Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The introduction of mechanical ventilation for the management of premature infants with severe respiratory distress syndrome (RDS) in the 1960s changed the natural course of the disease, resulting in increased survival of smaller and sicker infants, many of whom had severe chronic lung damage. Northway and associates were the first to describe this condition in 1967 and introduced the term bronchopulmonary dysplasia (BPD). All these infants had severe respiratory failure and received prolonged mechanical ventilation with high airway pressures and fraction of inspired oxygen (FiO 2 ). Since then, the advancements in the field of neonatal and perinatal medicine have resulted in increasing survival of extremely preterm infants and evolution in the clinical presentation of BPD to a milder form of chronic lung disease.
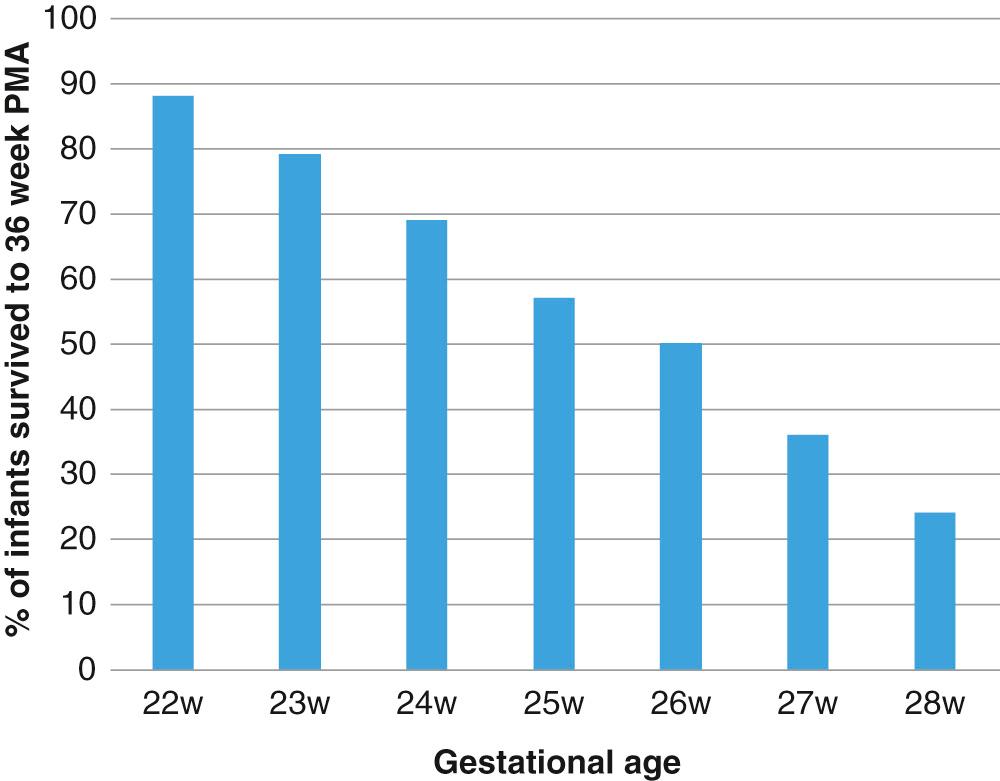
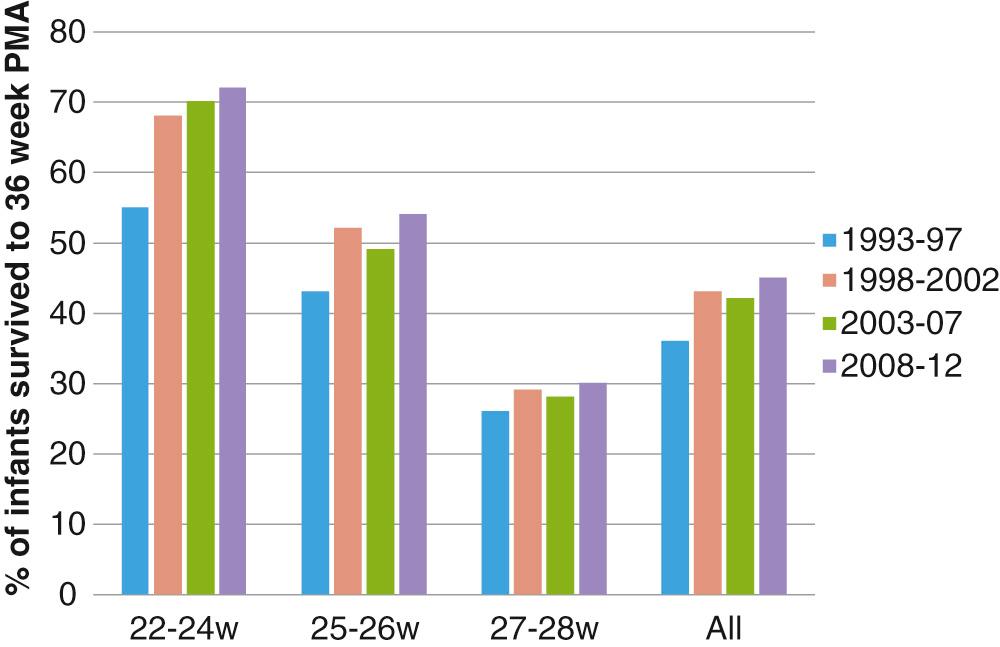
BPD continues to be the most important respiratory complication of preterm birth, and it is associated with long-term respiratory morbidities. Prematurity is the most important risk factor for BPD, with infants born at less than 28 weeks’ gestation being at greatest risk ( Fig. 69.1 ). According to the data from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN), the incidence of BPD in this group decreased from 45% in 2000 to 40% in 2008, with some increase in incidence of BPD over the last few years. The stable or increasing incidence of BPD is likely due to increasing survival of extremely preterm infants, with more infants surviving with milder forms of BPD as compared to before ( Fig. 69.2 ). The incidence of BPD varies widely among different centers not only due to differences in clinical practices and population characteristics but also according to the definition used.
