Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The parathyroid glands are derived from branchial pouches III and IV and number between two to six glands, although four is the usual number. In adults, each of these ovoid (bean-shaped) glands measures 4 to 6 mm × by 2 to 4 mm × 0.5 to 2 mm and weighs approximately 30 mg (the lower parathyroid glands are generally larger than the upper glands). They vary in color from yellow to tan, depending on vascularity and percentage of oxyphil cells and stromal fat.
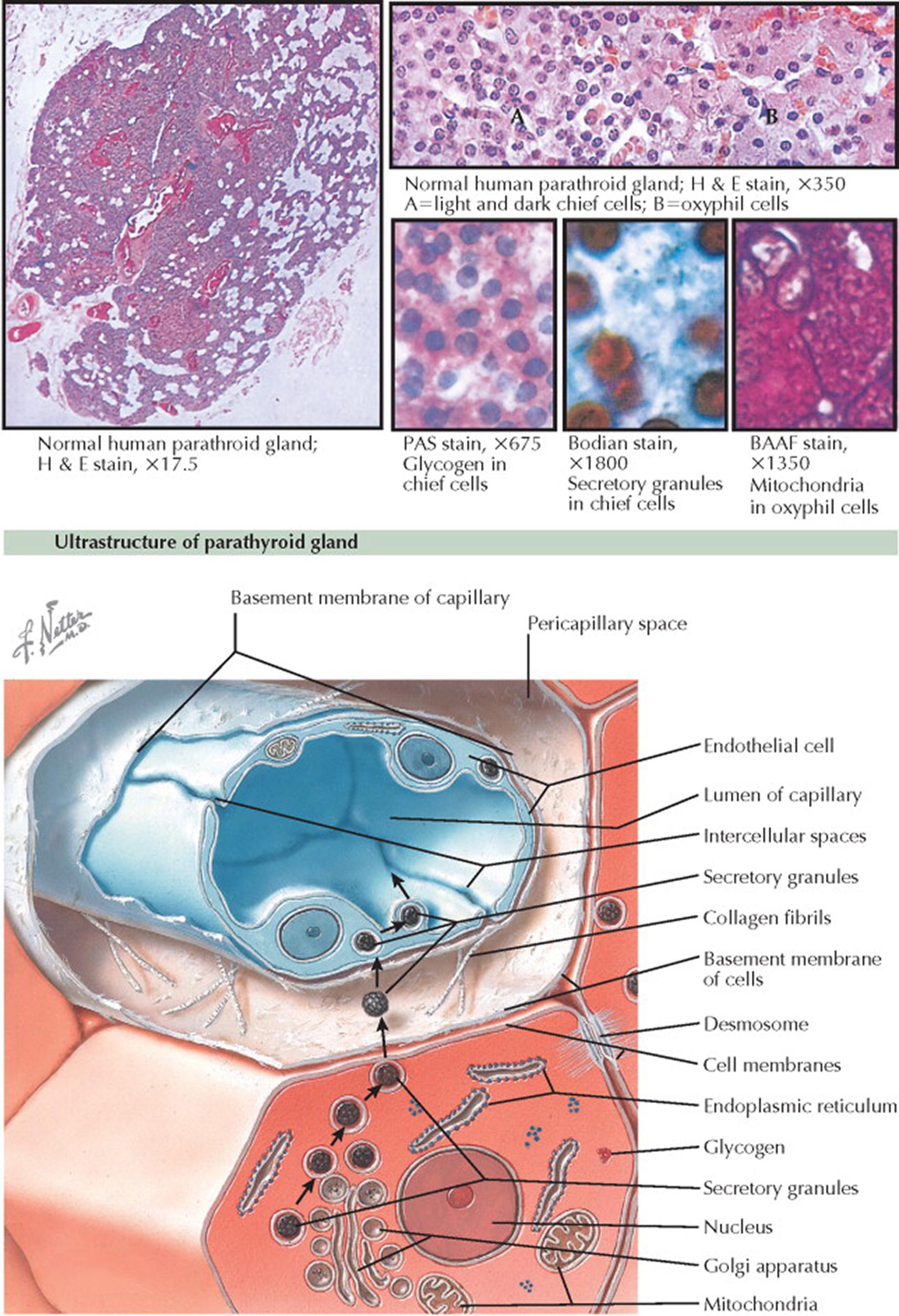
In infants and children, the glands are composed of sheets of closely packed chief cells, with little intervening stroma. Oxyphil (or oncocytic) cells first make their appearance at the time of puberty. Fat cells begin to appear in the stroma in late childhood. Both the oxyphil cells and the fat cells increase in number until they may occupy more than 50% of the volume of the glands during the fifth and sixth decades of life.
In adults, the glands are composed of cords, sheets, and acini of chief cells in a loose areolar stroma containing numerous mature fat cells. Chief cells appear in an active synthetic phase (“dark chief cell”) with well-formed endoplasmic reticulum and prominent Golgi apparatus or in a resting phase (“light chief cell”) with less well-developed endoplasmic reticulum. Scattered individually or in groups among these chief cells are the oxyphil cells. The chief cell measures approximately 8 μm in diameter. It has a well-defined cell membrane and a 4- to 5-μm centrally located nucleus. The chromatin is densely packed, appearing almost pyknotic, or it is finely fibrillar with peripheral margination. Nucleoli are rare. The cell cytoplasm is clear and amphophilic in hematoxylin and eosin (H&E) preparations. The periodic acid–Schiff (PAS) reaction reveals abundant glycogen in these cells. Chief cells also contain abundant intracytoplasmic neutral lipid droplets demonstrated with azure B or Erie garnet A procedures or with oil red O or Sudan IV stains. Immunohistochemical studies show stronger staining for parathyroid hormone in chief cells than in oxyphilic cells.
The oxyphilic cells are larger than chief cells (12–20 μm in diameter) and are polygonal in shape. The cell membranes are usually clear, and the nucleus is identical to that of the chief cell. The cytoplasm is composed of highly eosinophilic fine granules, which stain carmine with Bensley acid aniline fuchsin (BAAF) and dark blue with phosphotungstic acid hematoxylin. These cells contain tightly packed mitochondria filling the cytoplasm and have high levels of oxidative enzymes. Unlike chief cells, the oxyphilic cells have very little intracytoplasmic lipid or glycogen. Variants of the oxyphilic cells include transitional oxyphilic cells, which are smaller and contain less eosinophilic cytoplasm.
The ultrastructure of the active form of the chief cell and the mode of secretion are schematized in the illustration. The chief cells are arranged in cords and nests and separated from the interstitium by basal laminae. The chief cells have straight plasma membranes and are attached to other cells by desmosomes. During the active phase, in addition to the usual organelles, the Golgi apparatus enlarges, numerous vacuoles and vesicles appear in the Golgi apparatus, and many mature secretory granules (50–300 nm in diameter) appear in the cell. The mature secretory granule is oval to dumbbell-shaped and has a single membrane surrounding a thin clear space inside of which is a dense area composed of short rodlike profiles. The granule migrates out of the cell through the basement membrane into the wide pericapillary space. It then goes through the capillary basement membrane and into the fenestrated endothelial cells that line the capillaries, from which parathyroid hormone is liberated into the bloodstream.
Secretion of parathyroid hormone (PTH) from the four parathyroid glands is regulated by the blood level of ionized calcium (Ca 2+ ). Serum ionized calcium concentrations below the reference range stimulate PTH secretion, and levels above the reference range inhibit PTH secretion. The principal action of PTH is the regulation and maintenance of a normal serum total calcium level between 8.9 and 10.1 mg/dL. The calcium-sensing receptors (CaSRs) in the parathyroid glands are responsible for maintaining this calcium-dependent regulation of PTH secretion. The CaSRs in the kidneys serve to adjust tubular calcium reabsorption independent of PTH.
