Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Over the past decade, a wealth of studies has provided the scientific rationale to warrant the early screening and preemptive antithrombotic management of blunt cerebrovascular injuries (BCVIs). In the 1990s, BCVIs were thought to have unavoidable, devastating neurologic outcomes, but several reports suggested anticoagulation improved neurologic outcome in patients suffering ischemic neurologic events (INEs). If untreated, carotid artery injuries (CAIs) have a stroke rate up to 50% depending on injury grade, with increasing stroke rates correlating with increasing grades of injury; vertebral artery injuries (VAIs) have a stroke rate of 20% to 25%. Subsequently, screening protocols, based on patient injury patterns and mechanism of injury, have been instituted to identify these injuries in asymptomatic patients to initiate treatment prior to neurologic sequelae. Current studies suggest that early antithrombotic therapy in patients with BCVI reduces stroke rate and prevents neurologic morbidity.
BCVIs were initially reported by Verneuil in 1872. The patient’s symptom of cerebral ischemia or the distribution of the symptoms usually indicates the underlying cerebrovascular lesion. These symptoms are also dependent upon the presence or absence of collateral circulation and the presence or absence of underlying cerebrovascular disease. The circle of Willis is incomplete in 80% of patients. CAIs generally result in contralateral sensorimotor deficit, which is generally defined as a stroke. Aphasia occurs when the dominant hemisphere is involved, but nondominant hemisphere strokes may result in hemineglect. VAIs typically manifest with more vague symptomatology, including ataxia, dizziness, vomiting, facial or body analgesia, and visual field defects. Symptoms of carotid-cavernous fistulas include orbital pain, exophthalmos, chemosis, and conjunctival hyperemia. There are several signs that are highly suggestive of BCVI and should be investigated promptly, including active arterial hemorrhage from the neck, mouth, nose, or ear; expanding cervical hematoma; cervical bruit in a patient younger than 50 years of age; and focal or lateralizing neurologic deficits.