Since Northway's first description of BPD for the clinical and radiographic course of a group of preterm infants with severe respiratory failure, the definition of BPD has been modified multiple times to reflect changes in patient population and to better predict pulmonary outcomes. Initial definitions of BPD consisted of oxygen use along with radiographic pictures consistent with BPD at 28 days. With increasing survival of more immature infants over time, the implication of oxygen use at 28 days was questioned, and oxygen requirement at 36 weeks was shown to be more accurate predictor of long-term pulmonary outcomes. In 2001, a workshop conducted by the National Institutes of Health (NIH) proposed a severity-based definition of BPD into three categories based on the duration and level of oxygen therapy required ( Table 69.1 ). The assessment of severity of BPD adds richness to the outcome measure and identifies a spectrum of adverse pulmonary and neurodevelopmental outcomes. As the severity of BPD increases, the incidence of adverse events also increases. The use of supplemental oxygen as a criterion to define severity of BPD has some limitations. The use of supplemental oxygen in these infants is often inconsistent and is not based on a physiologic assessment, which can greatly affect the reported incidence of BPD. To overcome this inconsistency, a physiologic test to standardize the need for supplemental oxygen has been used. Some of the other limitations of the current definition of BPD include failure to classify infants with nonpulmonary causes such as central apnea or airway complications for need for respiratory support, inability to classify infant dying secondary to severe respiratory failure prior to 36 weeks, or failure to appropriately categorize infants on more contemporary modes of respiratory support like high flow nasal cannula. Definitions of BPD are currently evolving in an attempt to categorize infants by severity of disease based on level of support.
