Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The contributions to this chapter by Dr. Andrew Horvai, Department of Pathology, University of California, San Francisco in the previous edition of this book are gratefully acknowledged.
Bone provides mechanical support for the body, transmits forces generated by muscles, protects viscera, provides a niche for blood cell progenitors, and is intimately involved in calcium and phosphate homeostasis. It is produced and maintained by specialized cells that elaborate and remodel the calcified matrix that makes up most of the substance of bone.
Bone matrix consists of an organic component called osteoid (35%) and a mineral component (65%), within which are embedded a variety of cells that maintain bone homeostasis. Osteoid consists predominantly of type I collagen and smaller amounts of glycosaminoglycans and other proteins. The mineral component of bone that is responsible for its hardness is the inorganic moiety hydroxyapatite . Bone matrix is synthesized in two forms, woven and lamellar bone ( Fig. 19.1 ). Woven bone is produced rapidly during fetal development and repair of fractures but has a haphazard arrangement of collagen fibers that impart less structural stability than the parallel collagen fibers found in lamellar bone. In an adult, the presence of woven bone is always abnormal, as it indicates ongoing repair of damaged bone.
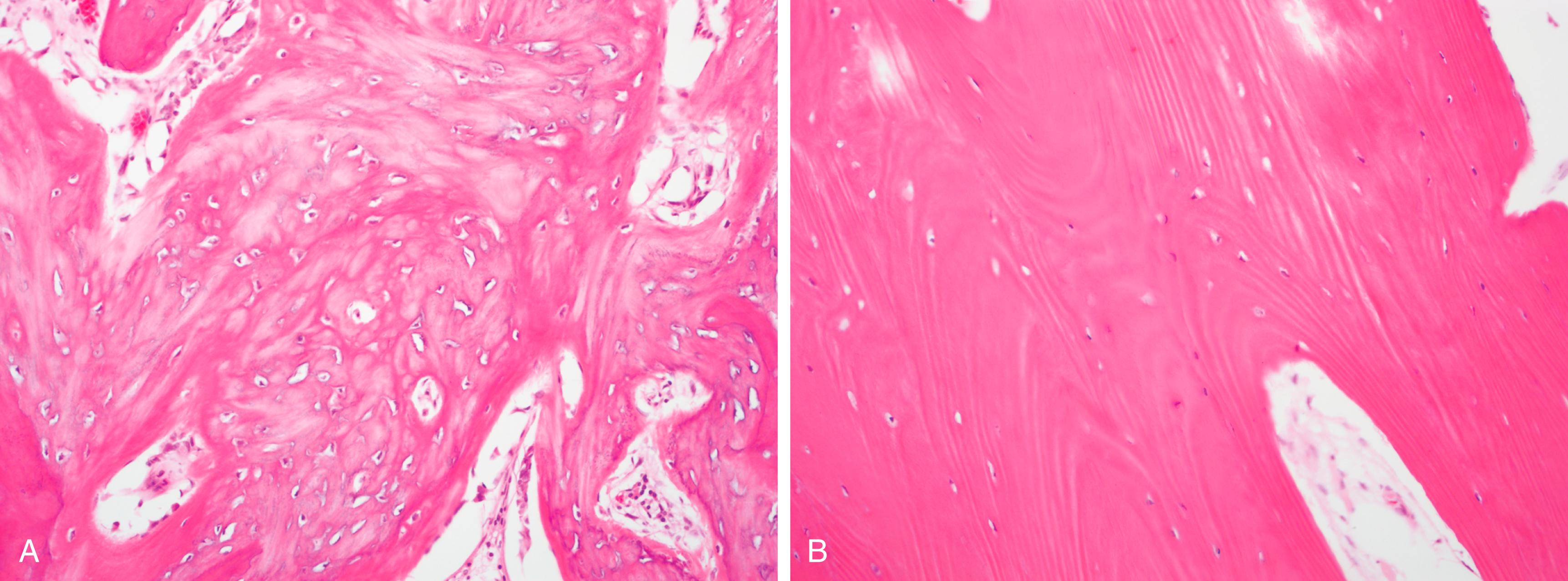
Embedded within the bone matrix are a variety of bone cells:
Osteoblasts, located on the surface of the matrix, synthesize and assemble bone matrix and regulate its mineralization ( Fig. 19.2 A ). They are derived from mesenchymal stem cells that are located under the periosteum in developing bone and in the medullary space later in life.
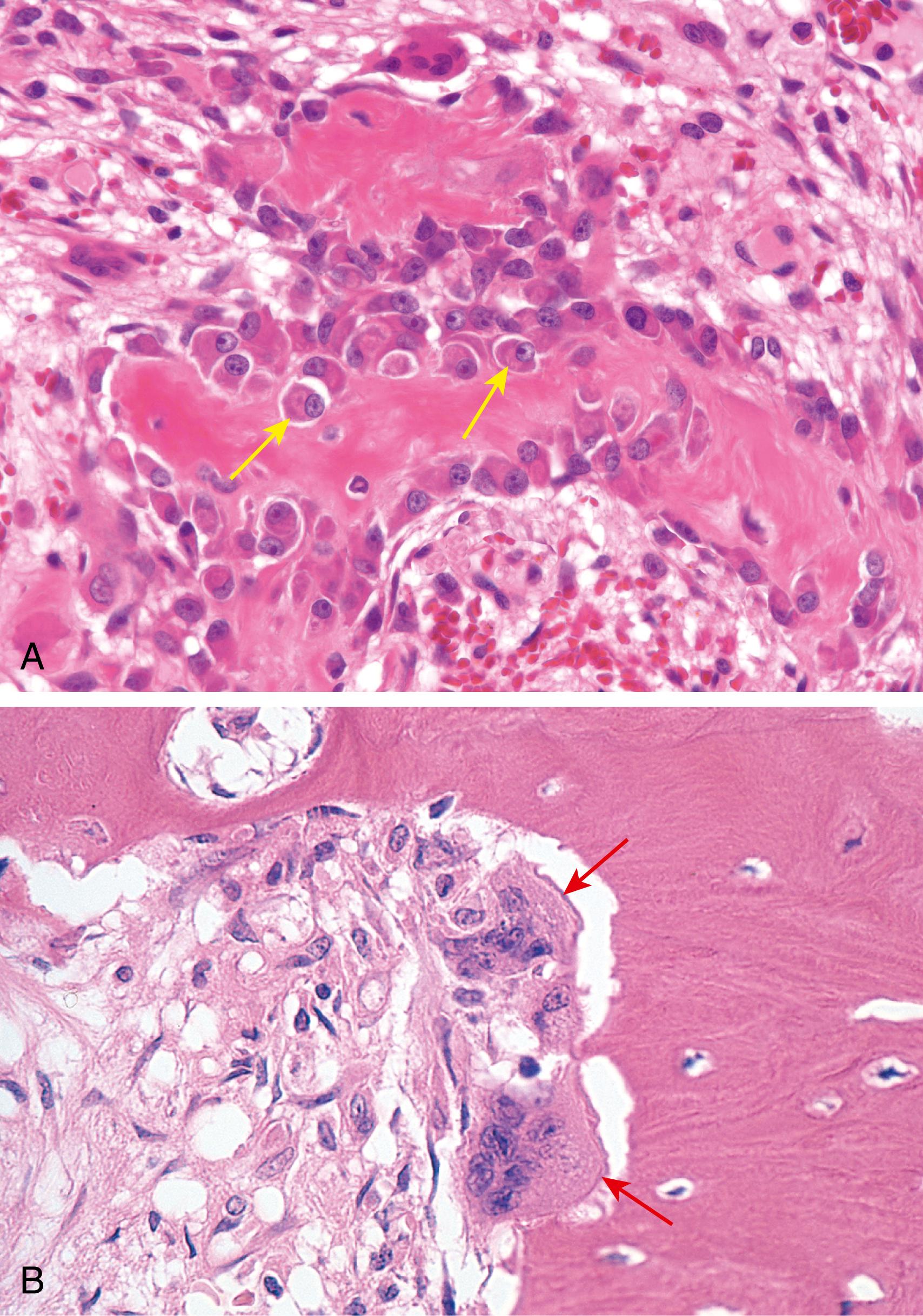
Osteocytes are derived from osteoblasts and are located within the bone; they are interconnected by an intricate network of tunnels (canaliculi) containing cytoplasmic processes. Osteocytes help control calcium and phosphate levels and detect and respond to mechanical forces by altering the remodeling of bone.
Osteoclasts , located on the surface of bone, are specialized multinucleate macrophages derived from circulating monocytes. Osteoclasts attach to proteins found in bone matrix and create a sealed extracellular trench (resorption pit) into which they secrete acid and neutral proteases (predominantly matrix metalloproteases [MMPs]), leading to bone resorption ( Fig. 19.2 B).
