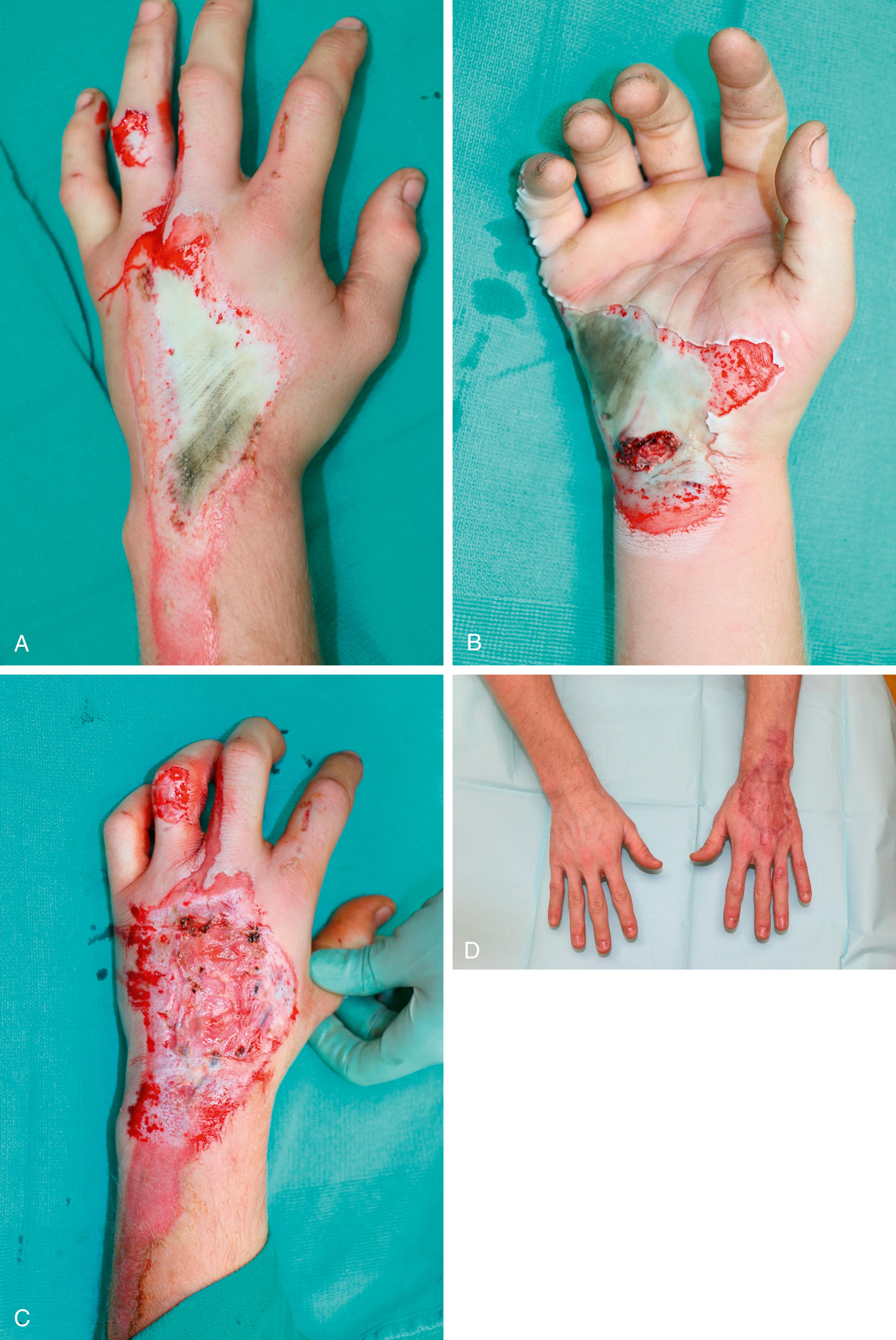
It is also important to provide coverage for exposed vital structures such as vasculature, tendons, etc. In the acute setting, this can be accomplished by several different methods depending on the extent of the injury and quality of surrounding tissues. In healthy wound beds requiring coverage of vital structures, Integra (Integra LifeSciences, Princeton, NJ, USA) , a dermal substitute, can be used which consists of a bilayer configuration of bovine collagen and glycosaminoglycans from shark forming a decellularized matrix with a semipermeable silicone membrane. This allows for fibroblasts to infiltrate the matrix to begin to form a neodermis. Revascularization of the neodermis takes approximately 2–3 weeks at which point it can be covered with a thin split-thickness skin graft (0.008–0.010 in). Integra is a useful temporizing dressing in traction or high energy burns where the depth is not yet known. Integra allows for surgeons to provide coverage over structures that would not typically accept a graft such as tendon without healthy overlying paratenon. In these cases, it is important to aggressively debride all necrotic tissue as well as a small rim of skin around the tendon ( Fig. 13.3 ). Amniotic membrane-based tissue substitutes can also be used to treat exposed tendon and will be discussed in detail later in this chapter. Similar to Integra, the success of these biologics depends on adequate debridement.
Recent advances in burn treatment have resulted in the addition of Epicel (Vericel, Cambridge, MA) and ReCell (Ativa Medical, Valencia, CA), which employ autologous cultured skin cells for grafting. Epicel is approved for patients with TBSA >30% which is comprised of deep dermal or full-thickness burns. A full thickness biopsy of the patient’s skin is needed and in approximately 17 days 96 grafts can be generated. ReCell similarly uses autologous cultured skin cells in the form of a spray-on suspension that is indicated for acute partial-thickness burns or in adjunct with meshed autographs for full-thickness burns.
In areas where there is unaffected surrounding tissue, local flaps (i.e., V–Y advancement, island advancement, rotation flaps or rhomboid flaps) can be used to advance or rotate healthy tissue to cover exposed structures. However, in the acute period, the zone of injury is usually beyond the immediate wound and thus early grafting with subsequent adjacent tissue rearrangements is preferred. In instances where the wound bed is not amenable to dermal substitutes or local flap options, free tissue transfer can be considered to bring in healthy vascularized tissue. Though not common, this is often required for burns with exposed bone.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here