Although some patients may present with symptoms of BCVI-related ischemia within an hour of injury, the majority exhibit a latent period. This asymptomatic phase has been inferred, based upon the time to onset of symptoms, in patients with defined injuries who did not receive antithrombotic therapy. This time frame appears to range from hours up to 14 years, but the majority seems to develop symptoms within 12 to 75 hours. The goal, then, is diagnosing BCVI during this “silent period” prior to the onset of stroke. Existing data affirm that if you diagnose these injuries during the asymptomatic period, you can effectively treat the patient to prevent stroke. Screening for BCVI during the asymptomatic period was initially suggested in the mid-1990s after the recognition that specific patterns of injuries were associated with BCVI. Although optimal screening criteria have yet to be defined, current screening algorithms include patients considered at high risk based on their injury pattern.
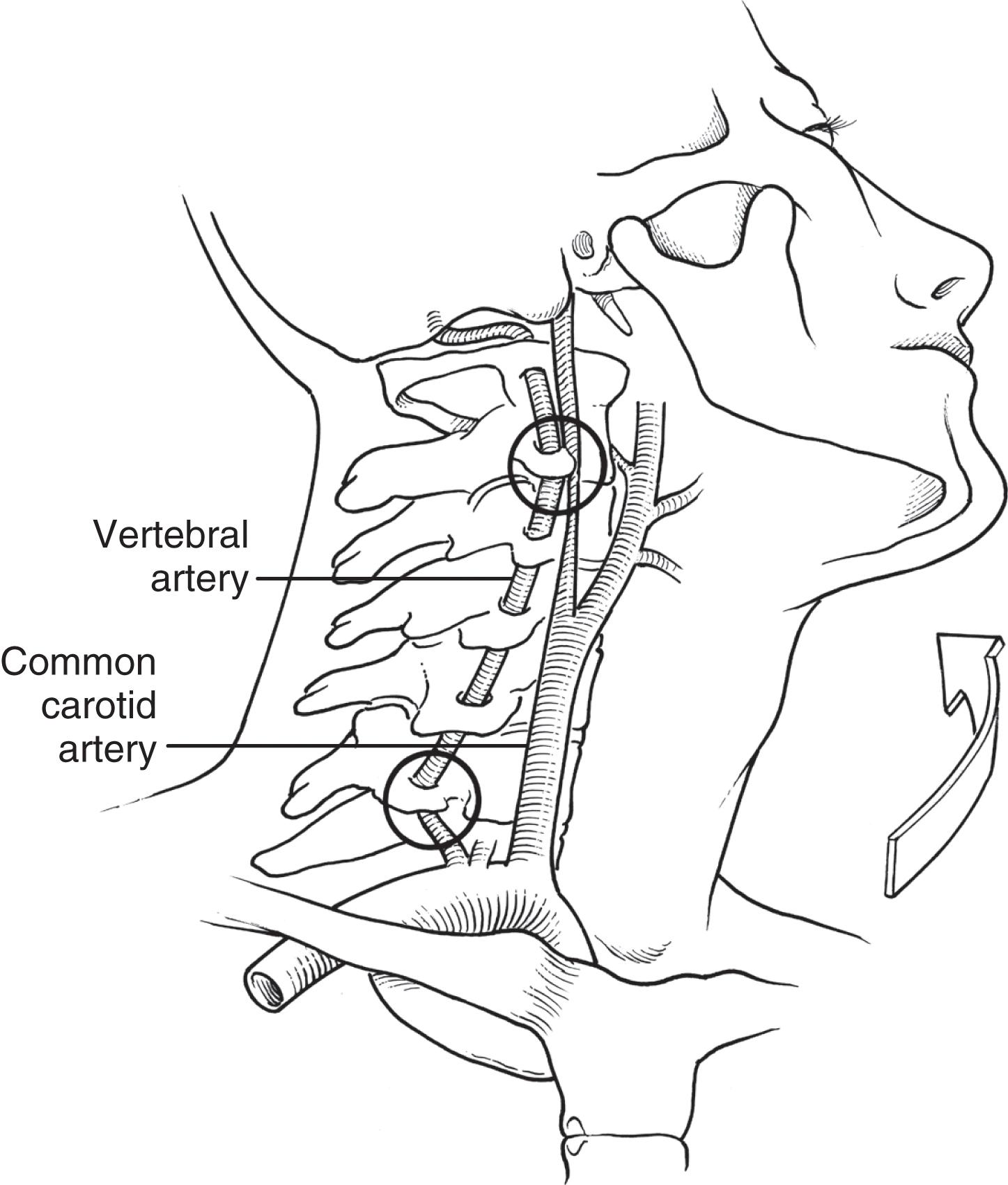
Crissey and Bernstein originally postulated three fundamental mechanisms of injury resulting in BCVI. The first is a direct blow to the neck. This mechanism is often seen with patients in motor vehicle collisions with inappropriately fitting seatbelts, indicated by a seatbelt sign across the neck; it can also be seen in recreational sports with a direct blow to the neck. The second proposed mechanism is hyperextension with contralateral rotation of the head and neck. This is the most common mechanism causing CAI with the hyperextension resulting in a stretching of the carotid artery over the lateral articular processes of C1–C3 ( Fig. 1 ). VAI may also be due to a hyperextension-stretch injury due to the tethering of the vertebral artery within the lateral masses of the cervical spine. The third mechanism of injury is a direct injury of the artery by adjacent fractures involving the sphenoid or petrous bones. Although originally described as the mechanism in association with CAI, this may also be the cause of VAI. With a fracture of the bony elements composing the vertebral foramen, the foramen transversarium, it is not surprising that the vertebral artery can also be injured directly. Regardless of the type of injury mechanism, there is intimal disruption of the carotid or vertebral artery. This intimal tear exposes subendothelial collagen and becomes a nidus for platelet aggregation that may lead to thrombosis or emboli and subsequent stroke. The intimal tear may also result in subintimal dissection causing luminal narrowing or acute occlusion of the affected vessel. Pseudoaneurysm formation is also possible, which may compress the vessel or develop thrombus with the potential for embolism. Finally, free rupture may result in intracranial or extracranial hemorrhage or the development of arteriovenous fistulae.