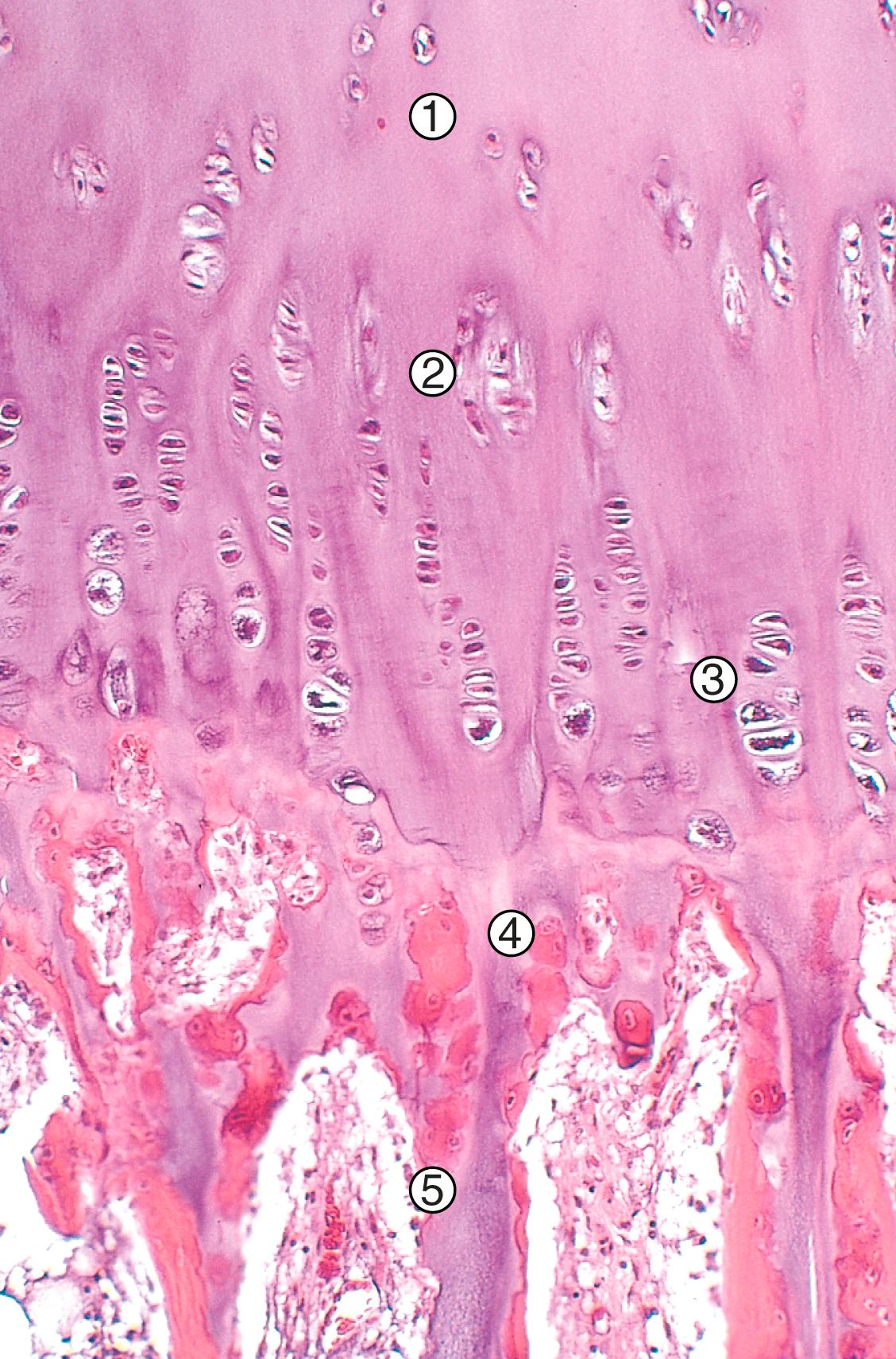
During embryogenesis, long bones develop from cartilage precursors by the process of endochondral ossification. A cartilage mold (anlage) is synthesized by mesenchymal precursor cells. At approximately 8 weeks of gestation the central portion of the anlage is resorbed, creating the medullary canal. Simultaneously at midshaft (diaphysis) osteoblasts begin to deposit the cortex beneath the periosteum, producing the primary center of ossification and initiating radial bone growth. At each end of the bone (epiphysis), endochondral ossification proceeds in a centrifugal fashion (secondary center of ossification). Eventually, a plate of cartilage becomes entrapped between the two expanding centers of ossification, forming the physis or growth plate ( Fig. 19.3 ). The chondrocytes within the growth plate sequentially proliferate, hypertrophy, and undergo apoptosis. In the region of apoptosis, the matrix mineralizes and is invaded by capillaries, providing the nutrients for osteoblasts, which synthesize osteoid. This process produces longitudinal bone growth.
Flat bones develop by a different process called intramembranous ossification. Bones of the cranium, for example, are formed by osteoblasts directly from a fibrous layer of tissue, without cartilage anlagen. The enlargement of flat bones is achieved by deposition of new bone on a preexisting surface.
The adult skeleton is constantly turning over in a tightly regulated process known as remodeling through coupled osteoblast and osteoclast activity, which together constitute the bone multicellular unit (BMU).
Remodeling is regulated by cell–cell interactions and cytokines ( Fig. 19.4 ). An important signaling pathway that controls remodeling involves three factors: (1) the transmembrane receptor activator of NF-κB (RANK), which is expressed on osteoclast precursors; (2) RANK ligand (RANKL), which is expressed on osteoblasts and marrow stromal cells; and (3) osteoprotegerin (OPG), a secreted “decoy” receptor made by osteoblasts that can block RANK interaction with RANKL. RANK signaling activates the transcription factor NF-κB, which is essential for the generation and survival of osteoclasts. Other important factors regulating remodeling include monocyte-colony stimulating factor (M-CSF), produced by osteoblasts, and WNT proteins, which are produced by various cells and trigger the production of OPG. The importance of these pathways is proven by rare but informative germline mutations in the OPG, RANK, and RANKL genes that cause severe disturbances of bone metabolism.
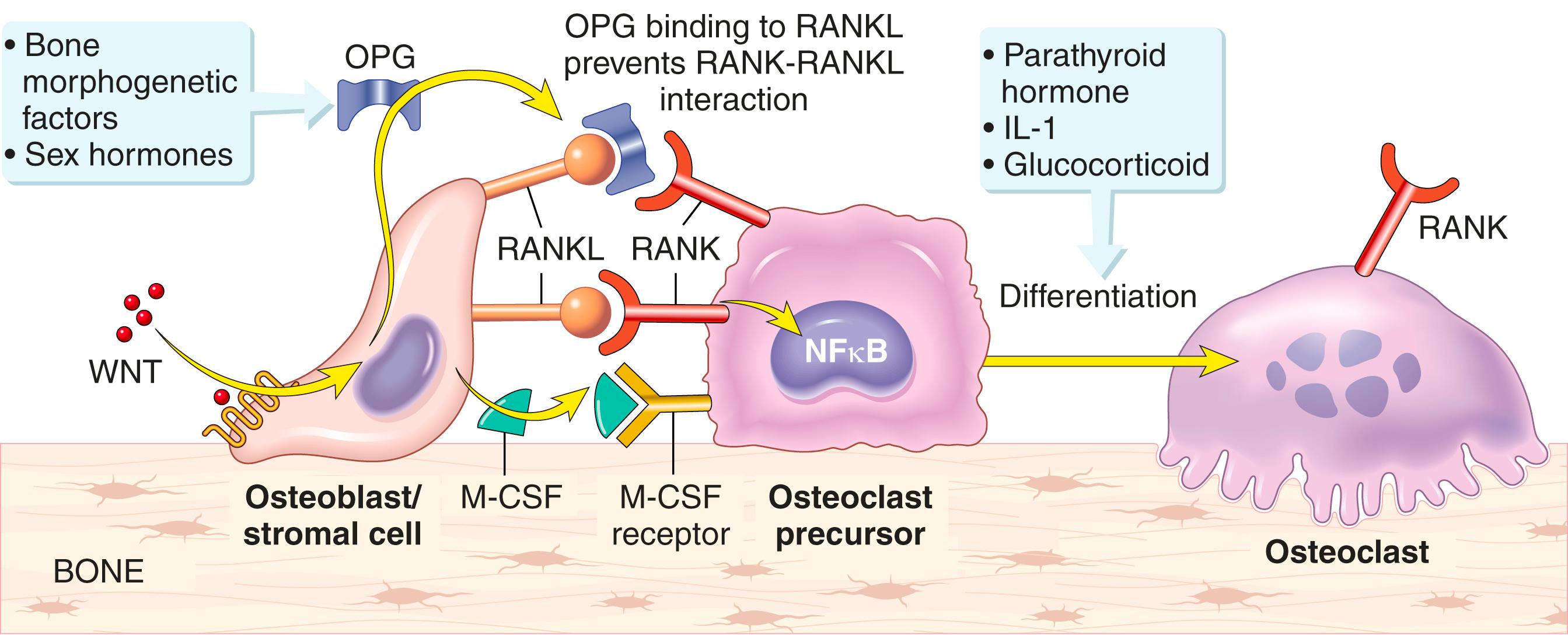
The balance between bone formation and resorption is modulated by RANK and WNT signaling. For example, because OPG and RANKL oppose one another, bone resorption or formation is favored by increasing or decreasing the RANK-to-OPG ratio, respectively. Systemic factors that influence this balance include hormones, vitamin D, inflammatory cytokines (e.g., IL-1), and growth factors (e.g., bone morphogenetic proteins [BMPs]). Through complex mechanisms, parathyroid hormone (PTH), IL-1, and glucocorticoids promote osteoclast differentiation and bone turnover. By contrast, BMPs and sex hormones (estrogen, testosterone) generally block osteoclast differentiation or activity by favoring OPG expression.
Peak bone mass is achieved in early adulthood after the cessation of skeletal growth. This set point is determined by many factors, including polymorphisms in the vitamin D receptor gene, nutrition, and physical activity. Beginning in the fourth decade, resorption exceeds formation, resulting in a decline in skeletal mass.
Developmental abnormalities of the skeleton frequently result from germline mutations and become apparent during the early stages of bone formation. The spectrum of developmental disorders of bone is broad, and there is no standard approach to their classification. Here, we categorize the major diseases according to their pathogenesis.
Developmental disorders may be caused by localized abnormalities in the migration and condensation of mesenchyme (dysostosis) or global disorganization of bone and/or cartilage (dysplasia). More than 350 skeletal dysostoses and dysplasias, most of them extremely rare, have been described. The most common dysostoses include complete absence of a bone or a digit (aplasia) , extra bones or digits (supernumerary digit), and abnormal fusion of bones (e.g., syndactyly, craniosynostosis ). Mutations in homeobox genes, genes encoding cytokines, and cytokine receptors are common causes of dysostoses. By contrast, dysplasias arise from mutations in genes that control development or remodeling of the entire skeleton (discussed below). It is important to note that as used in bone biology the term dysplasia refers to an abnormal pattern of growth rather than a premalignant lesion ( Chapter 6 ).
Achondroplasia, the most common skeletal dysplasia and a major cause of dwarfism, is an autosomal dominant disorder characterized by reduced endochondral cartilage growth. The disease is caused by gain-of-function mutations in fibroblast growth factor receptor 3 (FGFR3). FGFR3 typically inhibits endochondral growth; in achondroplasia, the receptor is constitutively active, causing a pathologic suppression of growth. Approximately 90% of cases stem from new mutations, almost all of which occur in the paternal allele. Affected individuals have shortened proximal extremities, a trunk of relatively typical length, and an enlarged head with a bulging forehead and conspicuous depression of the root of the nose. The skeletal abnormalities are usually not associated with changes in longevity or intelligence.
