Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In the context of neonatal care, invasive or noninvasive measurement of blood pressure can be accomplished with acceptable precision across all gestational age categories.
Gestational and postnatal-age-dependent population-based normative blood pressure ranges are available. However, their usefulness in the assessment of adequacy of circulatory status remains limited.
Interrogation of the individual components of the patient’s blood pressure may provide a greater understanding of the underlying physiology. This includes systolic, diastolic, mean blood pressure and pulse pressure.
Clinical, biochemical, and echocardiographic hemodynamic information in addition to individual BP components will provide a more complete assessment of cardiovascular well-being as we await more objective comprehensive hemodynamic monitoring and data acquisition systems to permit an individualized approach to care.
Historically blood pressure, in particular mean blood pressure, was the main determinant of circulatory well-being in the neonatal intensive care unit. Typically, it was the sole criterion used to initiate intervention, especially in preterm infants. The primary reason was the assumption that low blood pressure, however defined, was directly and strongly correlated with cardiac output and end-organ blood flow. While this practice remains commonplace, we now understand that as hemodynamic monitoring tools become more accessible and utilized at the bedside, the many shortcomings of our overreliance on blood pressure measurements to guide intervention is highlighted. However, rather than neglecting this measurement, we contend that it remains a critically important hemodynamic value and one of several other important measurements to be considered in the assessment of circulatory well-being.
In this chapter we have set out to address several factors: What is meant by blood pressure, how it is regulated, how it should be measured, what factors determine blood pressure, and how blood pressure should be factored into decision-making in commonly encountered conditions in the neonatal intensive care unit.
When the term blood pressure is used, it typically refers to the systemic arterial pressure. This is derived from the pumping action of the left ventricle (LV), and a characteristic aortic pressure waveform is generated with each contraction. This is the standard blood pressure waveform that we visualize in the neonatal unit every day, transduced from the aorta, displayed at the patient bedside. The peak pressure generated refers to the systolic pressure. The pressure subsequently falls to its lowest level in the cardiac cycle prior to the next contraction and is termed the diastolic pressure. The numerical difference between systolic and diastolic pressure is the pulse pressure (PP). An understanding of the individual components of the arterial pressure waveform can provide greater insights into cardiovascular status.