| Gestational Age | <32 Weeks | ≥32 Weeks |
|---|---|---|
| Time point of assessment | 36 weeks' PMA or discharge to home, whichever comes first | >28 days but <56 days' postnatal age or discharge to home, whichever comes first |
| Treatment With >21% Oxygen for at Least 28 Days PLUS | ||
| Mild BPD | Breathing room air by 36 weeks' PMA or discharge, whichever comes first | Breathing room air by 56 days' postnatal age or discharge, whichever comes first |
| Moderate BPD | Need for <30% oxygen at 36 weeks' PMA or discharge, whichever comes first | Need for <30% oxygen at 56 days' postnatal age or discharge, whichever comes first |
| Severe BPD | Need for ≥30% oxygen and/or positive pressure (PPV or NCPAP) at 36 weeks' PMA or discharge, whichever comes first | Need for ≥30% oxygen and/or positive pressure (PPV or NCPAP) at 56 days' postnatal age or discharge, whichever comes first |
BPD, as described originally by Northway and colleagues, resulted from injury to developing lung secondary to prolonged mechanical ventilation with high airway pressures and inspired oxygen concentration. In contrast, BPD currently is mostly seen in extremely preterm infants in late canalicular or early saccular stage of lung development at birth. Multiple pathogenic factors including pre- and postnatal infections, mechanical trauma from positive pressure ventilation, oxygen toxicity, and pulmonary edema secondary to increased pulmonary blood flow from patent ductus arteriosus (PDA) acts on the immature alveolar and vascular structure of the immature lung. The complex interplay of lung development, injury by pathogenic factors, and reparative processes results in the morphologic and functional lung alterations characteristic of BPD ( Fig. 69.3 ).
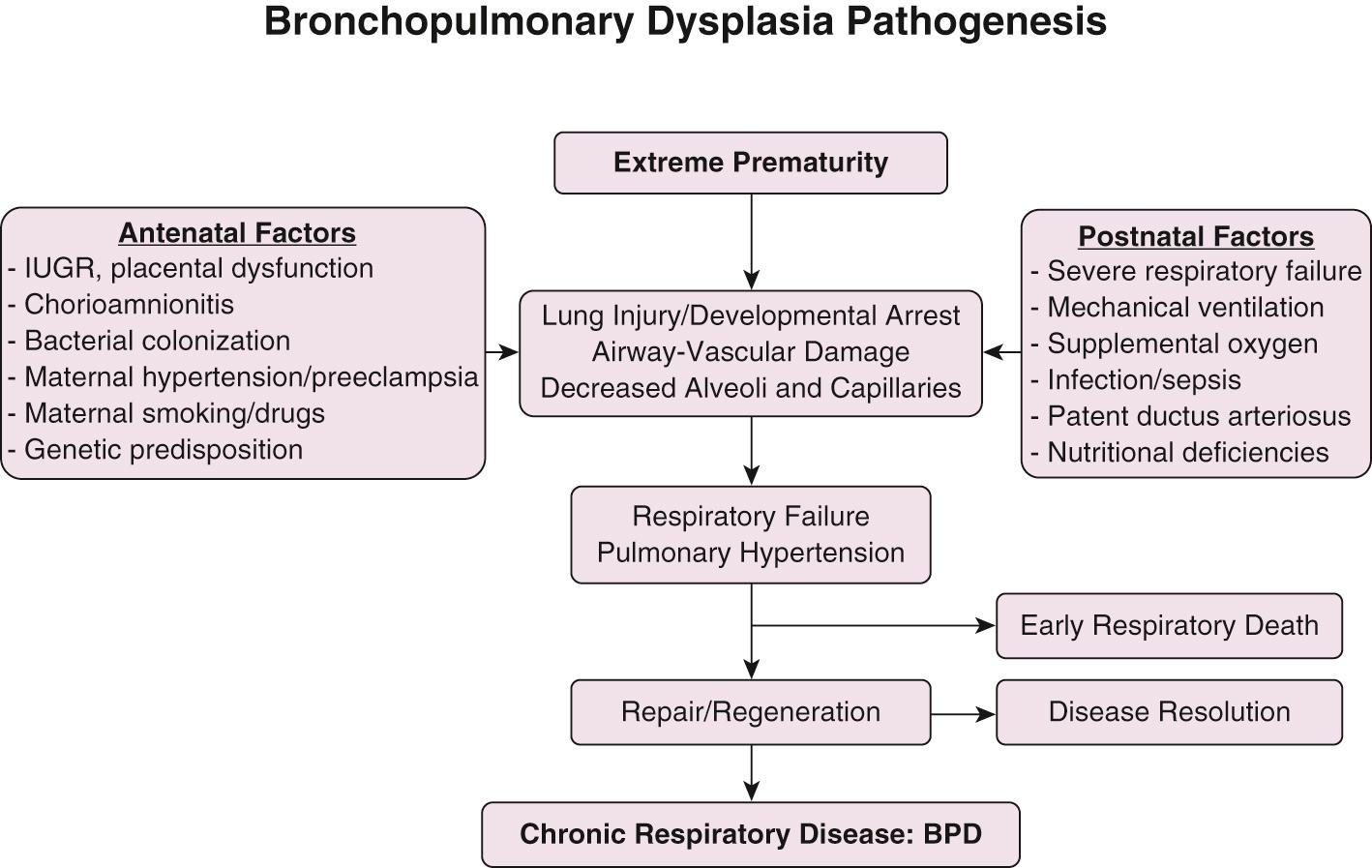
Prematurity is the most important risk factor for development of BPD. The prevalence of BPD is inversely related to gestational age and birth weight, strongly suggesting that incomplete development of the lungs plays a critical role in the pathogenesis of BPD. Epidemiologic studies have identified male gender, white race, and maternal smoking and hypertension as risk factors associated with increased risk for BPD. Intrauterine growth restriction is also associated with increased risk for BPD, likely secondary to impaired alveolar and vascular growth. The role of maternal chorioamnionitis on the risk for BPD has been unclear, with multiple studies showing increased risk for BPD in infants born to mothers with evidence for chorioamnionitis; others, however, have failed to show such association. The effect of chorioamnionitis on fetal lung varies from inflammation and lung injury to enhanced lung maturation depending on fetal inflammatory response, the organism causing the infection, and the severity or duration of infection.