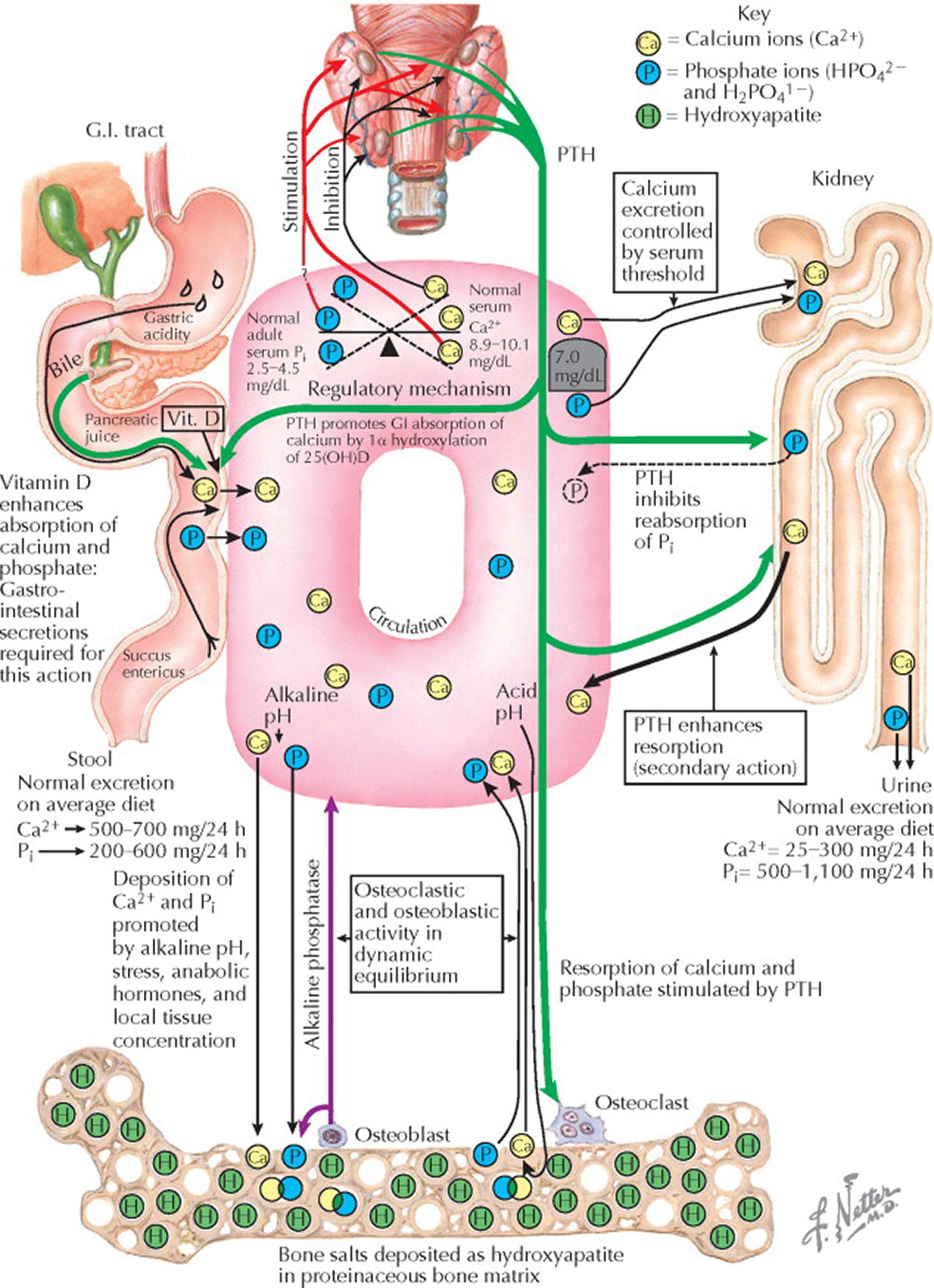
A normal serum concentration of ionized calcium is critical for many extracellular and cellular functions, and it is normally maintained in the very narrow range through a tightly regulated calcium–PTH homeostatic system. Neuromuscular activity is just one function that is dependent on calcium homeostasis; cytosolic free calcium serves as a key second messenger. Thus, disturbances in extracellular calcium concentration result in symptoms of abnormal neuromuscular activity. For example, hypercalcemia may cause muscle weakness and areflexia, anorexia, constipation, vomiting, drowsiness, depression, confusion, or coma. Hypocalcemia may result in anxiety, muscle twitching, Chvostek and Trousseau signs, carpal or pedal spasm, seizures, stridor, bronchospasm, or intestinal cramps.
The daily dietary calcium intake ranges between 300 and 1500 mg/d; total net gastrointestinal (GI) calcium absorption averages 200 mg/d. The urinary calcium excretion averages 200 mg/d (2% of the filtered load). Although the urinary calcium excretion is rather constant, the excretion of calcium in the stool depends greatly on the body's need and the dietary intake; normally, 500 to 700 mg of calcium is excreted per 24 hours (100–200 mg/d is endogenous fecal calcium that is unaffected by dietary or serum calcium). The average dietary intake of phosphate is 800 to 900 mg/d. GI tract phosphate absorption is enhanced by 1,25-dihydroxyvitamin D (1,25[OH] 2 D). Phosphate absorption is impaired by increasing dietary calcium. Whereas the fecal excretion of inorganic phosphate (P i ) is roughly 30% of dietary intake, the urinary phosphate excretion varies widely with intake and serum PTH concentration.
If dietary calcium is restricted in healthy individuals, a decrease in the blood calcium concentration leads to a compensatory increase in intestinal calcium absorption. This occurs because a small decrease in serum ionized calcium triggers the CaSR, and there is a prompt increase in PTH secretion. The increased blood PTH concentration leads to increased renal 1α hydroxylation of 25-hydroxyvitamin D (25[OH]D) to the more potent 1,25(OH) 2 D (calcitriol). Calcitriol acts on enterocytes to increase active transport of calcium. In addition, renal tubular calcium reabsorption is increased both by PTH and by a direct effect of hypocalcemia via the CaSRs in the loop of Henle. The direct actions of PTH and calcitriol at bone increase bone resorption and calcium release. Because of these three mechanisms of action, the serum ionized calcium concentration increases, and the serum PTH concentration decreases.
With increased dietary calcium exposure, there is suppression of PTH secretion, inhibition of the 1α hydroxylation of 25(OH)D, decreased intestinal absorption of calcium, increased renal excretion of calcium, decreased renal excretion of phosphate, and decreased bone resorption.
If the parathyroid glands are not functioning properly or are absent, the serum calcium level decreases, usually below the renal threshold of 7 mg/dL, and urinary calcium is absent. The presence of a large reservoir of calcium in the skeleton (∼1000 g) as hydroxyapatite (Ca 10 [PO 4 ] 6 [OH] 2 ), however, prevents the serum calcium from falling below 5 mg/dL even in the absence of the parathyroid glands.
In states of excessive PTH secretion, resorption of calcium and phosphate from bone matrix occurs through stimulation of the osteoclasts. The osteoclastic overresponse then evokes a tendency for the osteoblasts to become overactive and leads to bone repair, with the subsequent rise of the alkaline phosphatase level in the serum. Bone repair is promoted by enhanced absorption of calcium and phosphate from the GI tract facilitated in part by the increased serum concentration of 1,25(OH) 2 D.
Bone is composed of a collagen matrix, distributed in a lamellar pattern and strengthened by pyridinoline crosslinks between the triple-helical collagen molecules, on which calcium and phosphorus are deposited to form hydroxyapatite. The bone matrix also includes calcium-binding proteins such as osteocalcin. The resulting bone mineral is complex crystals of hydroxyapatite that contain fluoride and carbonate.
Bone modeling is the process of change in bone size and shape in childhood, where linear growth is the result of cartilaginous growth at the epiphyses, and bone width enlargement results from endosteal resorption and periosteal apposition. During puberty and early adulthood, endosteal apposition occurs, and peak bone mass is achieved.
Bone remodeling is the lifelong process of bone repair, which has three phases: resorption, reversal, and formation. The bone remodeling unit (osteon) involves a cycle of coupled osteoclastic and osteoblastic activities.
Osteoblasts develop from determined osteoblast progenitor cells that originate from mesenchymal stem cells. The osteoblast progenitor cells are localized to the periosteum and bone marrow. Osteoblasts have receptors for parathyroid hormone, 1, 25-dihydroxyvitamin D, testosterone, estrogen, glucocorticoids, growth hormone, thyroid hormone, and insulinlike growth factors. After osteoblasts lay down collagen and noncollagen proteins, some of the osteoblasts become osteocytes that are buried in the bone matrix. The remaining osteoblasts either become the less metabolically active, flattened lining cells or undergo apoptosis.