The pressure waveform can be separated into upstroke (anacrotic) and downstroke (dicrotic) limbs. The elements of the waveform consist of the systolic upstroke, the peak systolic pressure, the systolic decline, dicrotic notch, diastolic run-off, and end-diastolic pressure ( Figure 3.1 ). The area under the curve represents the mean arterial pressure and historically was calculated as the mean arterial pressure equal to the diastolic pressure plus one-third of the PP. However, this is a simplification of the calculation. In two patients the systolic and diastolic components can measure the same, but the area under the curve might be substantially different, resulting in significantly different mean arterial blood pressure measurements.
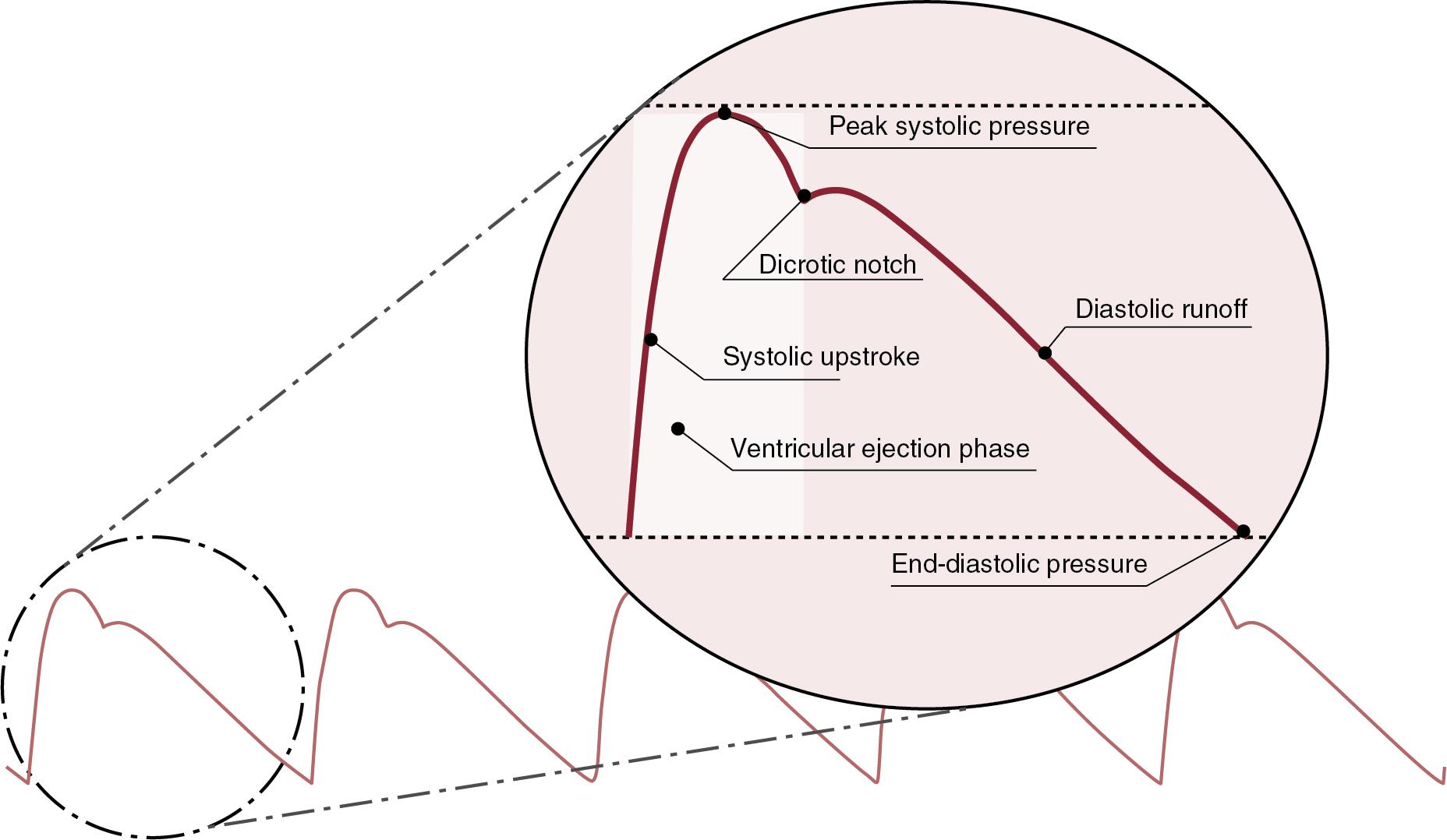
The systolic upstroke is a direct reflection of left ventricular (LV) ejection and corresponds to the peak aortic blood flow acceleration across the aortic valve. Thus factors that influence these will result in alterations of the upstroke. A prime example may be a slurred upstroke in the setting of aortic stenosis. The peak systolic pressure represents the maximum pressure in the central arteries and is directly related to LV contraction, the compliance of the arterial system, and reflected waves. Reflected waveforms refer to backward wave reflection as the resistance increases in the distal arteries. The effect of reflected waves is to increase the systolic blood pressure and alter the shape of the waveform. This phenomenon in the aorta is not typically seen in the neonate. As one moves further distally from the aorta, the phenomenon of distal systolic pulse amplification occurs as the systolic pressure increases due to augmentation of the reflected waves. However, the overall area of the waveform tends to decrease, resulting in a reduction in the overall mean blood pressure.
The systolic decline represents the drop in blood pressure as the ventricular contraction ceases and blood moves from the central arterial compartment faster than the influx from the later phase of ventricular contraction. Conditions such as LV outflow tract obstruction in the setting of hypertrophic obstructive cardiomyopathy result in a very rapid decline in this phase.
The dicrotic notch seen on the systolic decline represents a sudden small increase in blood pressure. This notch when measured in the aorta is referred to as the incisura because it “cuts into” the descending waveform. This increase in blood pressure represents aortic valve closure. As one moves further into the distal circulation, the dicrotic notch is more likely a reflection of the peripheral vascular resistance rather than aortic valve closure. Also, the latency between the peak systolic pressure and the dicrotic notch increases. Loss of the incisura/dicrotic notch on central invasive blood pressure monitoring may reflect transducer damping (discussed later).
Diastolic run-off is related to the cushioning effect of the elastic recoil of the large vessels and is often referred to as the Windkessel effect. In essence the large vessels act as reservoirs that distend during systole and recoil during diastole, permitting movement of blood throughout the cardiac cycle. The cushioning effect enables conversion of highly pulsatile flow from LV ejection into steady, non-pulsatile flow in downstream capillaries while also limiting large fluctuations in PP. Distal vessel structure contains a greater proportion of elastin. Otto Frank (of the Frank-Starling Law) pioneered work in this area. The end-diastolic pressure represents the pressure exerted primarily by the terminal resistance arterioles and is a very important factor in determining coronary blood flow.