Although high-peak inspiratory pressure is one of the factors implicated as a cause of BPD, it is difficult to separate the role of the high pressures from the underlying lung disease on the chronic lung damage. The damaging effect of high airway pressure and tidal volume on the surfactant-deficient lung was demonstrated in preterm lambs. Lung compliance was decreased after only a few breaths, with excessive tidal volumes given before surfactant replacement. Experimental evidence strongly suggests that excessive tidal volumes can damage the lung, initiate an inflammatory cascade, and interfere with normal lung development. The effect of prolonged ventilation was shown in newborn mice where ventilation for 24 hours resulted in increased apoptosis, inhibition of alveolarization, and angiogenesis in the lung.
Although injury from the mechanical ventilation continues to be an important factor in the pathogenesis of BPD, today most premature infants who require prolonged ventilation due to milder respiratory failure or poor respiratory effort receive lower airway pressures. Therefore, mechanical trauma appears to play a lesser role among the multiple factors acting concurrently on a premature lung to result in milder BPD.
Clinical and experimental evidence suggested that pulmonary oxygen toxicity was a major factor in the pathogenesis of the severe original BPD. Although many tissues can be injured by high oxygen concentrations, the lung is exposed directly to the highest partial pressure of oxygen. The precise concentration of oxygen that is toxic to the immature lung probably depends on a large number of variables, including gestational age, nutritional and endocrine status, and duration of exposure to oxygen and other oxidants. Although a safe level of inspired oxygen has not been established, any concentration in excess of room air might increase the risk of lung damage when administered over a long period of time.
The pulmonary changes of oxygen toxicity are nonspecific and consist of atelectasis, edema, alveolar hemorrhage, inflammation, fibrin deposition, and thickening of alveolar membranes. There is early damage to capillary endothelium in animals, and plasma leaks into interstitial and alveolar spaces. Pulmonary surfactant can be inactivated by protein in the airspaces, adding to the risk of atelectasis. Type 1 alveolar lining cells can be injured early, and bronchiolar and tracheal ciliated cells can also be damaged by oxygen. Total resolution after oxygen toxicity is possible if the initial exposure is not overwhelming.
Continued exposure to high inspired oxygen levels is accompanied by influx of polymorphonuclear leukocytes containing proteolytic enzymes. In addition, the antiprotease defense system is significantly impaired in infants exposed to prolonged high inspired oxygen levels, favoring proteolytic damage of structural elements in alveolar walls. This could be an important pathogenic factor in oxygen toxicity and BPD. High inspired oxygen concentration can also inhibit the normal process of alveolar and capillary formation in immature animals.
Although the cellular basis for oxygen toxicity has not been completely elucidated, the principal mechanisms involve the univalent reduction of molecular oxygen and formation of free radical intermediates. The latter can react with intracellular constituents and membrane lipids, thus initiating chain reactions that can cause tissue destruction.