Osteoclasts, derived from monocytes and macrophages, are multinucleated, large cells that dissolve bone mineral and degrade matrix. Osteoclast progenitors can be found in the bone marrow and the spleen. Osteoclastic differentiation is triggered by the production of macrophage colony–stimulation factor. Excessive osteoclastic activity is associated with Paget disease, hyperparathyroidism, and a subset of osteoporosis.
The bone remodeling cycle is activated by osteoblast cells that release collagenase, macrophage colony–stimulating factor, and receptor activator of NF-κ B (RANK) ligand. RANK ligand interacts with the RANK receptor and activates osteoclast formation and the initiation of bone resorption. RANK ligand also binds to osteoprotegerin, which is an osteoclastogenesis inhibitory factor. Macrophage colony–stimulating factor is also required for normal osteoclast activation. This initial osteoblast activation of osteoclasts can be affected by multiple hormones and factors. For example, parathyroid hormone induces the osteoblastic production of RANK ligand and inhibits production of osteoprotegerin. Vitamin D also increases the production of RANK ligand.
Osteoclasts enzymatically degrade bone matrix protein and remove mineral within cortical bone or on the trabecular surfaces. This process is self limited—perhaps by high local concentrations of calcium or bone matrix substances—and is completed over 2 weeks.
After osteoclastic resorption, the reversal phase is initiated by mononuclear cells that lay down a glycoprotein-rich material (cement) on the resorbed surface and signal for osteoblast differentiation. The new osteoblasts adhere to this material. The reversal phase is approximately 4 weeks in duration.
During the formation phase, osteoblasts lay down osteoid (collagen and matrix proteins) until the resorbed bone is completely replaced. The formation phase takes approximately 16 weeks to complete. Normally, the new bone formed is equivalent to what was resorbed. When the formation phase is complete, the bone surface is covered with bone-lining cells, and very little cellular activity occurs until another bone remodeling cycle begins.
In a normal bone remodeling unit, resorption and formation are tightly coupled. However, a mismatch between resorption and formation can lead to abnormally thin or dense bones. For example, if the osteoclastic resorption depth is excessive, it can perforate and weaken trabecular structure; if the osteoblasts do not completely fill the deep resorption cavity, bone density and quality decline. If the resorptive process is incomplete because the osteoclasts are not fully activated (e.g., with macrophage colony–stimulating factor deficiency) or if they are dysfunctional, excessively dense bones may result (osteopetrosis).
The annual incidence of primary hyperparathyroidism (HPT) is approximately four in 100,000. HPT is twice as common in women, and most patients are diagnosed after age 45 years. Primary HPT is caused by a single parathyroid adenoma (89%), multiple (“double”) parathyroid adenomas (4%), multigland parathyroid hyperplasia (6%), or parathyroid carcinoma (1%). The distinction between these forms of primary HPT directs the surgical approach.
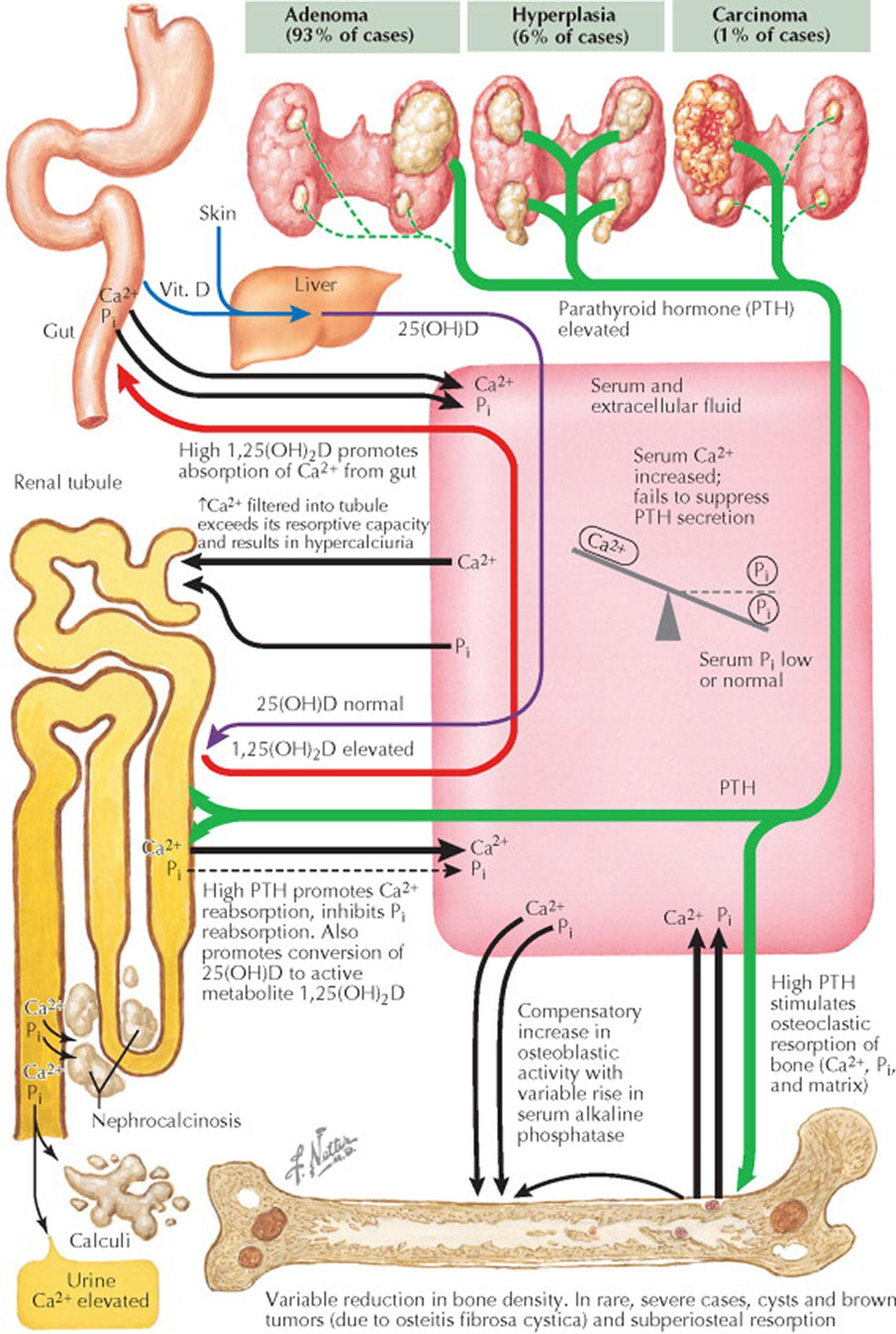
Most parathyroid adenomas are encapsulated and arise from chief cells; the others are composed of oxyphilic cells. Parathyroid adenomas are usually localized to the neck, but ectopic parathyroid tumors may arise anywhere in the anterior mediastinum or even in the posterior mediastinum. The cells in the adenomas are monoclonal and are a result of somatic mutations in genes that control growth. For example, the cyclin D1 proto-oncogene ( CCND1 ) encodes a major regulator of the cell cycle. Overexpression of cyclin D1 is found in approximately 30% of parathyroid adenomas. Multiple endocrine neoplasia (MEN) type 1 (see Plate 8-1 ) is caused by germline mutations in MEN1 , a tumor suppressor gene, and somatic mutations are found in approximately 15% of sporadic parathyroid adenomas.
Multiple-gland hyperplasia, characterized by enlargement of all four glands, is occasionally mistaken for multiple adenomas. Chief cell hyperplasia is much more common than clear cell hyperplasia (the latter is associated with hyperplasia primarily of the superior parathyroid glands). Parathyroid hyperplasia may be sporadic or associated with a familial syndrome (e.g., MEN 1, MEN 2A, HPT–jaw tumor syndrome, or familial isolated hyperparathyroidism). Distinguishing between parathyroid gland hyperplasia and normal parathyroid tissue can be difficult for the pathologist. In general, abnormal parathyroid glands are increased in size and contain less fat. Primary HPT affects almost all patients with MEN 1, and the hypercalcemia is typically present by the third decade of life, but primary HPT affects only 10% of patients with MEN 2A and tends to occur later in life. Parathyroid adenomas and hyperplasia are multiple and cystic in patients with HPT–jaw tumor syndrome. The jaw tumor is usually fibrous in nature.