Mean arterial BP (MAP) is the product of cardiac output and systemic vascular resistance. Accordingly, it is the dependent variable determined by the two independent variables in the circulation. It is in essence the hydrodynamic form of Ohm’s law (voltage difference is equal to current times resistance), where pressure difference is equal to flow times resistance.
Changes in cardiac output and systemic vascular resistance can result in significant alterations in blood pressure values. An understanding of each of these factors and their interrelatedness is essential to interpreting blood pressure, particularly in the first postnatal hours and days when significant changes in each may occur. The problem to date has been the fact that measuring cardiac output was previously challenging; however, with the use of echocardiography and noninvasive cardiac output monitoring, this has become feasible. Chapters 9 , 10 , and 12 deal with these assessments in much greater detail.
Briefly, cardiac output is the amount of blood pumped with each contraction and is the product of heart rate and stroke volume (SV). SV is influenced by several factors including preload, afterload, inotropy, ventricular compliance, and chronotropy. Preload represents the sarcomere length just prior to contraction (at the end of diastole) and as this cannot be measured, we rely on indirect measures such as end-diastolic volume. This in turn is dependent on ventricular compliance and pressure. An increase in venous return will result in an increase in ventricular filling, end-diastolic volume, and thus in preload. This increase in preload results in an increase in the force of the contraction and thus an increase in SV. This is the Frank-Starling mechanism described by Otto Frank and Ernest Starling. In newborns the curve is relatively flat across a range of normal filling pressures. A typical example might be an increase in cardiac output following a bolus of fluid.
Afterload is the load against which the heart must contract to eject blood. An increase in afterload (for whatever reason) will typically result in a decrease and shift to the right in the Frank-Starling curve and ultimately a decrease in SV and cardiac output. Both ventricles are particularly sensitive to increases in afterload. However, a sudden increase in afterload may result in an increase in preload and thus a Frank-Starling response resulting in short-term increased SV (Anrep effect), highlighting the interdependency of preload and afterload.
Inotropy (activation of the contractile proteins) occurs independent of sarcomere length. Changes in inotropy will result in changes in the Frank-Starling relationship such that an increase in inotropy effects an increase in SV. An increase in heart rate may also result in an increase in inotropy termed the Bowditch effect or force frequency relationship. This refers to the increase in inotropy that occurs with an increase in heart rate. This effect is most likely due to an increase in intracellular calcium. This phenomenon has not been investigated to any great extent in the newborn. However, an increase in heart rate may have a negative effect on diastolic filling time and thus negatively impact preload. These factors are one example of the interdependency of preload, afterload, and inotropy that need to be considered in the newborn, especially where medications which may have differing effects on each are utilized in preterm or term infants with low blood pressure.
An understanding of the vascular system and its function is essential to understanding blood pressure and blood pressure regulation. The vascular system is essentially made up of three types of vessels: distributive, exchange, and capacitance vessels. The distributive vessels include the aorta, large, and small arteries. The primary function of the aorta is to distribute blood into the arterial system and dampen the pulsatile flow. The small arteries and arterioles are the primary resistance vessels and account for approximately 70% of the systemic vascular resistance. These vessels are innervated by the autonomic nervous system and constrict and dilate depending on the underlying situation. The pressure within the arterial system is greatest in the aorta and large arteries. The greatest drop in pressure occurs in the small arterioles due to the increased resistance to flow.
The exchange vessels include the capillaries and small venules. These have a very large surface area and therefore flow across these vessels is low; hence these vessels are the primary sites for exchange to occur. To a lesser extent, they also contribute to the systemic vascular resistance. The capacitance vessels include the large venules, large veins, and venae cavae. Approximately 70–80% of the blood volume at any given point is in the venous system. These vessels have smooth muscle and therefore also contribute to the total systemic vascular resistance by approximately 15%. Venous constriction will result in an increase in venous return and ultimately an increase in SV.
Vascular tone is determined by the interaction between extrinsic and intrinsic factors acting on the smooth muscle of the blood vessel. Extrinsic factors include autonomic nervous system innervation and circulating hormones such as catecholamines, angiotensin, and vasopressin. The intrinsic factors include various endothelial factors, local metabolites, and local hormones among others (see Chapter 2 for a more detailed discussion). Systemic vascular resistance is expressed in mmHg/mL/min or dyn/s/cm –5 , where 1 dyn is 1330 mmHg. While somewhat of an oversimplification, vasoconstrictor elements can be thought of as maintaining SVR and MAP, whereas vasodilator elements are more related to organ blood flow.