Although the mechanism of injury is important for determining patients at high risk for BCVI, the patient’s associated injuries are also critical to determine which asymptomatic patients need screening for BCVI. Aggressive screening for BCVI was initially suggested after recognition that specific patterns of injuries were associative. Current screening algorithms include patients with signs or symptoms, as well as those considered at high risk by injury pattern ( Table 1 ). With the availability of noninvasive diagnosis using computed tomographic angiography (CTA), and the recognition that historical protocols missed 20% of BCVI, broadening BCVI screening guidelines has been suggested. Injury patterns not originally included that are now potential triggers for diagnostic imaging include mandible fracture, complex skull fracture, traumatic brain injury with thoracic injuries, scalp degloving, thoracic vascular injuries, upper rib fractures, and clothesline type injury/seatbelt abrasion with significant swelling, pain, or altered mental status. Additionally, screening protocols should include all patients with cervical spine fractures, ligamentous injury, or spinal cord injury to rule out BCVI. Finally, screening with noninvasive CTA for all high-energy transfer mechanisms is advocated based upon the morbidity of a missed injury compared to the risk of imaging.
| Signs/symptoms of BCVI |
|
| Risk factors for BCVI |
|
Using defined screening protocols, high-risk patients undergo imaging to identify BCVI. Historically, four-vessel digital subtraction arteriography (DSA) was the gold standard to diagnose BCVI. However, many clinicians appropriately questioned the need for subjecting patients to angiography. Angiography is labor-intensive, costly, and not without risks; if not available at smaller hospitals, angiography requires emergent transfer of a patient for definitive evaluation.
CTA is an attractive alternative to catheter-based diagnostic imaging. In addition to being a noninvasive imaging modality, the majority of patients undergoing screening for BCVI have indications for CT scanning of other regions. Hence, imaging can often be accomplished with only one “road trip” or via whole-body multidetector CT. Due to its ready availability, CTA can reduce the time to BCVI identification and permits earlier treatment than historical angiography.
Although the accuracy of early-generation one- to four-slice CTA was relatively poor for diagnosing BCVI (sensitivity 47% to 68% and specificity of 67%), identification of injuries improved with the introduction of multidetector-row CTA. Five published studies and an additional report have evaluated the accuracy of 16-slice CTA compared with arteriography. Eastman and colleagues evaluated 162 patients with CTA, of whom 146 agreed to angiography. With a screening yield of 28%, they reported 100% sensitivity of 16-slice CTA for CAI, and 96% sensitivity for VAI, with one false-negative CTA of a grade I injury. The Harborview study illustrates the importance of experience in identifying BCVI on a CTA; they performed arteriography on 82 patients who had had a normal screening CTA and found that CTA missed seven BCVIs, for a negative predictive value of 92%. However, retrospective review of the CTA images found that the injuries were evident in six of the seven patients and that the seventh patient’s abnormality was most likely not traumatic in origin. Moreover, all missed injuries occurred in the first half of the study period. A very similar finding was noted by the Medical College of Virginia group; they screened 119 patients with 92 undergoing confirmatory angiography. They reported a 43% false-positive rate and 9% false-negative rate for CTA, but all of the missed BCVIs occurred in the first half of the study period. In the second half of the study, the sensitivity and negative predictive value of CTA was 100%. Each of these studies recognize that injuries in the region of the skull base appear to be the most difficult to identify, underlining the importance of carefully examining this high-risk region. In a 2009 study by Goodwin and colleagues, the reported sensitivity of 16-slice CTA was 29% and only 54% for 64-slice CTA. These are by far the worst results for high-resolution CTA; however, without quality control it is difficult to understand how best to interpret this study’s impact on screening options for BCVI. DiCocco and colleagues from Memphis reported a similar 51% sensitivity for 32-slice CTA compared to DSA. Two recent studies from the Memphis group suggest an alternative approach to evaluation of these injuries. The first suggests that 64-slice CTA may replace DSA with a significantly improved sensitivity of 68% and over 60% of false-negatives comprising low-grade injuries. The second coupled 64-slice CTA with confirmatory DSA and found that 55% of injuries on CTA were confirmed on subsequent DSA, leading the authors to conclude that screening 64-slice CTA with confirmatory DSA should be employed to avoid unnecessary anticoagulation in 45% of patients. But, as mentioned previously, DSA requires transfer to a center with these capabilities and has potential embolic complications. The 2020 Practice Management Guidelines from the Eastern Association for the Surgery of Trauma recommend screening using CTA in patients with high-risk cervical spine injuries and conditionally recommend CTA for patients with low-risk cervical spine injuries.