Thanatophoric dysplasia, the most common lethal form of dwarfism, is due to diminished proliferation of chondrocytes and disorganization in the zone of proliferation. Like achondroplasia, it is also caused by gain-of-function mutations in FGFR3 , but in thanatophoric dysplasia the mutations cause even greater increases in signaling activity. Thanatophoric dysplasia occurs in about 1 of every 20,000 live births. Affected individuals have shortening of the limbs, frontal bossing, relative macrocephaly, a small chest cavity, and a bell-shaped abdomen. The underdeveloped thoracic cavity leads to respiratory insufficiency and affected individuals usually die at birth or soon thereafter.
Osteogenesis imperfecta (OI), the most common inherited disorder of connective tissue, is a phenotypically diverse disorder caused by mutations that impair the synthesis of type I collagen. OI principally affects bone and other tissues rich in type I collagen (e.g., joints, eyes, ears, skin, and teeth). It is genetically heterogeneous, but usually results from an autosomal dominant mutation in the genes that encode either the α1 or α2 chains of type I collagen. These mutations cause misfolding of collagen polypeptides, which interferes with the assembly of wild-type collagen chains (a dominant negative loss of function).
The fundamental abnormality in OI is synthesis of too little bone, resulting in extreme skeletal fragility. Other findings include blue sclerae, as the decreased collagen content of the sclerae allows visualization of the underlying choroid, which imparts a bluish color; hearing loss related to a sensorineural deficit and impeded conduction due to abnormalities in the bones of the middle ear; and small, misshapen, blue-yellow teeth, secondary to dentin deficiency.
OI is separated into multiple clinical subtypes that vary widely in severity; while some phenotypes are uniformly fatal in utero or during the perinatal period, other variants are associated with a normal life span despite a susceptibility to fractures, particularly during childhood.
Osteopetrosis, also known as marble bone disease, refers to a group of rare genetic diseases that are characterized by reduced bone resorption and diffuse symmetric skeletal sclerosis resulting from impaired formation or function of osteoclasts. Although the term osteopetrosis refers to the stonelike quality of the bones, the bones are abnormally brittle and fracture easily.
Osteopetrosis is classified into variants based on both the mode of inheritance and the severity of clinical findings. Most mutations interfere with the process of acidification of the osteoclast resorption pit, which is required for the dissolution of calcium hydroxyapatite.
Autosomal dominant osteopetrosis is typically the mildest type of the disorder. It may not be detected until adolescence or adulthood, when it is discovered on radiographic studies done to evaluate repeated fractures. These individuals may also have mild cranial nerve deficits and anemia. The involved bones lack a medullary canal and the ends of long bones are bulbous (Erlenmeyer flask deformity) and misshapen. The neural foramina are small and compress exiting nerves. The primary spongiosa, which is normally removed during growth, persists and fills the medullary cavity, leaving no room for hematopoietic marrow resulting in anemia.
Severe infantile osteopetrosis is autosomal recessive and is often fatal due to leukopenia, despite extensive extramedullary hematopoiesis that can lead to prominent hepatosplenomegaly.
Osteopenia refers to decreased bone mass, while osteoporosis is defined as osteopenia that is severe enough to significantly increase the risk of fracture. Radiographically, osteoporosis is diagnosed when bone mass is at least 2.5 standard deviations below mean peak bone mass, whereas osteopenia is 1 to 2.5 standard deviations below the mean. The disorder may be localized to a certain bone or region (e.g., disuse osteoporosis of a limb) or generalized, involving the entire skeleton. Although osteoporosis can be secondary to endocrine disorders (e.g., hyperthyroidism), gastrointestinal disorders (e.g., malnutrition), or drugs (e.g., corticosteroids), most osteoporosis is primary.
The most common forms of osteoporosis are the senile and postmenopausal types. The following discussion relates largely to these forms of osteoporosis.
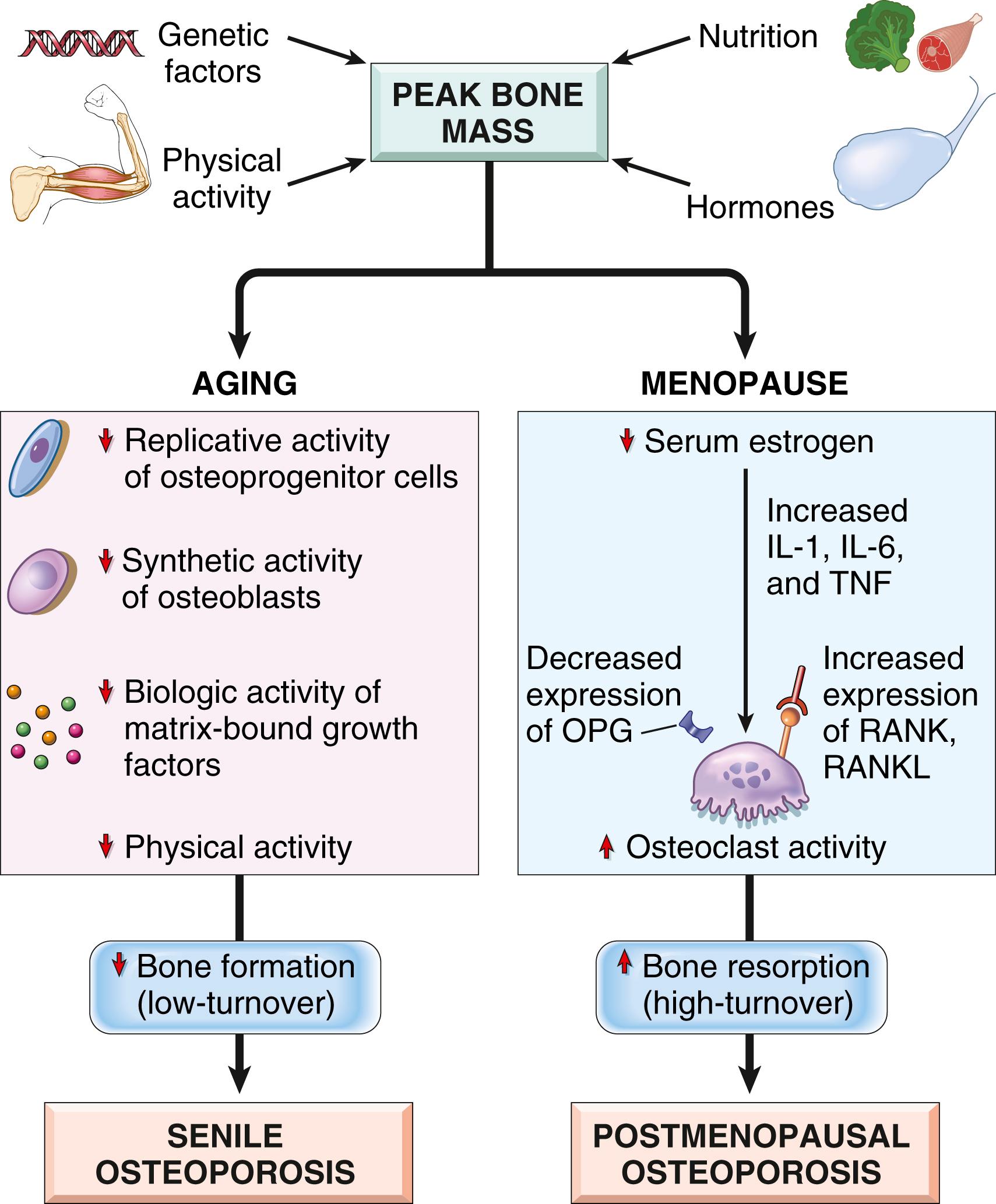
Peak bone mass is achieved during young adulthood. The height of this peak is influenced by hereditary factors, especially polymorphisms in the genes that affect bone metabolism (discussed later). Physical activity, muscle strength, diet, and hormonal state also contribute. After maximal skeletal mass is attained, bone turnover leads to an average loss of 0.7% of bone mass per year. Several factors underlie the pathogenesis of osteoporosis ( Fig. 19.5 ):
Age-related changes. Compared to younger individuals, osteoblasts from older individuals have reduced proliferative and biosynthetic potential and reduced response to growth factors, resulting in a diminished capacity to make bone. This form of osteoporosis, known as senile osteoporosis, is considered low-turnover osteoporosis.
Reduced physical activity. The decreased physical activity associated with aging results in reduced bone mass. Osteocytes respond to mechanical load by stimulating or inhibiting osteoblasts and osteoclasts to remodel normal bone, as evidenced by localized bone loss in an immobilized or paralyzed extremity or increased bone density in athletes. The type of exercise is important, as load magnitude influences bone density more than the number of load cycles. Thus, resistance exercises such as weight training are more effective stimuli for increasing bone mass than repetitive endurance activities such as bicycling.
Genetic factors. Single gene defects account for only a small fraction of osteoporosis cases. Polymorphisms in certain genes have been linked to osteoporosis by genome-wide association studies. These include sequence variants in RANK , RANKL , and OPG , all of which encode key osteoclast regulators; the HLA locus (for unknown reasons); and the estrogen receptor gene.
Calcium nutritional state. Adolescents (particularly girls) tend to have low dietary calcium intake, a factor that restricts peak bone mass. Calcium deficiency, increased PTH concentrations, and reduced levels of vitamin D may also play a role in the development of senile osteoporosis.
Hormonal influences. Estrogen promotes bone density through a variety of mechanisms including inhibiting the apoptosis of osteoblasts, stimulating the apoptosis of osteoclasts, and suppressing RANKL production. In the decade after menopause, up to 2% of cortical bone and 9% of cancellous bone may be lost each year. Estrogen deficiency plays the major role in this process, and close to 40% of postmenopausal women are affected by osteoporosis. Although decreased estrogen increases both bone formation and resorption, the latter dominates, resulting in high-turnover osteoporosis. Estrogen loss also induces increased secretion of inflammatory cytokines, such as IL-6, TNF, and IL-1, by innate immune cells in the blood and marrow through unknown mechanisms (see Fig. 19.5 ). These cytokines increase RANKL and decrease OPG, stimulating osteoclast recruitment and activity.