Blood pressure regulation is a complex process involving input from multiple sources, including baroreceptors, volume receptors, and chemoreceptors located either peripherally or centrally. Each of these feedbacks to the cardiovascular center is located in the region of the pons and medulla (nucleus tractus solitarius [NTS]). These regions also receive input from the hypothalamus (e.g., temperature regulation), the cortex, and the limbic system (e.g., stress). Efferents from the cardiovascular center act primarily on the myocardium and peripheral vasculature to alter blood pressure as necessary. Sympathetic effects include increased inotropy, increased heart rate, increased conduction velocity (dromotropy) within the heart, and increased vascular smooth muscle activation resulting in vasoconstriction. Vagal activation has the opposite effect.
Arterial baroreceptors are located in the carotid sinus and the aortic arch. When systemic arterial blood pressure drops, there is a decrease in the stretch in the vessel resulting in a reduction in baroreceptor firing to the NTS. As a result, sympathetic activity increases and basal vagal activity decreases. This typically results in an increase in cardiac output and systemic vascular resistance to increase the arterial blood pressure.
Cardiopulmonary baroreceptors are located primarily in the atria or venoatrial area and are often referred to as low pressure receptors or volume receptors. A drop in venous return may result in a reduction in signals from the receptors and thus an increase in sympathetic activity, similar to the arterial baroreceptors response.
Chemoreceptors (central and peripheral) influence cardiovascular function. Hypoxia typically results in an increase in sympathetic activity leading to an increase in arterial blood pressure. Pulmonary stretch receptor activation can result in an inhibition in sympathetic activity and a drop in arterial blood pressure. This is something that should be considered in the setting of mechanical ventilation and overdistension. Humoral factors also have a very important role to play in blood pressure regulation, either directly affecting cardiovascular function or indirectly via alterations in blood volume. These include circulating catecholamines, atrial natriuretic peptide, vasopressin, and renin-angiotensin system (RAS). Some result in relatively quick changes in blood pressure such as circulating catecholamines, whereas others result in gradual increases or decreases in blood pressure over time such as occurs with the RAS via hormonal-mediated mechanisms.
It is not too difficult to appreciate that maturation of these systems will vary with many factors such as in utero environment, gender, gestational age, and postnatal age. , One measure of autonomic function is baroreceptor sensitivity (BRS). BRS can be calculated from spontaneous oscillations of systolic BP and pulse intervals by the cross-correlation sequence method. Javorka et al. studied this among preterm infants matched for postconceptual age (approximately 34 weeks) but who differed in gestational age at birth. Those born more preterm had markedly decreased values of BRS despite having similar postconceptional age. The more mature the infant, the higher the BRS and diastolic and mean blood pressure. This would suggest that in very preterm infants, cardiovascular maturation occurs faster in utero, or alternatively that neonatal interventions may negatively impact on this expected maturation. Other studies have confirmed that BRS in preterm infants is lower than term matched controls. One of these evaluated BRS weekly, and while they did find a significant increase in BRS with postnatal age, this was reduced compared to term controls.
Golder et al. studied autonomic control in the setting of hypotension during the first postnatal days in preterm infants. They evaluated heart rate variability (HRV), blood pressure variability (BPV), and BRS. The assumption was that reduced BRS could potentially be harmful. The study was limited by relatively small numbers of subjects (23 preterm infants). The authors found that gestational age correlated with all measures of HRV but not with BPV and BRS. In the group who received inotropes BRS was lower (3.8 ± 0.9 vs 6.9 ± 1.6 ms/mmHg), and the low-frequency/high-frequency HRV ratio was also lower (5.7 ± 1.3 vs 13.6 ± 2.8, P < 0.05). This ratio may reflect decreased sympathetic activity in those infants who receive inotropes.
Vesoulis assessed BPV in preterm infants (40 in total) who were either normotensive or hypotensive as a method to assess vasomotor function. Hypotensive infants (9 in total) who received inotropes had decreased low-frequency variability at baseline compared with normotensive infants, which did increase after inotrope initiation. However, low-frequency power did not change for those with treatment failure. This points to the fact that vasomotor dysregulation is associated with cardiovascular instability and that vasomotor dysregulation is a potential measure of treatment failure.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here