The diagnosis of parathyroid carcinoma is based on local invasion of contiguous tissues or metastases (lymph node or distant). Both sporadic and germline inactivating mutations in CDC73 (previously known as HRPT2 ) are responsible for HPT–jaw tumor syndrome, which is associated with an increased risk for parathyroid carcinoma.
A normal serum concentration of ionized calcium (Ca 2+ ) is critical for many extracellular and cellular functions, and it is normally maintained in the very narrow range through a tightly regulated calcium–PTH homeostatic system. In healthy individuals, a small decrease in serum ionized calcium triggers the calcium-sensing receptor, and there is a prompt increase in PTH secretion. The increased blood PTH concentration leads to an immediate increase in bone resorption and renal 1α-hydroxylation of 25-hydroxyvitamin D to the more potent 1, 25-dihydroxyvitamin D (calcitriol). Calcitriol leads to increased intestinal calcium absorption over several days. Finally, PTH leads to an immediate decrease in urinary calcium excretion by stimulating calcium reabsorption at the distal renal tubule. Because of these three mechanisms of action, the serum ionized calcium concentration increases, and the serum PTH concentration decreases.
Patients with primary HPT have abnormal regulation of PTH secretion by calcium, a finding partly caused by an elevation in the set point. The set point for calcium-dependent feedback suppression of PTH release is 15% to 30% above normal. It is important to note that PTH secretion in primary HPT is not completely autonomous and can usually be partially inhibited by a further rise in serum calcium. The excessive production of PTH leads to hypercalcemia by increased stimulation of the osteoclastic activity of bone (with the release of calcium and phosphate), increased absorption of calcium from the gut, and increased reabsorption of calcium by the renal tubules. In addition, PTH inhibits the tubular reabsorption of inorganic phosphate (P i ), causing an excessive loss of phosphate. The net effect of these chemical changes is an increase in serum calcium and a decrease in serum phosphate, with increasing amounts of both calcium and phosphate being excreted in the urine. This predisposes to the formation of calcium phosphate and calcium oxalate renal stones. At times, there may be precipitation of calcium in the soft tissues of the kidneys, producing nephrocalcinosis.
Roughly 25% of patients with primary HPT have evidence of bone disease, with marked bone resorption and a compensatory increase in osteoblastic activity. Bone mineral is formed by small hydroxyapatite crystals that contain carbonate, magnesium, sodium, and potassium.
Approximately 80% of patients with primary hyperparathyroidism (HPT) are asymptomatic, and hypercalcemia is detected by routine biochemical testing. Less commonly, primary HPT is diagnosed during the evaluation of symptomatic hypercalcemia, renal lithiasis, osteoporosis, or osteitis fibrosa cystica. Although most patients with primary HPT do not have overt symptoms, it may be responsible for more subtle symptomatology (e.g., weakness, fatigue, depressed mood, or mild cognitive dysfunction).
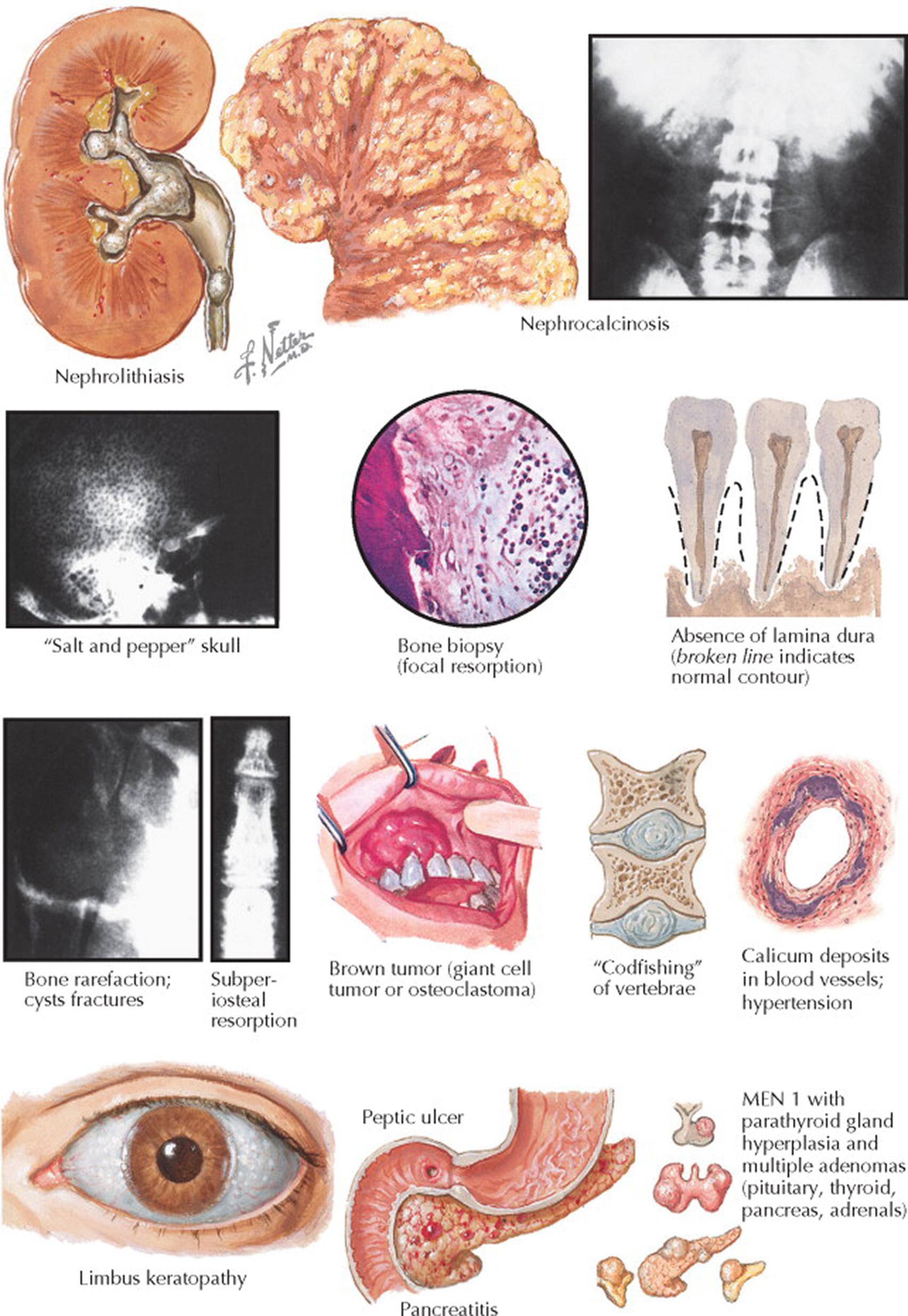
The more overt signs and symptoms of long-standing, untreated primary HPT have been referred to as “bones, stones, abdominal moans, and groans.” The “stones” refer to nephrolithiasis, which occurs in 20% of patients with primary HPT. The nephrolithiasis is caused by the hypercalciuria (a result of increased filtered calcium in the setting of hypercalcemia) that predisposes to the development of calcium oxalate stones. The most common effect on bones is osteopenia and osteoporosis. Less common bone-related sequelae of severe, long-standing primary HPT include absence of the lamina dura around the teeth; subperiosteal resorption of the bone, especially around the radial margins of the phalanges, around the sternal end of the clavicle, and along the margins of other bones; diffuse “salt-and-pepper” decalcification of the skull, resembling multiple myeloma; fractures of the terminal phalanges with telescoping, giving the appearance of pseudoclubbing (the phalangeal joints may show increased flexibility); large bone cysts (osteitis fibrosa cystica) in various locations (fractures through these cysts or fractures through rarefied bone may occur); diffuse demineralization of the skeleton, especially of the spine, with “codfishing” of the vertebral bodies; and brown tumors (also known as giant cell tumors, osteoclastoma, or epulis), which are radiolucent bone tumors that may develop in the jaw and other bones.