The hallmark of osteoporosis is a decreased quantity of histologically normal bone. The entire skeleton is affected in postmenopausal and senile osteoporosis, but certain bones tend to be more severely affected. In postmenopausal osteoporosis, the increase in osteoclast activity affects mainly bones or portions of bones with increased surface area, such as the cancellous compartment of vertebral bodies ( Fig. 19.6 ). The trabecular plates become perforated and thinned and lose their interconnections ( Fig. 19.7 ), leading to progressive microfractures and eventual vertebral collapse.
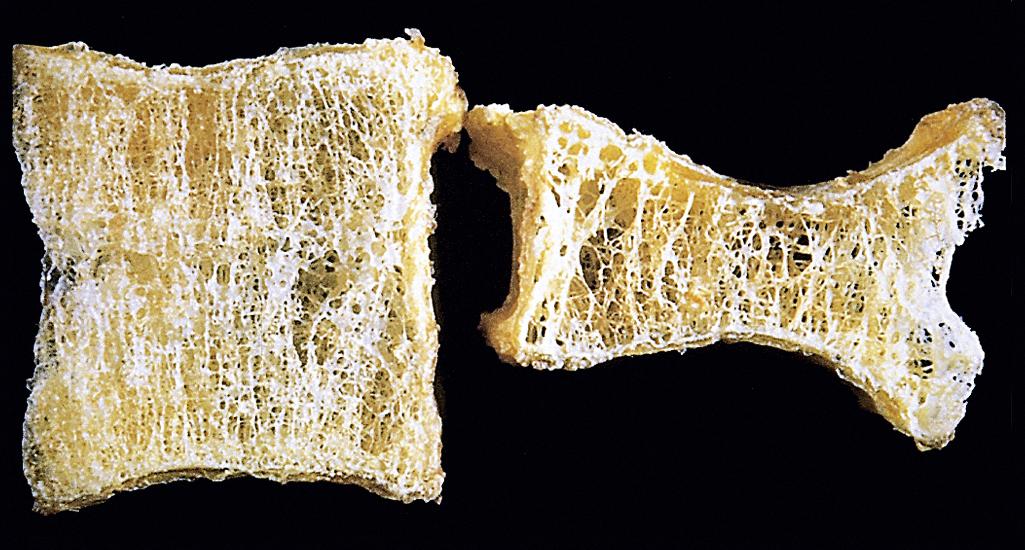
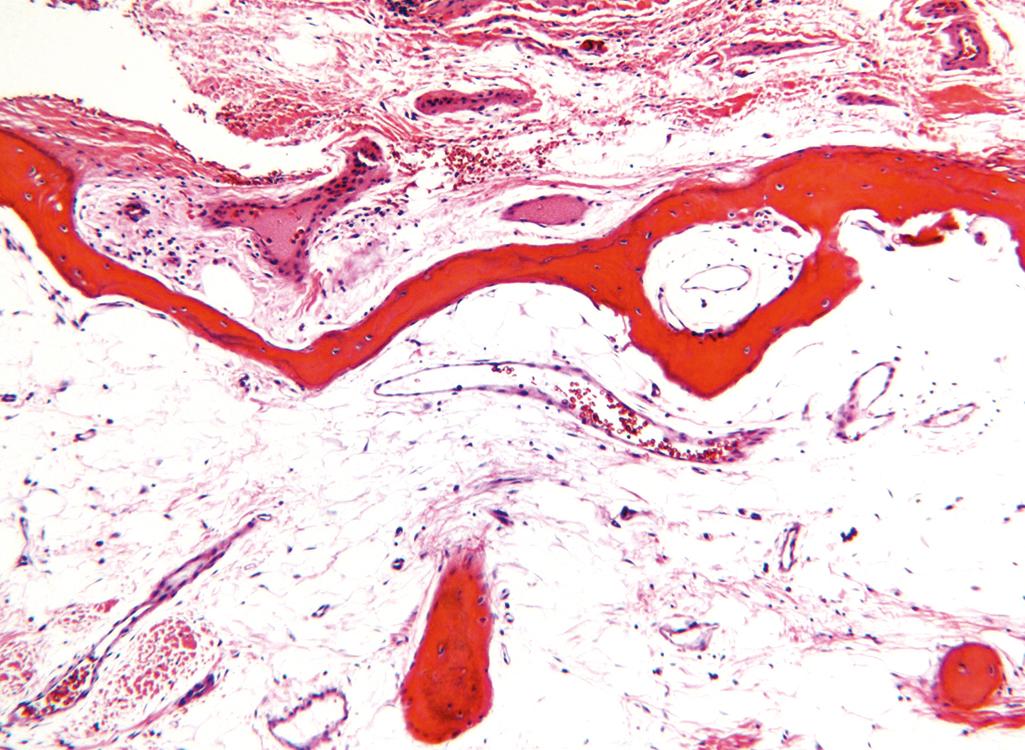
The clinical manifestations of osteoporosis depend on the bones that are involved. Vertebral fractures in the thoracic and lumbar regions are painful, and, when multiple, can cause significant loss of height and various deformities, including lumbar lordosis and kyphoscoliosis . Immobility following fractures of the femoral neck, pelvis, or spine results in complications such as pulmonary embolism and pneumonia, accounting for 40,000 to 50,000 deaths per year.
Osteoporosis cannot be reliably detected in plain radiographs until 30% to 40% of the bone mass has been lost, and measurement of blood levels of calcium, phosphorus, and alkaline phosphatase are not diagnostic. Osteoporosis is thus a difficult condition to screen for in asymptomatic people. The best estimates of bone loss are specialized radiographic imaging techniques, such as dual-energy x-ray absorptiometry and quantitative computed tomography, both of which measure bone density.
The prevention and treatment of senile and postmenopausal osteoporosis include exercise, appropriate calcium and vitamin D intake, and pharmacologic agents that decrease bone resorption (e.g., bisphosphonates). Denosumab, an anti-RANKL antibody that blocks osteoclast activation, is another effective therapy for postmenopausal osteoporosis. Menopausal hormone therapy with estrogen receptor agonists can slow bone loss, but complications, particularly deep venous thrombosis, stroke, and an increased risk of breast cancer, have limited the use of estrogen in the treatment of osteoporosis.
Both rickets and osteomalacia are manifestations of vitamin D deficiency or its abnormal metabolism ( Chapter 7 ). Rickets refers to the disorder in children, in whom it interferes with the deposition of bone in the growth plates. Osteomalacia is the adult counterpart, in which bone formed during remodeling is undermineralized, causing a predisposition to fractures. The fundamental defect is an impairment of mineralization and a consequent accumulation of unmineralized matrix.
Excess production and activity of parathyroid hormone (PTH) cause increased osteoclast activity, bone resorption, and osteopenia. Although the entire skeleton is affected, osteopenia in some bones (e.g., phalanges) is more conspicuous radiographically. Isolated hyperparathyroidism peaks in middle adulthood and slightly earlier if presenting as a component of multiple endocrine neoplasia syndromes (MEN-1 and MEN-2A) ( Chapter 18 ).
As discussed in Chapter 18 , PTH plays a central role in calcium homeostasis through the following effects:
Osteoclast activation , increased bone resorption, and calcium mobilization by increased RANKL expression on osteoblasts
Increased resorption of calcium by the renal tubules
Increased urinary excretion of phosphates
Increased synthesis of active vitamin D , 1,25(OH) 2 -D, by the action of renal alpha-1 hydroxylase, which in turn enhances calcium absorption from the gut and mobilizes bone calcium by inducing RANKL on osteoblasts
The net result of these actions is an elevation in serum calcium, which, under normal circumstances, inhibits further PTH production. Excessive or inappropriate levels of PTH may stem from autonomous parathyroid secretion (primary hyperparathyroidism) or may occur in the setting of underlying renal disease (secondary hyperparathyroidism) ( Chapter 18 ). PTH is directly responsible for the bone changes seen in primary hyperparathyroidism. Abnormalities stemming from chronic renal insufficiency that contribute to bone disease in secondary hyperparathyroidism include inadequate 1,25(OH) 2 -D synthesis, hyperphosphatemia, and metabolic acidosis.
Symptomatic, untreated primary hyperparathyroidism manifests with three interrelated skeletal abnormalities: osteoporosis, brown tumors, and osteitis fibrosa cystica. Osteoporosis is generalized but is most severe in the phalanges, vertebrae, and proximal femur. Osteoclasts may tunnel into and dissect centrally along the length of the trabeculae, creating a railroad track appearance that is referred to as dissecting osteitis ( Fig. 19.8 ), a misnomer, as this is not an inflammatory lesion. The marrow spaces around the affected surfaces are replaced by fibrovascular tissue. Radiographs show a decrease in bone density.
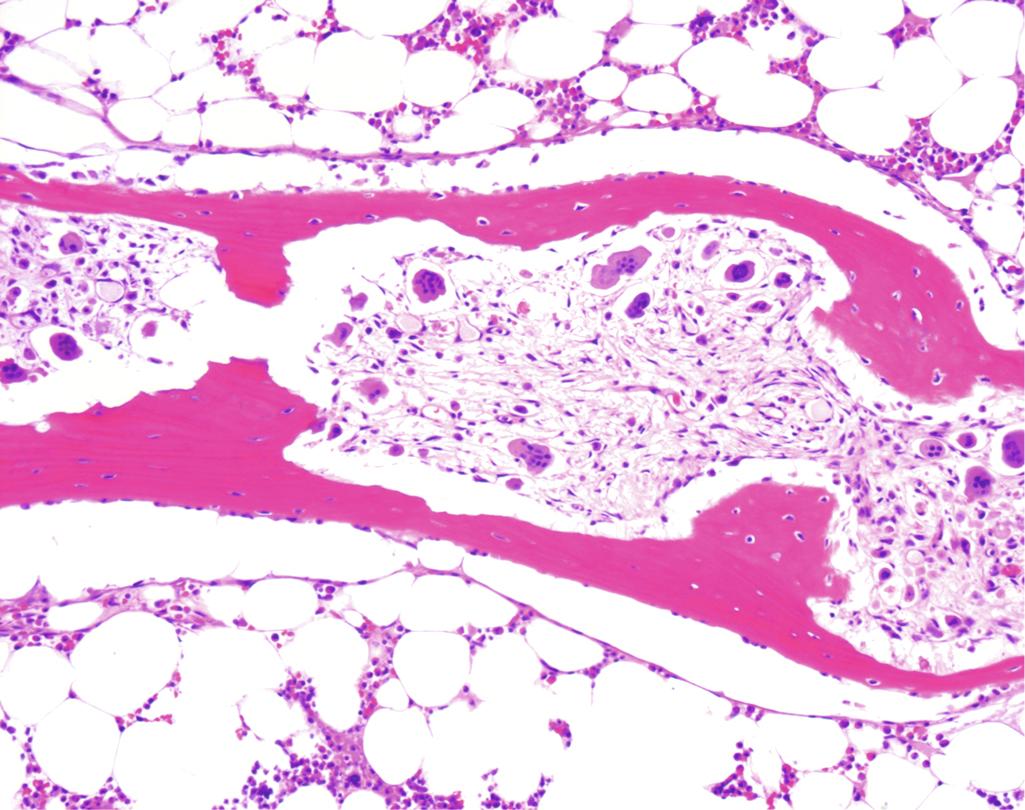
Bone loss predisposes to microfractures and secondary hemorrhages that elicit an influx of macrophages and an ingrowth of reparative fibrous tissue, creating a mass of reactive tissue known as a brown tumor. The brown color is the result of the vascularity, hemorrhage, and hemosiderin. These lesions often undergo cystic degeneration. The combination of increased osteoclast activity, peritrabecular fibrosis, and cystic brown tumors is the hallmark of severe hyperparathyroidism and is known as generalized osteitis fibrosa cystica.