Whole-body multidetector-row CT is being increasingly employed in trauma centers due to the advantages of more rapid imaging and utilizing a single dose of contrast for the entire study. Sliker and colleagues have demonstrated equivalent accuracy of whole-body multidetector-row CT to 16-slice CTA; however, further studies are warranted.
All patients with indications for screening should undergo imaging as soon as possible. Patients with documented BCVI often undergo repeat imaging 7 to 10 days after their initial diagnostic study or sooner for any change in neurologic status. The importance of routine follow-up imaging is particularly salient in patients with grade I and II injuries; over half of grade I injuries completely heal, allowing cessation of antithrombotic therapy. Conversely, less than 10% of all grade II, III, and IV injuries heal, with injury progression rates of approximately 12% for all treated BCVI in historical cohorts. Therefore, a pragmatic approach to repeat imaging is based on the number of BCVIs, the grade of each BCVI, and whether the patient requires antithrombotic therapy for other indications. For example, a patient with a single grade I CAI should undergo repeat imaging with an approximate 50% rate of healing and hence cessation of antithrombotics. Conversely, a patient with a grade III VAI, a grade II right CAI, and a grade I left carotid is unlikely to demonstrate healing or resolution of all three injuries; repeat imaging at 7 days may not be warranted and rather a repeat CTA planned for 3 to 6 months after initiation of treatment. A more recent study from our group investigating the rate of resolution on repeat imaging showed that 20% and 17% of grade II CAI and VAI, respectively, resolve on repeat imaging. A subsequent study from Baltimore found a 50% rate of improvement or resolution for grade II BCVI on repeat imaging of 379 patients with 509 BCVIs; however, up to 30% of lesions progressed. Patients with carotid or vertebral artery occlusions are not as critical to reimage, as over 80% display no change on follow-up imaging. On the other hand, some authors have advocated an endovascular approach to pseudoaneurysms, hence supporting the use of repeat imaging to reassess grade II and III injuries for progression.
Other imaging modalities have been employed to varying degrees to screen for BCVI. While DSA is still considered the gold standard for diagnosis, it has largely been supplanted by CTA as the latter is less invasive and associated with fewer complications. Still, DSA is required in certain circumstances, including when the suspicion for BCVI is high despite negative or equivocal noninvasive testing. Some also advocate the use of DSA for confirming positive findings on other studies to reduce the risk of unnecessary anticoagulation. Magnetic resonance angiography (MRA) and duplex ultrasonography have also been studied for use in BCVI screening. MRA is not an effective screening modality given the length of time required to perform the examination and its low sensitivity and specificity for BCVI. Similarly, duplex ultrasonography has several shortcomings that preclude its use for BCVI screening. These include the inability to visualize injuries at the base of the skull, which is where the majority of these injuries occur; the need to remove a cervical collar in a group of patients that are high risk for cervical spine injuries; and operator dependence for high quality studies.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here