Hypercalcemia may also cause nausea, anorexia, constipation, nephrogenic diabetes insipidus (polyuria and polydipsia), glucose intolerance, peptic ulcer disease, pancreatitis, and hypertension. Parathyroid crisis occurs with severe hypercalcemia (calcium >15 mg/dL), and affected patients present with central nervous system dysfunction (e.g., confusion, coma). Parathyroid crisis—typically precipitated by volume depletion—is treated with volume repletion with isotonic saline and an agent to decrease bone resorption (e.g., a bisphosphonate).
Physical examination typically reveals no specific findings for primary HPT. Parathyroid adenomas are not palpable; when a parathyroid tumor is palpable, it is usually parathyroid carcinoma. With a slit-lamp examination of the eyes, calcium phosphate deposition may be seen in a semicircular form around the limbus of the cornea and is termed band keratopathy .
Laboratory abnormalities in patients with primary HPT include increased serum total and ionized calcium concentrations, decreased serum phosphorus concentration (parathyroid hormone [PTH] inhibits the proximal renal tubular reabsorption of phosphate), serum PTH concentration that is either above the reference range or inappropriately (in the setting of hypercalcemia) within the reference range, and increased serum calcitriol concentration (a result of PTH-stimulated renal hydroxylation of 25-hydroxyvitamin D). Serum creatinine concentrations can be increased in patients with marked and long-standing primary HPT and can be associated with nephrocalcinosis. Patients with severe primary HPT may also have a normochromic normocytic anemia.
Vitamin D deficiency is frequently present in patients with primary HPT, a state that can attenuate the degree of hypercalcemia. Treating the vitamin D deficiency in this setting can aggravate the hypercalcemia and hypercalciuria.
The treatment of primary HPT rests on the surgical removal of the parathyroid adenoma (for single-gland disease) or, rarely, of 3.5 hyperplastic parathyroid glands in the setting of diffuse parathyroid hyperplasia (e.g., with multiple endocrine neoplasia [MEN] type 1).
The most common causes of hypercalcemia are primary hyperparathyroidism (HPT) and malignancy. The first question the clinician should ask is whether the hypercalcemia is a persistent finding; thus, a serum calcium (Ca 2+ ) concentration should be remeasured. If levels of serum calcium have been measured in the past, they should be reviewed. Whereas long-standing mild hypercalcemia (<11 mg/dL) is typical of primary HPT, an abrupt onset of severe hypercalcemia (>13 mg/dL) is more typical of hypercalcemia of malignancy. The patient's diet and medications should be reviewed for clues suggestive of milk–alkali syndrome and medication-related hypercalcemia.
For patients with persistent hypercalcemia, the first step is to determine whether it is parathyroid hormone (PTH) mediated. Whereas PTH-mediated hypercalcemia is primary HPT, non–PTH-mediated hypercalcemia is usually caused by underlying malignancy, granulomatous disease, or vitamin D intoxication.
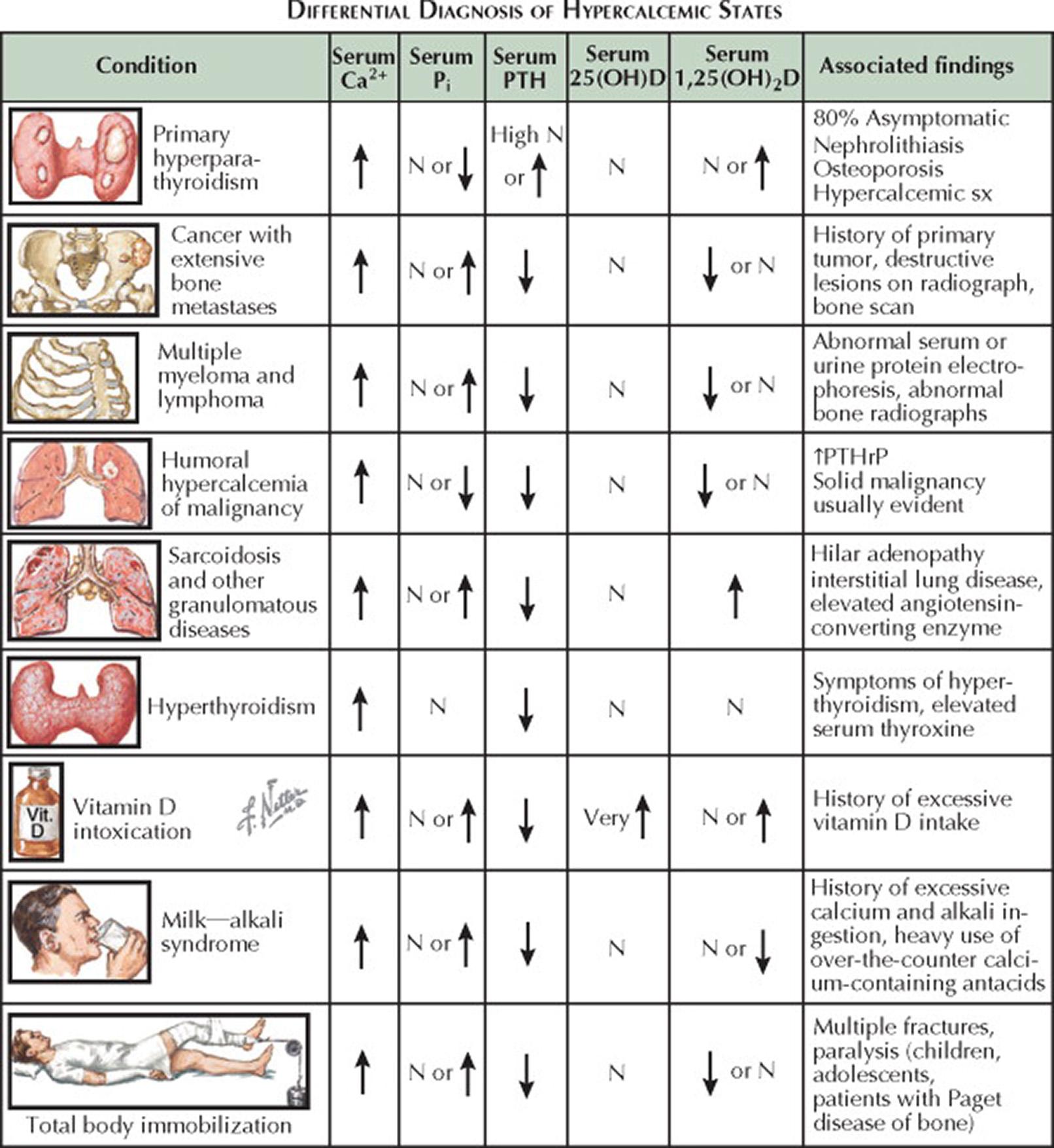
Most patients with primary HPT have serum PTH concentrations above the reference range. However, 20% of patients with primary HPT have serum PTH concentrations in the upper portion of the reference range, but they are inappropriately in the reference range for the setting of hypercalcemia. Measurement of serum inorganic phosphate (P i ) concentration and 24-hour urinary excretion of calcium are usually helpful in confirming PTH-mediated disease. Hypophosphatemia results from the PTH-related inhibition of renal proximal tubular phosphate reabsorption. Although hypophosphatemia may also be seen with PTH-related protein (PTHrP)–mediated hypercalcemia, it is not seen with the other forms of non–PTH-mediated hypercalcemia. The 24-hour urinary excretion of calcium is either at the high end of the reference range or above the reference range in most patients with primary HPT. However, this finding is not specific to primary HPT and is seen with most other causes of hypercalcemia. The 24-hour urinary excretion of calcium is typically less than 100 mg in patients with milk–alkali syndrome or familial hypocalciuric hypercalcemia or in patients treated with thiazide diuretics. Serum 1,25-dihydroxyvitamin D (calcitriol; 1,25[OH] 2 D) concentrations are usually increased in patients with primary HPT because PTH increases the 1α-hydroxylation of 25-hydroxyvitamin D (calcidiol; 25[OH]D) in the kidney.
When the serum PTH concentration is low in a patient with hypercalcemia, additional stepwise testing to consider includes measurement of 25(OH)D, 1,25(OH) 2 D, PTHrP, serum thyrotropin (TSH) (for hyperthyroidism), and vitamin A and performance of serum protein electrophoresis (for multiple myeloma).