As bone mass decreases, affected patients are increasingly susceptible to fractures, bone deformation, and joint problems. Osteitis fibrosa cystica is now rarely encountered because hyperparathyroidism is usually diagnosed on routine blood tests (that include serum calcium) and treated at an early stage. In symptomatic patients, restoration of PTH levels to normal by surgical removal of parathyroid glands can completely reverse the bone changes. Secondary hyperparathyroidism is usually not as severe or as prolonged as primary hyperparathyroidism; hence, the skeletal abnormalities tend to be milder. Asymptomatic cases that are increasingly recognized by routine blood testing are followed by measurement of serum calcium, creatinine, and bone mineral density.
Paget disease is associated with increased, but disordered and structurally abnormal, bone formation. This unique skeletal disease appears in three sequential phases: (1) an initial osteolytic stage; (2) a mixed osteoclastic–osteoblastic stage, which ends with a predominance of osteoblastic activity; and (3) a final burned-out quiescent osteosclerotic stage ( Fig. 19.9 ).

Paget disease usually begins in late adulthood and its incidence increases with age. An estimated 1% of the U.S. population older than age 40 is affected. Paget disease is relatively common in the United Kingdom, central Europe, and Greece, as well as in areas colonized by European immigrants (e.g., the United States, Australia). By contrast, the disease is rare in the native populations of Scandinavia, China, Japan, and Africa. The exact incidence is difficult to determine because many affected individuals are asymptomatic.
Current evidence suggests both genetic and environmental factors are involved in Paget disease. Approximately 50% of familial Paget disease cases and 10% of sporadic cases have mutations in SQSTM1 , a gene that encodes a protein known as sequestosome-1. Mutations in SQSTM1 appear to lead to increased NF-κB activity, which in turn increases osteoclast activity. Activating mutations in RANK and inactivating mutations in OPG account for some cases of juvenile Paget disease. The geographic distribution is suggestive of involvement of some unknown environmental factor. In vitro studies have raised the possibility that chronic infection of osteoclast precursors by measles or other RNA viruses may also play a role.
Paget disease shows remarkable histologic variation over time and from site to site. The initial lytic phase is characterized by numerous large osteoclasts and resorption pits. The osteoclasts may have 100 or more nuclei. Osteoclasts persist in the mixed phase, but many of the bone surfaces are also lined by prominent osteoblasts. The hallmark, seen in the sclerotic phase, is a mosaic pattern of lamellar bone ( Fig. 19.10 ). The jigsaw puzzle–like appearance is produced by unusually prominent cement lines, which join haphazardly oriented units of lamellar bone. In the sclerotic phase, the bone is thickened but lacks structural stability, making it vulnerable to deformation and fracture.
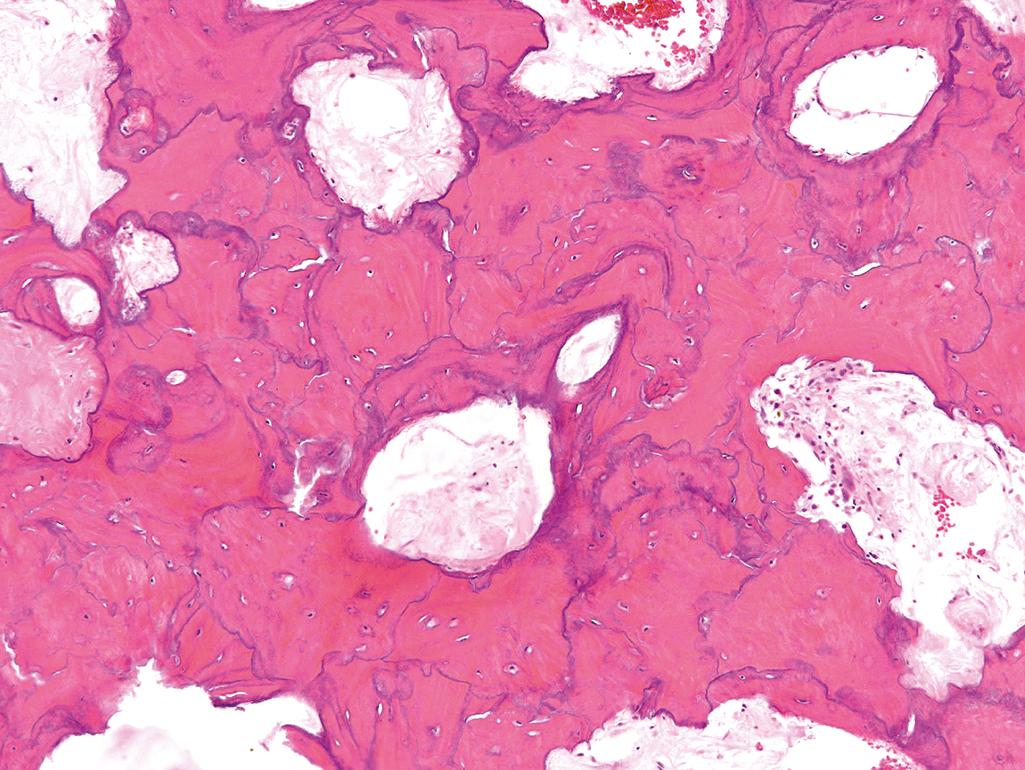
Paget disease is monostotic (about 15% of cases) or polyostotic (about 85%). The axial skeleton or proximal femur is involved in up to 80% of cases. Most cases are asymptomatic and are discovered as an incidental radiographic finding. Pain localized to the affected bone is a common symptom and may be due to microfractures or compression of spinal and cranial nerve roots by overgrown bone. Enlargement of the craniofacial skeleton may produce leontiasis ossea (lion face) and a cranium so heavy that is difficult for the person to hold the head erect. The weakened pagetic bone may lead to invagination of the skull base (platybasia) and compression of the posterior fossa. Weight bearing causes anterior bowing of the femurs and tibias ( eFig. 19.1 ) and distorts the femoral heads, resulting in the development of severe secondary osteoarthritis . Chalk stick–type fractures are another frequent complication and usually occur in the long bones of the lower extremities. Compression fractures of the spine result in kyphosis and spinal cord injury. Rarely, the hypervascularity of pagetic bone warms the overlying skin, and in severe polyostotic disease increased blood flow can act as an arteriovenous shunt, occasionally leading to high-output heart failure or exacerbation of underlying cardiac disease. Many affected individuals have elevated serum alkaline phosphatase levels; serum calcium and phosphate levels are normal.

A variety of tumors develop in pagetic bone. Secondary osteosarcoma occurs in less than 1% of all individuals with Paget disease but affects 5% to 10% of those with severe polyostotic disease, in whom it is rapidly fatal. In the absence of malignant transformation, Paget disease is usually not a serious or life-threatening disease. Most affected individuals have mild symptoms that are readily suppressed by treatment with antiresorptive agents such as calcitonin and bisphosphonates, which act by reducing osteoclast activity.
A fracture is defined as loss of bone integrity resulting from mechanical injury and/or diminished bone strength. Fractures are the most common pathologic conditions affecting bone. The following descriptors are used to describe fracture types:
Simple: the overlying skin is intact
Compound: the bone communicates with the skin surface
Comminuted: the bone is fragmented
Displaced: the ends of the bone at the fracture site are not aligned
Stress: a slowly developing fracture that follows a period of increased physical activity in which the bone is subjected to repetitive loads
Greenstick: extending only partially through the bone, common in infants and young children whose bones are soft
Pathologic: involving bone weakened by an underlying disease process, such as a tumor
At the time of fracture, rupture of blood vessels produces a hematoma that fills the fracture gap and surrounds the area of bone injury ( Fig. 19.11 ). The clotted blood seals off the fracture site and creates a fibrin scaffold that guides the influx of inflammatory cells and the ingrowth of fibroblasts and new capillaries. Simultaneously, degranulated platelets and migrating inflammatory cells release PDGF, TGF-β, FGF, and other factors that activate osteoprogenitor cells in the periosteum, medullary cavity, and surrounding soft tissues and stimulate osteoclastic and osteoblastic activity. By the end of the first week, a mass of predominantly uncalcified tissue—called the soft callus or procallus —bridges the ends of the fractured bones. By approximately 2 weeks, the soft callus is converted to a bony callus by the deposition of woven bone by osteoblasts. In some cases, the activated mesenchymal cells in the soft tissues and bone surrounding the fracture line also differentiate into chondrocytes to make fibrocartilage and hyaline cartilage. The newly formed cartilage along the fracture line undergoes endochondral ossification, forming a contiguous network of bone with newly deposited bone trabeculae in the medulla and beneath the periosteum. In this fashion, the fractured ends are bridged (see Fig. 19.11 ).