To resist the detrimental effects of oxygen, the organism has evolved a number of antioxidant systems. Antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase seem to play an important role in preventing the toxic effects of oxygen. Other elements, such as vitamin E, glutathione, and selenium, are also part of the endogenous antioxidant mechanisms. The capacity for synthesizing these enzymes in some animal species follows a maturational trend similar to the production of surfactant; therefore, animals born prematurely have lower concentrations of antioxidant enzymes than those born at term.
Loss of mucociliary function may be an additional pathogenic factor in BPD, because exposure to high oxygen concentrations results in a reduction of ciliary movements.
There is ample evidence supporting the role of infections in the development of BPD, especially in very small infants in whom nosocomial infections are associated with a marked increase in the risk of BPD. Evidence suggests that postnatal adenovirus and cytomegalovirus infection might also increase the risk for BPD. Pulmonary colonization with ureaplasma has been evaluated as one of the possible risk factors for BPD. Experimental studies in animal models have shown that colonization of fetal lung with ureaplasma resulted in increased inflammation, fibrosis, and impaired alveolar development. Several studies have suggested an association between Ureaplasma urealyticum tracheal colonization and the development of BPD in infants with very low birth weight. Surprisingly, trials evaluating treatment with macrolide antibiotics have so far shown inconsistent effect on BPD.
There is an association between the presence of a PDA and an increased risk for BPD ( Fig. 69.4 ). This may be secondary to increased pulmonary blood flow producing an increase in interstitial fluid and a decrease in pulmonary compliance and increased airway resistance. This can prolong the need for mechanical ventilation with higher ventilatory pressures and oxygen concentrations, increasing the risk for BPD. Moreover, the increased pulmonary blood flow can damage the pulmonary capillary endothelium and induce neutrophil margination and activation in the lung, contributing to the progression of the inflammatory cascade. Data from studies in preterm baboons also showed decreased alveolarization in animals that remained for a longer time with an open ductus arteriosus, supporting the role of a persistent ductus arteriosus in the pathogenesis of BPD. Despite epidemiologic and experimental evidence of association between PDA and BPD, prospective clinical trials to evaluate early closure of the PDA have failed to show a decrease in the incidence of BPD.
![Fig. 69.4, Perinatal and postnatal risk factors for bronchopulmonary dysplasia (BPD) defined as 28 days’ duration of oxygen dependency during hospitalization. Obtained by logistic regression analysis from all extremely premature infants born at University of Miami Jackson Memorial Medical Center during the period 1995-2000. ( N = 505 alive at 28 days; birth weight [BW], 500-1000 g; gestational age [GA], 23-32 weeks). OR, Odds ratio; PDA, patent ductus arteriosus; RDS, respiratory distress syndrome. Fig. 69.4, Perinatal and postnatal risk factors for bronchopulmonary dysplasia (BPD) defined as 28 days’ duration of oxygen dependency during hospitalization. Obtained by logistic regression analysis from all extremely premature infants born at University of Miami Jackson Memorial Medical Center during the period 1995-2000. ( N = 505 alive at 28 days; birth weight [BW], 500-1000 g; gestational age [GA], 23-32 weeks). OR, Odds ratio; PDA, patent ductus arteriosus; RDS, respiratory distress syndrome.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/BronchopulmonaryDysplasiaintheNeonate/3_3s20B9780323567114000699.jpg)
Infants with BPD have a predisposition to fluid accumulation in their lungs. Possible causes are an increase in pulmonary vascular resistance, low plasma oncotic pressure, and increased capillary permeability that favor the extravascular accumulation of fluid. Pulmonary vascular pressure can be increased because of remodeling of the pulmonary vessels aggravated by hypoxemia and hypercapnia. Fluid accumulation can also be secondary to left ventricular dysfunction that has been described in patients with chronic respiratory failure. Capillary permeability might be increased secondary to the effects of high inspired oxygen concentration, mechanical trauma, increased flow caused by a PDA, and infection on the capillary endothelium. The abnormal accumulation of lung fluid in infants with BPD further compromises lung function, perpetuating a cycle in which more aggressive respiratory assistance is required, which produces further lung damage.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here