PTHrP—the hormone responsible for humoral hypercalcemia of malignancy—is an agonist at the PTH receptor and may be hypersecreted by solid malignancies. However, unlike PTH, PTHrP does not induce renal conversion of 25(OH)D to 1,25(OH) 2 D.
If the serum concentration of PTHrP is low, serum concentrations of 25(OH)D and 1,25(OH) 2 D should be measured. Whereas a markedly increased serum concentration of 25(OH)D is consistent with vitamin D intoxication, increased serum concentrations of 1,25(OH) 2 D may be seen with the enhanced extrarenal 1α-hydroxylation of 25(OH)D that occurs in granulomatous disorders and lymphoma. In this setting, sarcoidosis is usually evident on a plain chest radiograph or computed tomography; these images usually demonstrate bilateral hilar adenopathy and reticular pulmonary opacities.
If vitamin D and PTHrP levels are low in a patient with non–PTH-mediated hypercalcemia, then serum protein electrophoresis should be performed, and serum TSH and vitamin A concentrations should be measured. In this setting, hypercalcemia is usually associated with stimulation of bone resorption (e.g., multiple myeloma, hyperthyroidism, vitamin A intoxication, or immobilization) or increased calcium intake in the setting of renal insufficiency (milk–alkali syndrome).
Increased serum total calcium concentration is usually the result of an increased amount of free (ionized) calcium—the physiologically relevant fraction. Rarely, an increased measured amount of total blood calcium may be attributable to increased amounts of calcium bound to protein (e.g., in multiple myeloma with increased calcium-binding paraprotein) and the ionized fraction is normal; this is termed pseudohypercalcemia .
Familial hypocalciuric hypercalcemia (FHH) is a rare autosomal dominant disorder associated with an inactivating mutation in the gene encoding the calcium-sensing receptor. Typically identified because of incidental discovery of hypercalcemia, these patients have hypocalciuria with the fractional urinary excretion of calcium less than 1%. Patients with FHH have mild hypercalcemia with either normal or slightly increased concentrations of serum PTH. The mutation responsible for FHH makes the calcium-sensing receptor less sensitive to calcium. Thus, a higher than normal serum calcium concentration is required to reduce PTH release; an increase in tubular calcium and magnesium reabsorption in the kidney results in hypercalcemia, hypocalciuria, and hypermagnesemia. These patients are asymptomatic; FHH has a benign natural history, and no treatment is required. All first-degree relatives should be tested for hypercalcemia and alerted that it is not primary HPT and that no surgery is needed.
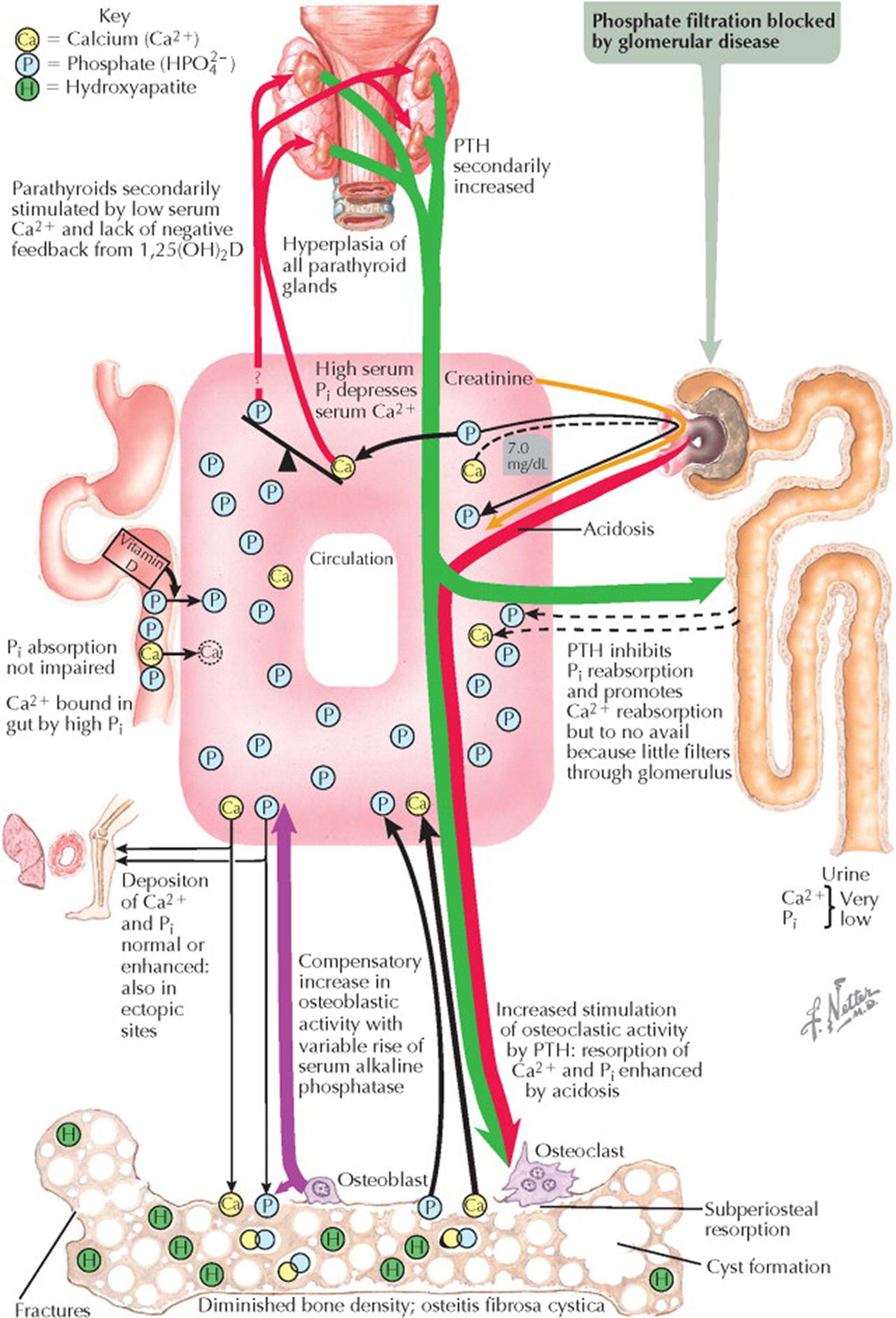
The kidneys have a central role in regulating mineral metabolism. Renal osteodystrophy refers to the bone morphology alterations found in patients with chronic kidney disease. Common forms of renal osteodystrophy in patients with progressive renal failure include high bone turnover with secondary or tertiary hyperparathyroidism (HPT) and the associated osteitis fibrosa cystica, low bone turnover with adynamic bone disease, low bone turnover combined with increased amounts of unmineralized bone (osteomalacia), bone cysts related to β2-microglobulin–associated amyloid deposits, and mixed osteodystrophy in which components of high and low bone turnover are found. The two key factors in renal osteodystrophy are decreased renal conversion of 25-hydroxyvitamin D(25[OH]D) to 1,25-dihydroxyvitamin D (1,25[OH] 2 D) and decreased ability to excrete inorganic phosphate (P i ).
As renal failure progresses, the glomerular filtration rate (GFR) decreases, resulting in a decreased filtered load of phosphate. As the serum phosphate concentration rises, the serum calcium (Ca 2+ ) concentration decreases, and the serum parathyroid hormone (PTH) secretion increases. The secondary HPT state is also a result of decreased blood concentration of 1, 25(OH) 2 D; when GFR decreases below 30 mL/min, the decreased renal mass leads to decreased renal 1α-hydroxylation of 25(OH)D. Over the short term, the increased serum PTH concentrations provide a temporary and partial correction in the biochemical abnormalities by decreasing renal tubule reabsorption of filtered phosphate, increasing bone reabsorption of calcium, and stimulating the renal 1α-hydroxylation of 25(OH)D. However, over the long term, these effects are pathologic as renal failure progresses, and PTH effects have no influence on the failed kidneys. This maladaptive situation is made worse by the renal failure–associated hyperphosphatemia directly decreasing the renal conversion of 25(OH)D to 1,25(OH) 2 D, leading to less 1,25(OH) 2 D inhibition on PTH secretion.