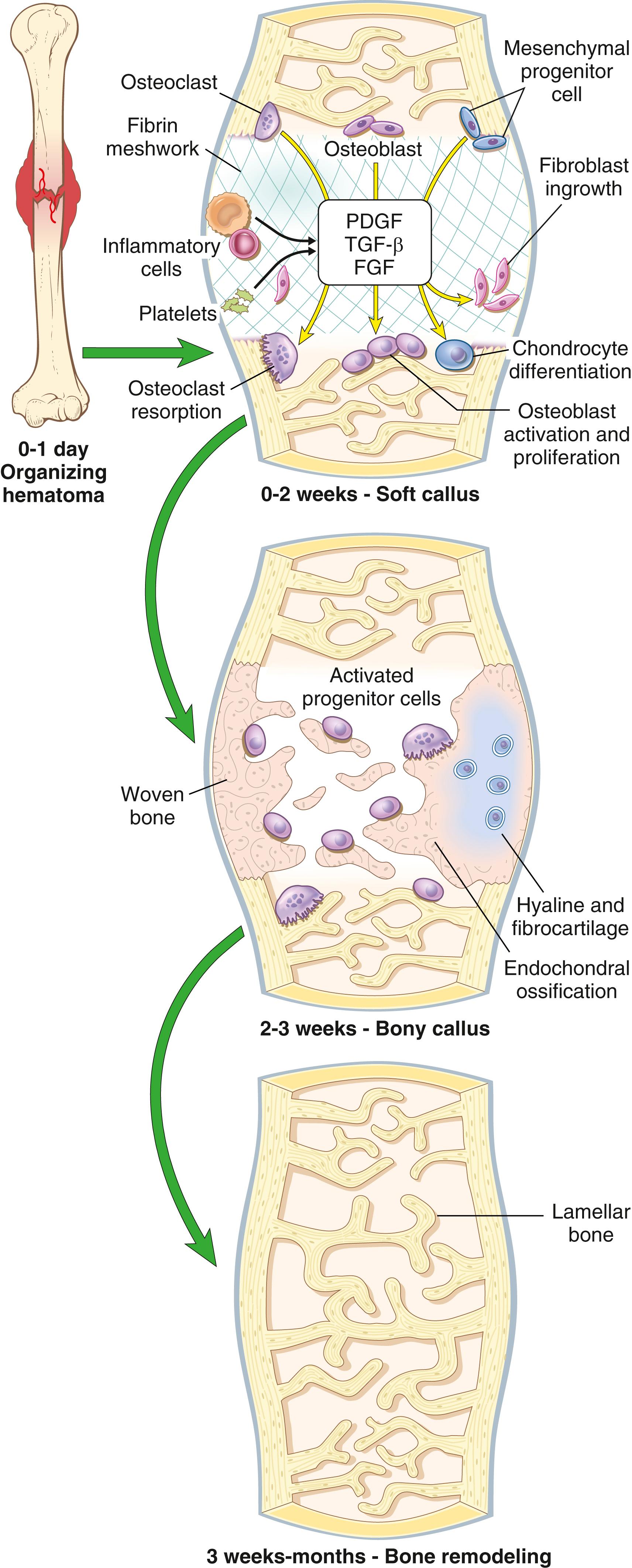
As the callus matures and is subjected to weight-bearing forces, portions that are not physically stressed are resorbed. This remodeling reduces the size of the callus until the shape and outline of the fractured bone are reestablished as lamellar bone . The healing process is complete with restoration of the medullary cavity.
The healing of a fracture may be impeded by a number of factors .
Displaced and comminuted fractures frequently result in some deformity.
Inadequate immobilization results in movement of the callus and prevents its normal maturation, resulting in delayed union or nonunion.
If a nonunion persists, the malformed callus undergoes cystic degeneration, and the luminal surface may become lined by synoviallike cells, creating a false joint or pseudoarthrosis.
Infection of the fracture site is a serious obstacle to healing and is especially common in open fractures.
Malnutrition, diabetes, and skeletal dysplasia (e.g., osteoporosis) also hinder fracture healing; surgical immobilization may be needed in such settings.
Osteonecrosis refers to infarction (ischemic necrosis) of bone and marrow. A diverse set of conditions predispose to bone ischemia, including vascular injury (e.g., trauma, vasculitis), drugs (e.g., corticosteroids), systemic disease (e.g., sickle cell crisis), and radiation. In about 25% of cases, the cause is unknown. Mechanisms of disease include mechanical disruption of vessels, thrombotic occlusion, and extravascular compression. Osteonecrosis peaks in middle adulthood and accounts for about 10% of hip replacements in the United States.
Bone infarcts may be medullary or subchondral. Regardless of etiology, medullary infarcts involve the trabecular bone and marrow but spare the cortex, which is protected by collateral blood flow from the periosteum. In subchondral infarcts, a triangular or wedge-shaped segment of tissue with the subchondral bone plate as its base undergoes necrosis. The overlying articular cartilage remains viable due to nutrients in the synovial fluid. Dead bone is recognized histologically by the presence of empty lacunae lacking osteocytes. The remaining viable trabeculae act as scaffolding for deposition of new bone, while osteoclasts resorb necrotic trabeculae. Because the pace of repair in subchondral infarcts is slow, there is collapse of the necrotic bone, with fracture and sloughing of the articular cartilage. ( Fig. 19.12 ).

Symptoms depend on the location and extent of the infarct. Typically, subchondral infarcts cause pain that is initially associated only with activity but then becomes constant. As mentioned above, subchondral infarcts often collapse and therefore may lead to severe, secondary osteoarthritis. Treatment ranges from conservative measures (limited weight bearing, immobilization) to surgery.
Osteomyelitis is bone and marrow inflammation, virtually always secondary to infection. Osteomyelitis may be a complication of any systemic infection but frequently manifests as a primary solitary focus. All types of organisms, including viruses, parasites, fungi, and bacteria, can produce osteomyelitis, but infections caused by certain pyogenic bacteria and mycobacteria are the most common.
Pyogenic osteomyelitis is almost always caused by bacterial infection. Organisms may reach the bone by (1) hematogenous spread; (2) extension from a contiguous site; and (3) direct implantation after compound fractures or orthopedic procedures. In otherwise healthy children, most osteomyelitis is hematogenous in origin and is more common in the long bones. In adults, osteomyelitis is most often a complication of open fractures, surgical procedures, or diabetes, the latter particularly predisposing to infections of the feet.
The etiologic agent varies depending on the anatomic location and clinical setting (e.g., diabetic foot, surgical site). Overall, Staphylococcus aureus is the most common pathogen identified in culture-positive pyogenic osteomyelitis. Staphylococcal cell wall proteins bind to bone matrix components, such as collagen, which facilitates adherence to bone. In the neonatal period, group B streptococci and E. coli are likely pathogens, whereas in older children gram-positive organisms such as S. aureus are the most likely cause. Mixed bacterial infections are common in the setting of direct spread, surgery, or open fractures. In patients with sickle cell anemia, areas of bone necrosis, which are susceptible to bacterial seeding, and loss of splenic function increases the risk of developing osteomyelitis, Salmonella and other gram-negative organisms being the most frequent culprits. Culture samples should be obtained from bone specimens whenever possible to increase the chances of identifying a causative organism. No specific organism is identified in nearly 50% of patients.
Changes associated with osteomyelitis depend on the stage (acute, subacute, or chronic) and location of the infection. In the acute phase, bacteria proliferate and induce a neutrophilic inflammatory reaction. Necrosis of bone cells and marrow ensues within the first 48 hours. The bacteria and inflammation spread longitudinally and radially throughout the Haversian systems to reach the periosteum. In children, the periosteum is loosely attached to the cortex and may be detached by the inflammatory infiltrate, leading to the formation of a subperiosteal abscess that may dissect along the bone surface. Lifting of the periosteum further impairs the blood supply to the affected region, contributing to the necrosis. Rupture of the periosteum may produce a soft tissue abscess that tracks to the skin, creating a draining sinus. Epiphyseal infection may spread through the articular surface or along capsular and tendoligamentous insertions into a joint, producing septic or suppurative arthritis, which can cause destruction of the articular cartilage and permanent disability.
After the first week, chronic inflammatory cells release cytokines that stimulate osteoclastic bone resorption, ingrowth of fibrous tissue, and the deposition of reactive bone at the periphery. The dead bone is known as a sequestrum . The newly deposited bone can form a shell of living tissue, known as an involucrum, around the segment of devitalized infected bone ( Fig. 19.13 ). The histologic findings of chronic osteomyelitis are more variable but typically involve marrow fibrosis, sequestrum, and an inflammatory infiltrate of lymphocytes and plasma cells.

Hematogenous osteomyelitis may present acutely as a systemic illness with malaise, fever, chills, leukocytosis, and throbbing pain over the affected region. In other instances, the presentation is subtle, with only unexplained fever (infants) or localized pain (adults). Plain radiographs characteristically show a lytic focus of bone destruction and associated reactive bone; MRI is more specific and sensitive for identifying the changes of osteomyelitis. Biopsy and microbial cultures are required to identify the pathogen in most instances. The combination of antibiotics and surgical drainage is usually curative.
In 5% to 25% of cases, acute osteomyelitis fails to resolve, particularly in the setting of delayed diagnosis, weakened host defenses, extensive bone necrosis, or inadequate antibiotic therapy or surgical debridement. The course of such chronic infections may be punctuated by spontaneous acute flare-ups, sometimes after years of dormancy. Complications of chronic osteomyelitis include pathologic fracture, secondary (reactive) amyloidosis, endocarditis, sepsis, and the development of squamous cell carcinoma in draining sinus tracts or sarcoma in the infected bone.
Mycobacterial osteomyelitis, historically a problem in lower-income countries, has risen in incidence worldwide due to increases in immigration and in the number of patients who are immunocompromised. Overall, approximately 1% to 3% of individuals with pulmonary or extrapulmonary tuberculosis develop osseous infection.
The organisms are usually bloodborne and originate from a focus of active visceral disease elsewhere. Direct extension (e.g., from a pulmonary focus into a rib or from tracheobronchial nodes into adjacent vertebrae) may also occur. The bone infection may persist for years before being recognized. Typically, affected individuals present with localized pain, low-grade fevers, chills, and weight loss. Infection is usually unifocal except in individuals who are immunocompromised. The principal histologic feature, the presence of caseating granulomas, is typical of tuberculosis at other sites. Mycobacterial osteomyelitis tends to be more destructive and resistant to control than pyogenic osteomyelitis.