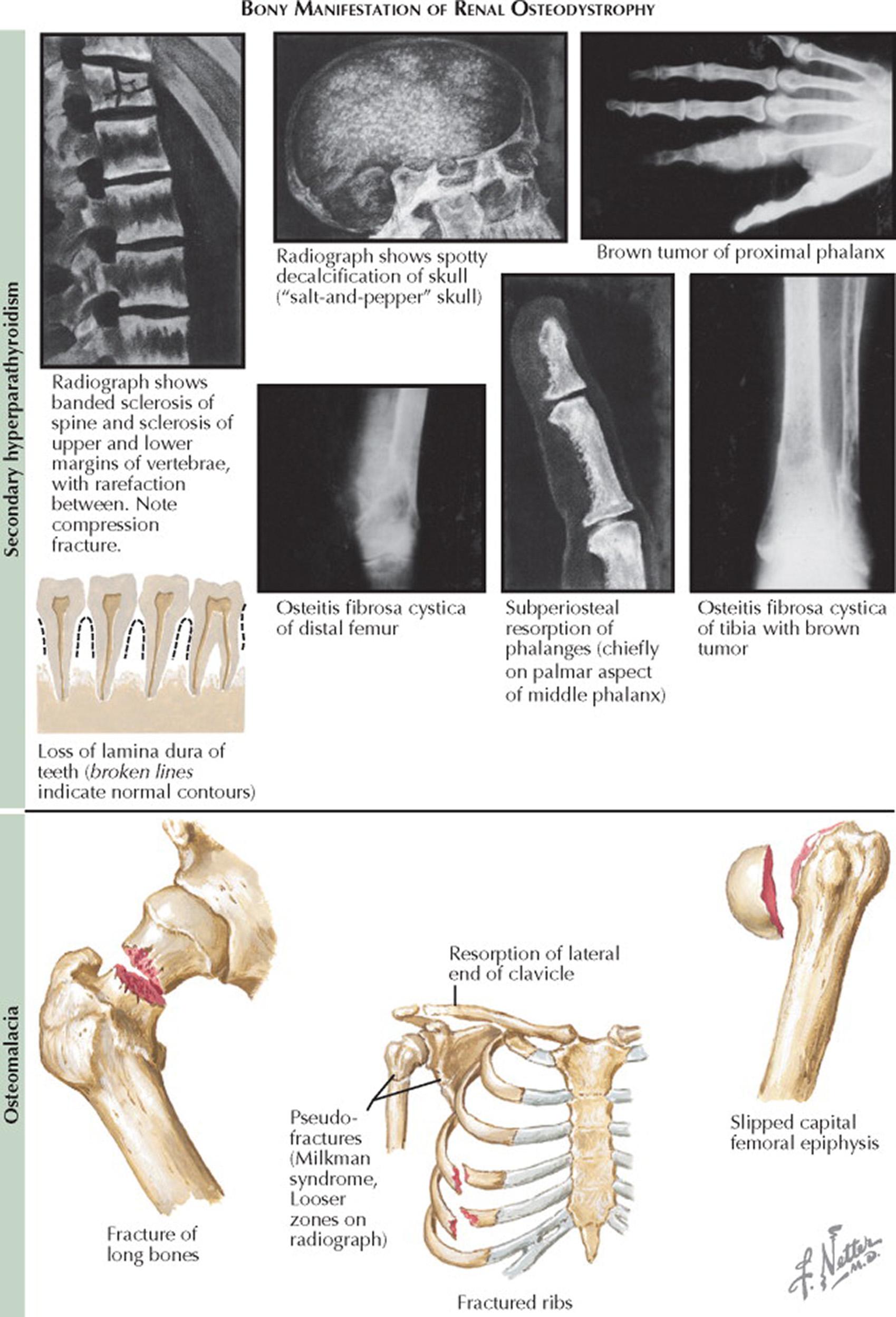
Tertiary hyperparathyroidism results when there is refractory hypersecretion of PTH associated with severe parathyroid gland hyperplasia and neoplastic transformation with monoclonal parathyroid adenomas. Because of the limited effect of PTH on enhancing phosphate excretion in failing kidneys and the continued effect on reabsorption of calcium and phosphate from bone, hypercalcemia develops. The increased calcium–phosphate product leads to metastatic calcification with calcium–phosphate precipitation into soft tissues, joints, viscera, and arteries. Blood vessel involvement can lead to ischemia and gangrene. Tertiary HPT results in subperiosteal resorption, cyst formation, variably diminished bone density, osteitis fibrosa cystica with brown tumors, and fractures. Spine radiographs may show banded sclerosis of the upper and lower margins of the vertebral bodies with rarefaction between. Skull radiographs may show spotty decalcification (“salt-and-pepper” skull).
Subperiosteal resorption of the phalanges may be evident—primarily on the palmar aspect of the middle phalanx. In children, renal osteodystrophy results in growth retardation and skeletal deformities.
The most common bony abnormality in patients treated with peritoneal dialysis or hemodialysis is adynamic bone disease—bone turnover does not occur, and bone cell activity is absent. Unlike what is observed with osteomalacia, osteoid formation does not increase. Adynamic bone disease is, in part, a result of excess PTH suppression with calcium-based phosphate binders and vitamin D analogues. Adynamic bone disease predisposes to bone fractures (e.g., hip fracture). This disorder can be confirmed by bone biopsy or by a serum PTH concentration less than 100 pg/mL. The key to treatment is to allow PTH to increase by decreasing or discontinuing calcium-based phosphate binders and vitamin D analogues.
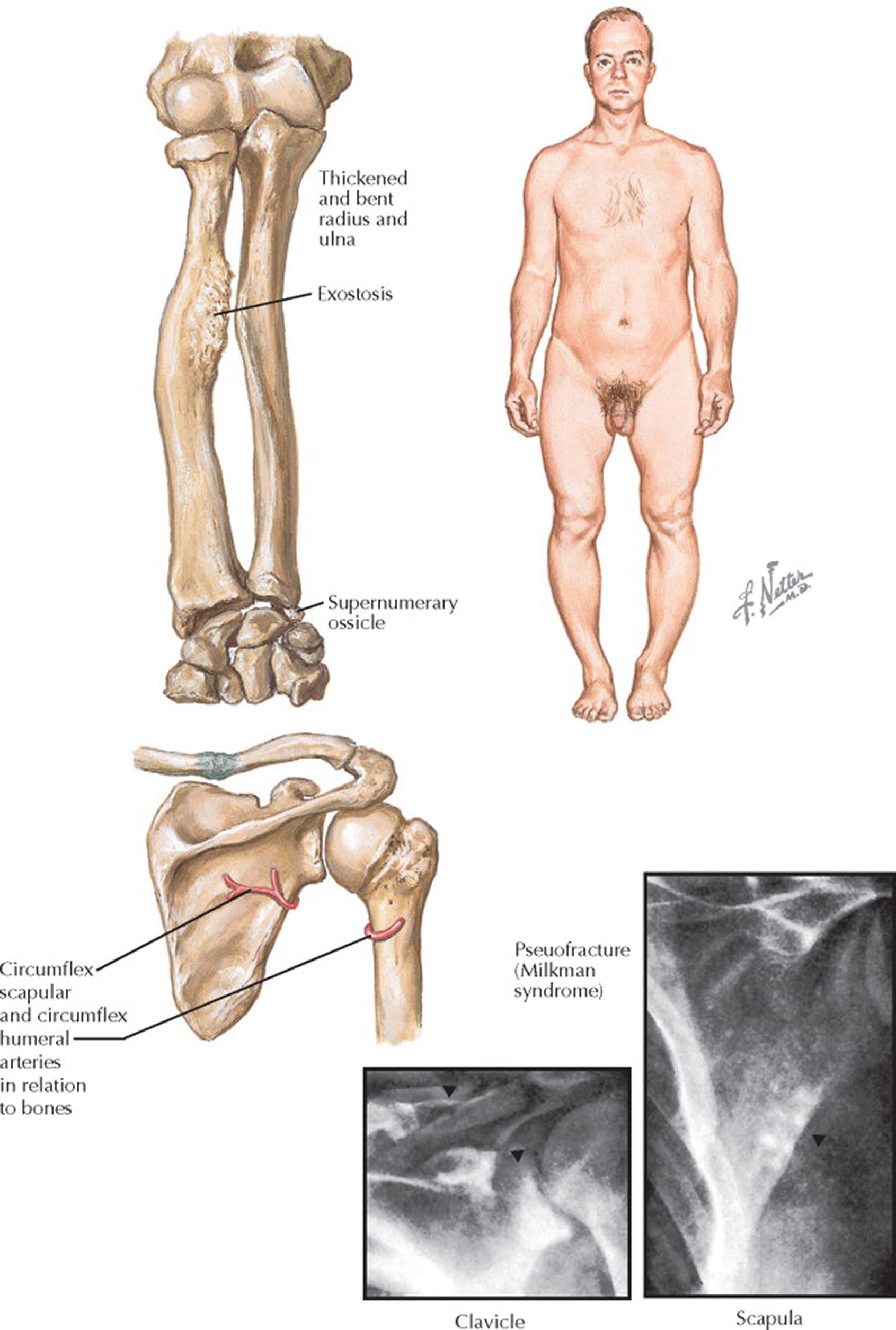
Osteomalacia is the result of decreased bone turnover and an increase in unmineralized bone caused by either vitamin D deficiency or aluminum intoxication (the latter from aluminum-containing antacids). There is decreased bone density with thinning of the cortex. Looser zones are a characteristic radiologic finding in osteomalacia (see Plates 6-8 and 6-22 ). Looser zones are pseudofractures or narrow radiolucent lines (2–5 mm in width) with sclerotic borders that lie perpendicular to the cortical margin. Frequently bilateral and symmetric, they are located in the scapula, femoral neck, medial part of the femoral shaft, ribs, pubic and ischial rami, and clavicle. Bone resorption may be seen at the lateral ends of the clavicles. Milkman syndrome refers to the findings of bilateral and symmetric pseudofractures in a patient with osteomalacia. Fractures may occur with minimal or no trauma and usually involve the long bones (e.g., hip), ribs, and vertebral bodies. A bone biopsy—usually obtained from the iliac crest after the administration of tetracycline markers to determine the rate of new bone formation (see Plate 6-22 )—may be needed to determine the pathogenesis of renal osteodystrophy.