The spine is involved in 40% of cases of mycobacterial osteomyelitis (Pott disease). Infection breaks through intervertebral discs to affect multiple vertebrae and extends into the soft tissues where it may give rise to a psoas abscess ( eFig. 19.2 ). Destruction of discs and vertebrae frequently results in permanent compression fractures that produce scoliosis or kyphosis and neurologic deficits secondary to spinal cord and nerve compression.

The rarity of primary bone tumors and the disfiguring surgery often required to treat bone malignancies make this group of disorders especially challenging. About 2400 primary bone sarcomas are diagnosed annually in the United States. Therapy aims to optimize survival while maintaining the function of affected body parts. The predilection of specific types of tumors for certain age groups and particular anatomic sites provides diagnostic clues. For example, osteosarcoma peaks during adolescence and most frequently involves the knee, whereas chondrosarcoma affects older adults and arises most often in the pelvis and proximal extremities.
Bone tumors may present in a variety of ways: benign lesions are often asymptomatic incidental findings while other tumors cause pain or are identified as a slow-growing mass or pathologic fracture. Radiographic imaging defines the location and extent of the tumor and can detect features that narrow the differential diagnosis, but biopsy is necessary for definitive diagnosis in almost all cases.
Bone tumors are classified according to the normal cell types they recapitulate or the matrix they produce ( Table 19.1 ). Lesions that do not have normal tissue counterparts are grouped according to their clinicopathologic features. Benign tumors greatly outnumber malignant tumors and occur with greatest frequency within the first three decades of life. In older adults, a bone tumor is more likely to be malignant.
| Category | Behavior | Tumor Type | Common Locations | Age (yr) | Morphology |
|---|---|---|---|---|---|
| Cartilage forming | Benign | Osteochondroma | Metaphysis of long bones | 10–30 | Bony excrescence with cartilage cap |
| Chondroma | Small bones of hands and feet | 30–50 | Circumscribed intramedullary hyaline cartilage nodule | ||
| Malignant | Chondrosarcoma (conventional) | Pelvis, shoulder | 40–60 | Extends from medullary canal through cortex into soft tissue, chondrocytes with increased cellularity and atypia | |
| Bone forming | Benign | Osteoid osteoma | Metaphysis of long bones | 10–20 | Cortical, interlacing microtrabeculae of woven bone |
| Osteoblastoma | Vertebral column | 10–20 | Posterior elements of vertebra, histology similar to osteoid osteoma | ||
| Malignant | Osteosarcoma | Metaphysis of distal femur, proximal tibia | 10–20 | Extends from medullary canal to lift periosteum, malignant cells producing woven bone | |
| Unknown origin | Benign | Giant cell tumor | Epiphysis of long bones | 20–40 | Destroys medullary canal and cortex, sheets of osteoclasts |
| Aneurysmal bone cyst | Proximal tibia, distal femur, vertebra | 10–20 | Hemorrhagic spaces separated by cellular, fibrous septa | ||
| Malignant | Ewing sarcoma | Diaphysis of long bones | 10–20 | Sheets of primitive small round cells |
Tumors in this category produce unmineralized osteoid or mineralized woven bone.
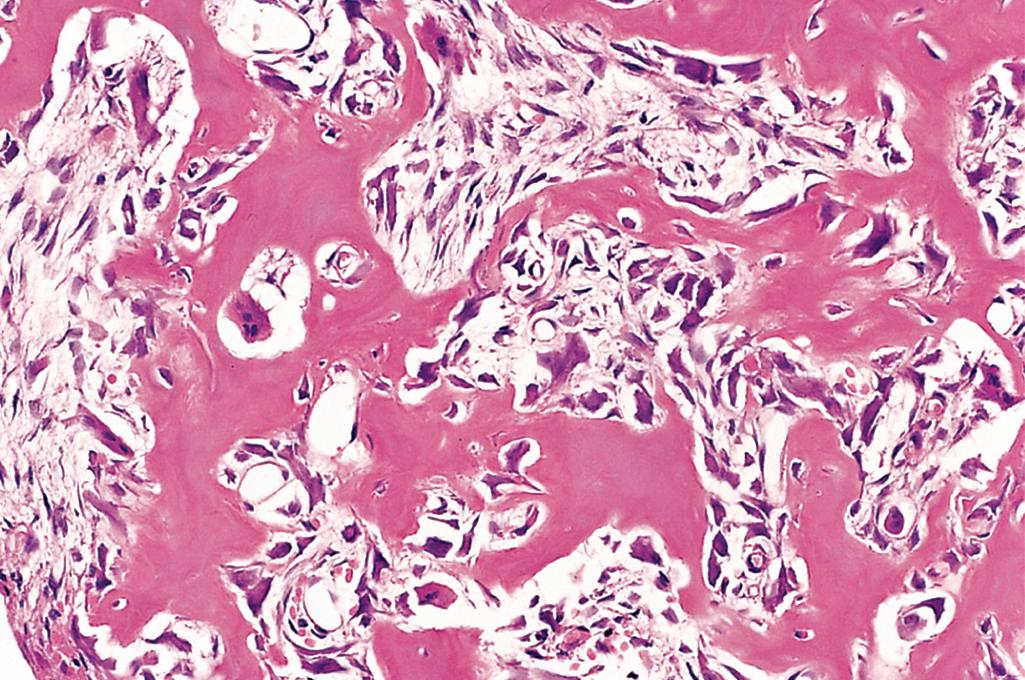
Osteoid osteoma and osteoblastoma are benign bone-producing tumors that have similar histologic features but differ clinically and radiographically. By definition, osteoid osteomas are less than 2 cm in diameter. They are most common in young men. About 50% of cases involve the cortex of the femur or tibia. A thick rim of reactive cortical bone may be the only radiographic clue. Despite their small size, they present with severe nocturnal pain that is probably caused by prostaglandin E 2 produced by osteoblasts and is relieved by aspirin and other nonsteroidal antiinflammatory drugs. Osteoblastomas are larger than 2 cm and more frequently involve the posterior components of the vertebrae (laminae and pedicles). Any associated pain is unresponsive to aspirin and the tumor usually does not induce a marked bony reaction. Osteoid osteoma can be treated by radiofrequency ablation, whereas osteoblastoma is usually curetted or removed by en bloc excision.
Osteoid osteoma and osteoblastoma are round-to-oval masses of hemorrhagic gritty tan tissue. They are well circumscribed and composed of delicate interconnecting trabeculae of woven bone that are rimmed by a single layer of osteoblasts ( Fig. 19.14 ). The stroma surrounding the neoplastic bone consists of loose connective tissue with abundant dilated and congested capillaries. The relatively small size, well-defined margins, and benign cytologic features of the neoplastic osteoblasts help distinguish these tumors from osteosarcoma.
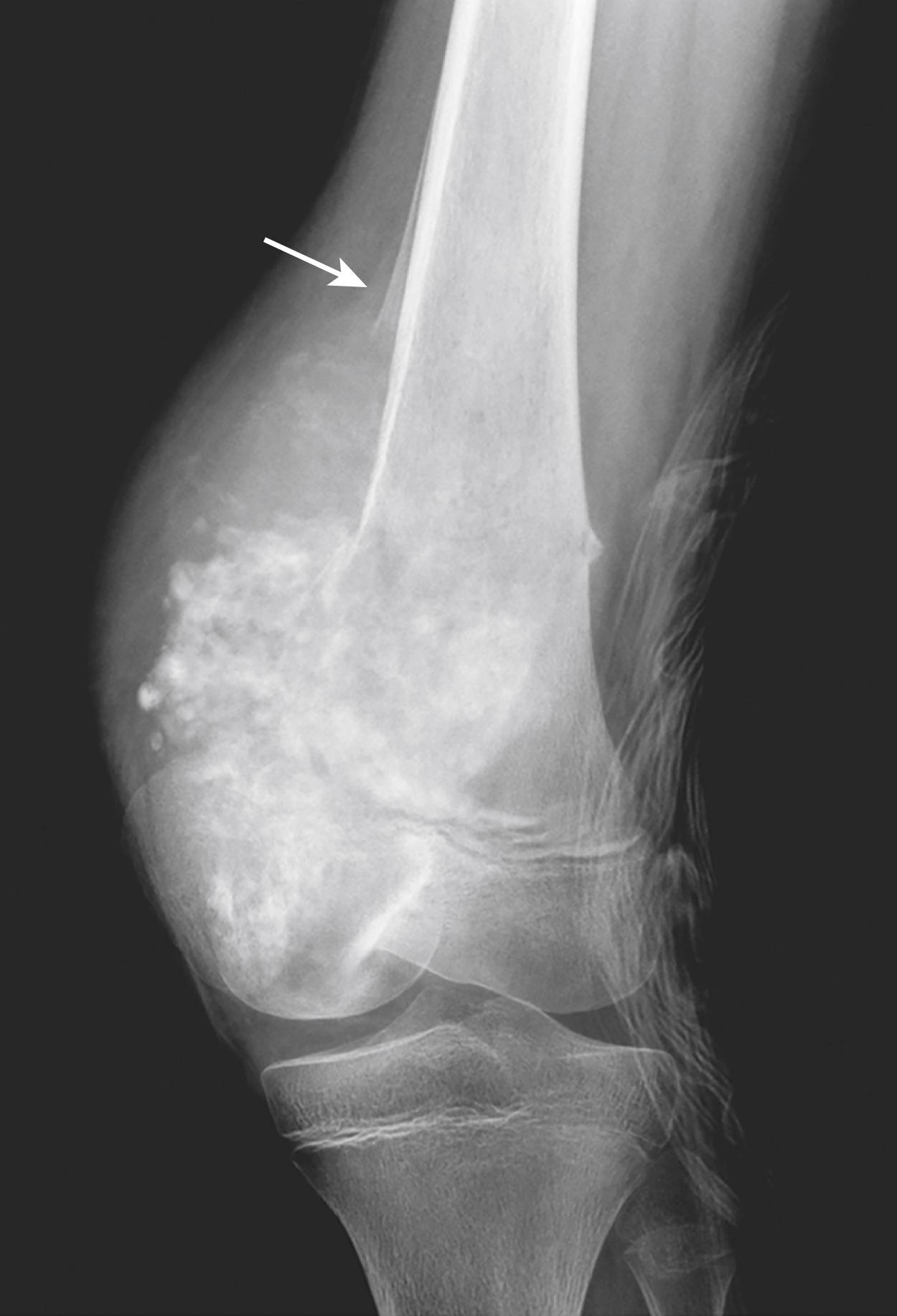
Osteosarcoma is a malignant tumor that produces osteoid matrix or mineralized bone. Excluding hematopoietic tumors, osteosarcoma is the most common primary malignant tumor of bone. The age distribution is bimodal, with about 75% occurring before 20 years of age, while a second smaller peak occurs in older adults in the setting of predisposing factors (secondary osteosarcomas) such as Paget disease, bone infarcts, and previous radiation. Men are more commonly affected than women (1.6 : 1). Although any bone can be involved, in adolescents tumors usually arise in the metaphyseal region of the long bones; almost 50% are near the knee in the distal femur or proximal tibia.
Osteosarcomas present as painful, progressively enlarging masses. A pathologic fracture may be the first indication. Typically, radiologic imaging reveals a large, destructive, mixed lytic and sclerotic mass with infiltrative margins ( Fig. 19.15 ). The tumor frequently breaks through the cortex and lifts the periosteum, resulting in a triangular wedge of reactive subperiosteal bone formation (Codman triangle) . This finding is indicative of an aggressive tumor but is not pathognomonic of osteosarcoma.
The peak incidence of osteosarcoma is during the adolescent growth spurt. The tumor occurs most frequently near the growth plate of rapidly growing bones, where increased proliferation may predispose to mutations that drive oncogenesis. Osteosarcomas are characterized by complex karyotypes with numerous chromosomal aberrations and with mutations in well-known tumor suppressor genes and oncogenes ( Chapter 6 ), including the following:
RB mutations are present in up to 70% of sporadic osteosarcomas; germline RB mutations increase risk of osteosarcoma 1000-fold.
TP53 is mutated in the germline of patients with Li-Fraumeni syndrome, who have a greatly increased incidence of osteosarcoma. Mutations affecting the TP53 gene are common in sporadic tumors.
MDM2 and CDK4 , which inhibit p53 and RB function, respectively, are overexpressed in many low-grade osteosarcomas.
CDKN2A (also known as INK4a ), which encodes two tumor suppressors (p16 and p14), is inactivated in many osteosarcomas.
MYC amplification is seen in up to half of cases and may be associated with a particularly poor prognosis.
Osteosarcomas are bulky tumors that are gritty and tan-white, often with areas of hemorrhage, and tend to destroy the surrounding cortices and invade into soft tissue ( Fig. 19.16 ). Extensive intramedullary spread infiltrates and replaces the marrow. Infrequently, the tumor penetrates the epiphyseal plate and enters the adjacent joint.
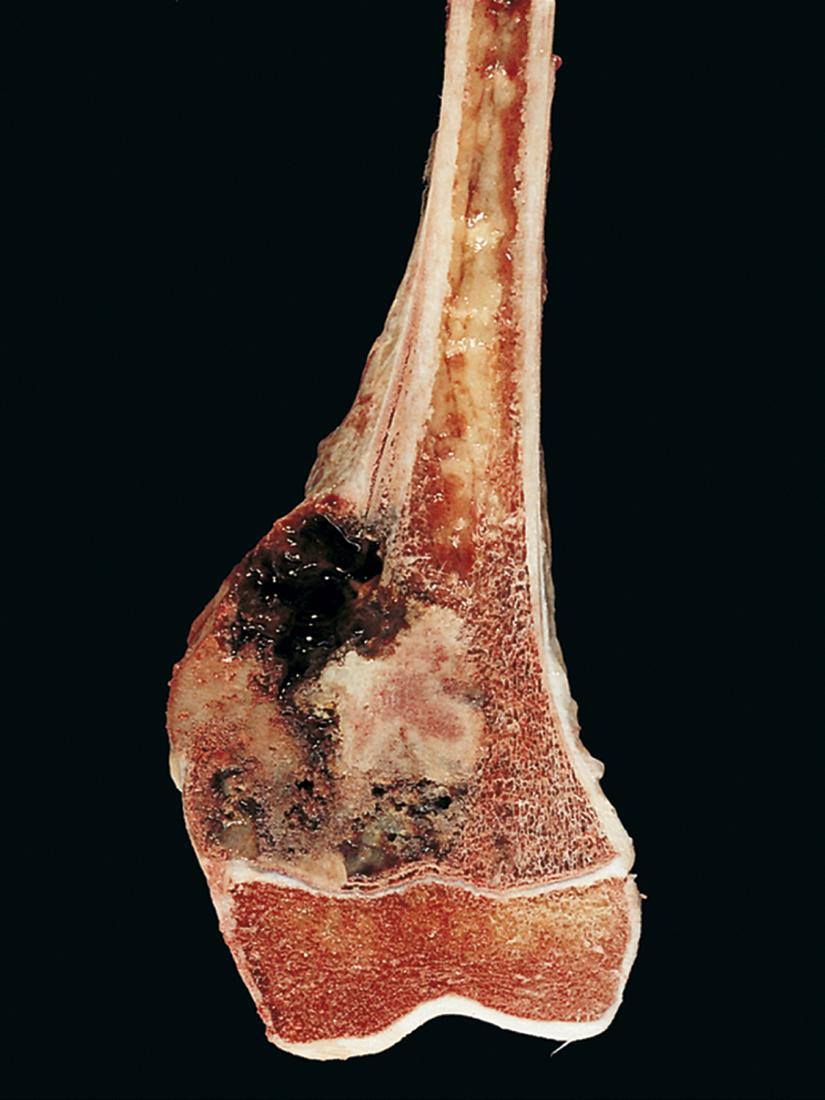
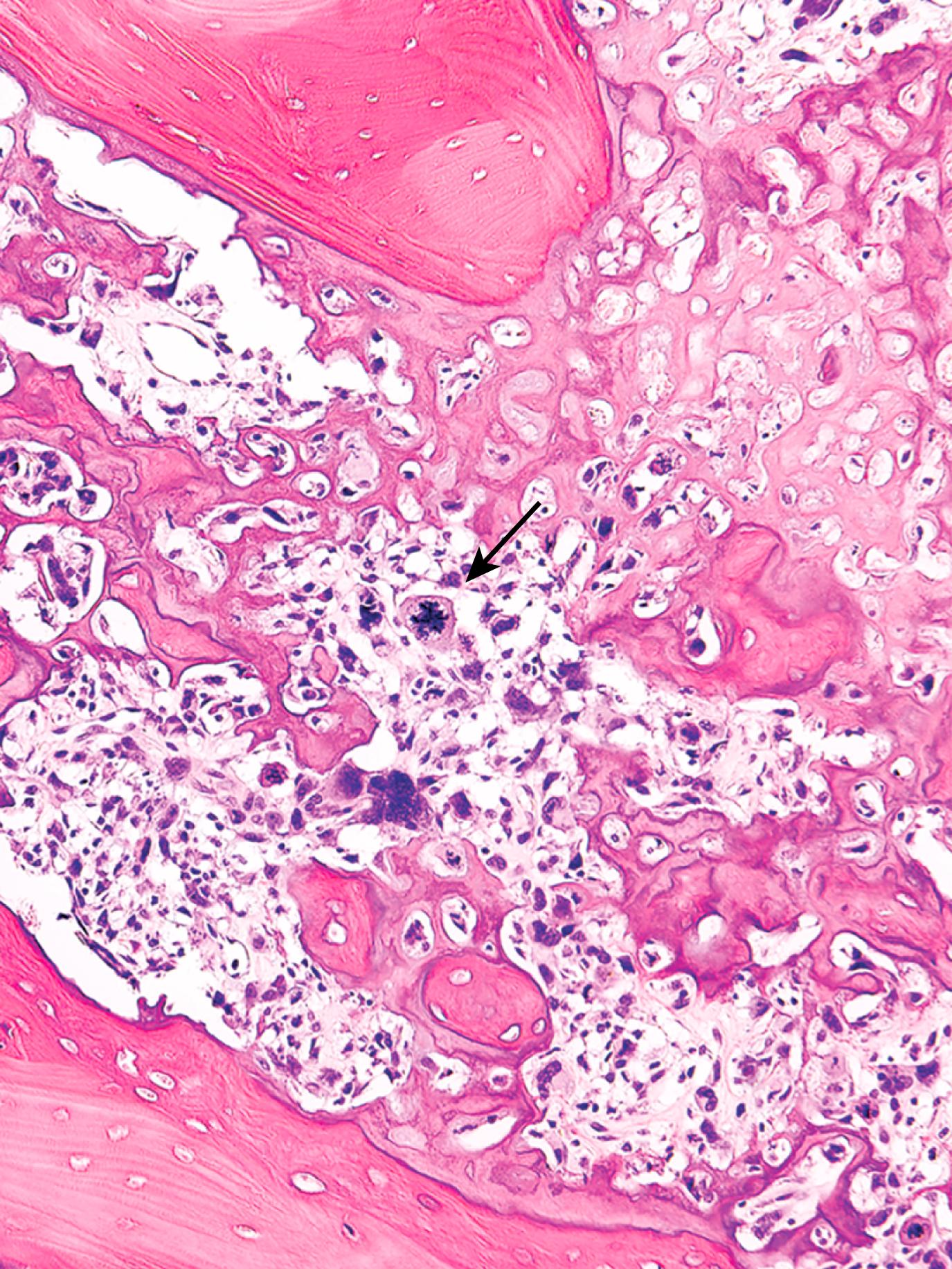
The tumor cells demonstrate pleomorphism, large hyperchromatic nuclei, bizarre tumor giant cells, and abundant mitoses including abnormal (e.g., tripolar) forms. Extensive necrosis and intravascular invasion are also common. Diagnosis of osteosarcoma requires the presence of malignant tumor cells producing unmineralized osteoid or mineralized bone ( Fig. 19.17 ), which is typically fine and lacelike but can also appear as broad sheets or primitive trabeculae.
All osteosarcomas are assumed to have occult metastases at the time of diagnosis. As a result, treatment generally includes neoadjuvant chemotherapy, surgery, and postoperative adjuvant chemotherapy. Chemotherapy has greatly improved osteosarcoma prognosis, with 5-year survival reaching 70% in individuals without overt metastases at initial diagnosis. Osteosarcoma metastasizes hematogenously to the lungs, bones, brain, and other sites. The outcome for those with clinically evident metastases, recurrent disease, or secondary osteosarcoma remains guarded, with a 5-year survival rate less than 20%.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here