Other contributing factors to bone disease in patients with renal failure include vitamin K deficiency (required for carboxylation of bone matrix proteins) and bone morphogenetic protein-7 (required for normal osteoblast differentiation and produced in the normal kidney).
The goals of treatment should be to maintain normal serum calcium and phosphorus concentrations while minimizing the exposure to aluminum. These targets are achieved by a low-phosphate diet, addition of phosphate binders when GFR is below 25% of normal, and vitamin D supplementation to maintain a normal blood concentration of 1,25(OH) 2 D. With advanced renal failure and tertiary HPT, parathyroidectomy may be indicated.
Primary hyperparathyroidism (HPT) is caused by a single parathyroid adenoma (89%), multiple (“double”) parathyroid adenomas (4%), multigland parathyroid hyperplasia (6%), or parathyroid carcinoma (1%).
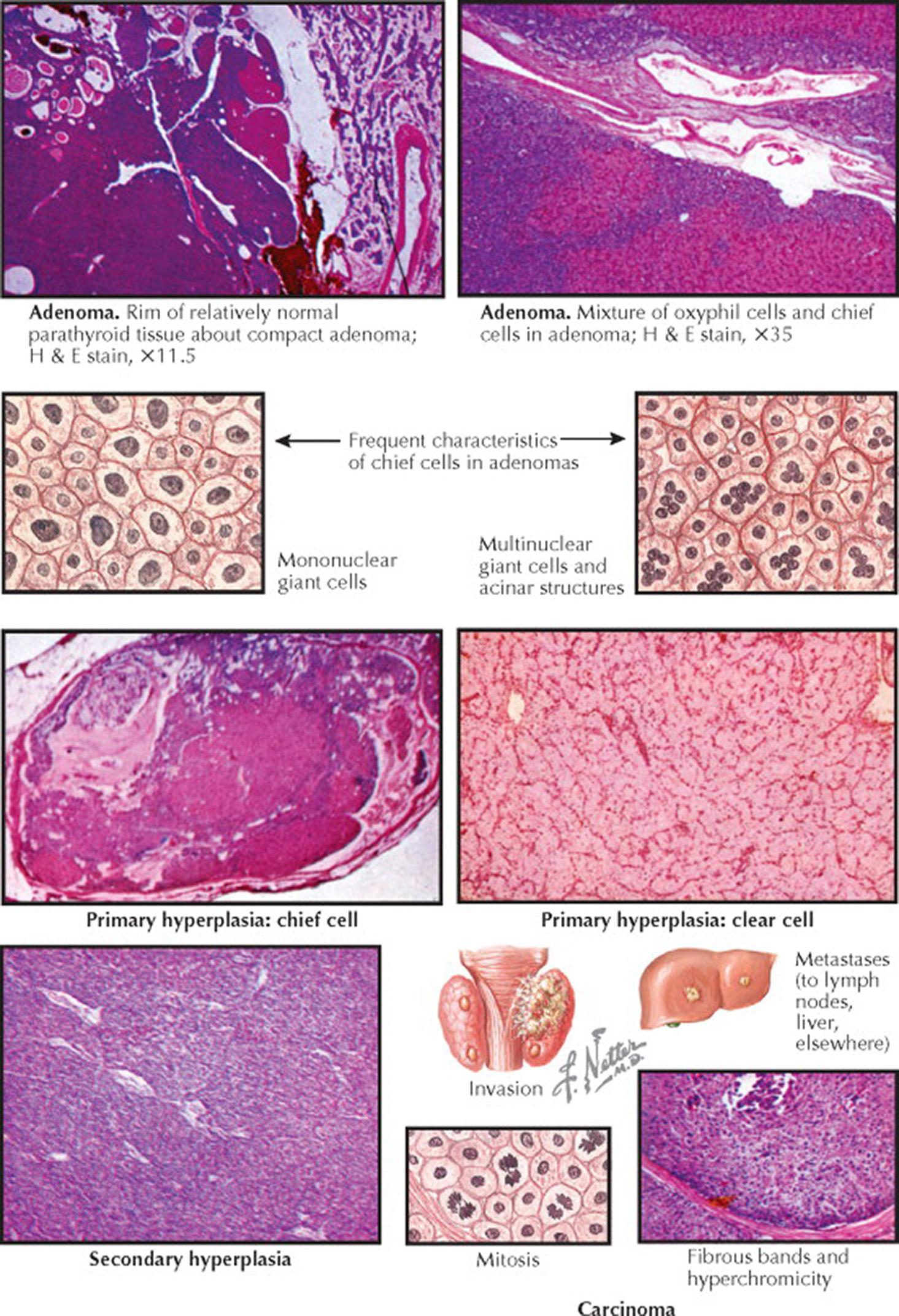
Most parathyroid adenomas are encapsulated and arise from chief cells; the others are composed of oxyphilic cells. The adenoma is composed of tightly packed sheets, cords, and acini of predominantly chief cells. Clear cells and oxyphilic cells, singly and in groups, are often present, and some adenomas may be composed entirely of oxyphilic cells. The tumor may be homogeneous or nodular. The chief cell of an adenoma is usually somewhat larger than a normal chief cell. The nuclei are variable in size, and mononuclear giant cells with hyperchromatic nuclei, not indicative of malignancy, are often present. Multinuclear giant cells are frequently seen in adenomas. Immunohistochemical staining is typically positive for parathyroid hormone (PTH) and chromogranin A. The most important criterion for differentiating an adenoma from chief cell hyperplasia is the identification of normal parathyroid tissue in a patient with an adenoma; this may occur either as a rim outside the capsule of the adenoma or in another gland. This “normal” parathyroid in the presence of an adenoma is composed almost entirely of large, light, inactive chief cells, with abundant glycogen, small Golgi apparatuses, and rare secretory granules. The cells in the adenomas are monoclonal and result from somatic mutations in genes that control growth. For example, the cyclin D1 proto-oncogene ( CCND1 ) encodes a major regulator of the cell cycle. Overexpression of cyclin D1 is found in approximately 30% of parathyroid adenomas. Multiple endocrine neoplasia (MEN) type 1 is caused by germline mutations in MEN1 , a tumor suppressor gene, and somatic mutations are found in approximately 15% of sporadic parathyroid adenomas.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here