Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Living cells respond to physical and biochemical cues, e.g., hormones, cell–cell signaling molecules, and with the physical environment through the extracellular matrix (ECM), enabling them to adapt to changes in the physiological environment. Regulation of cell function (growth, migration, proliferation, and differentiation) is dependent upon contact or adhesion between cells and the substrate (ECM). ECM has three key components: collagen – the structural framework of ECM – proteoglycans, and adhesive matrix proteins (e.g., fibronectin, vitronectin). Initial contact of cells with a solid substrate is mediated by adsorbed adhesive proteins. Cell surface receptors subsequently form bonds leading to cytoskeletal reorganization and progressive spreading of the cell on the substrate. These receptors transduce biochemical signals to the nucleus by activating the same intracellular signaling pathways that are used by growth factor receptors. In addition to biochemical cues, mechanical forces play an important role in cell adhesion, growth, and possibly differentiation. These responses are mediated through internal cell receptors that respond to mechanical forces. Cell binding to ECM through specific cell–substrate contacts is critical to cell-growth control through mechanical forces resulting in alterations in cell shape and cytoskeletal tension.
A biomaterial is a nonpharmaceutical, nonviable material in intimate contact with living tissue in order to treat, augment, or replace any tissue, organ or function of the body. Ideal properties of a biomaterial include: biocompatibility, noncarcinogenicity, lack of allergenicity, cost-effectiveness, and ease of handling. Biomaterials or tissue engineering scaffolds contain chemical and structural information that may control tissue formation in a manner similar to cell–cell communication and cell–ECM interaction ( Fig. 3.1 ). An appropriate 3D scaffold should facilitate normal cellular organization and behavior, define and maintain the desired tissue volume, whilst also promoting host integration and implant vascularization.
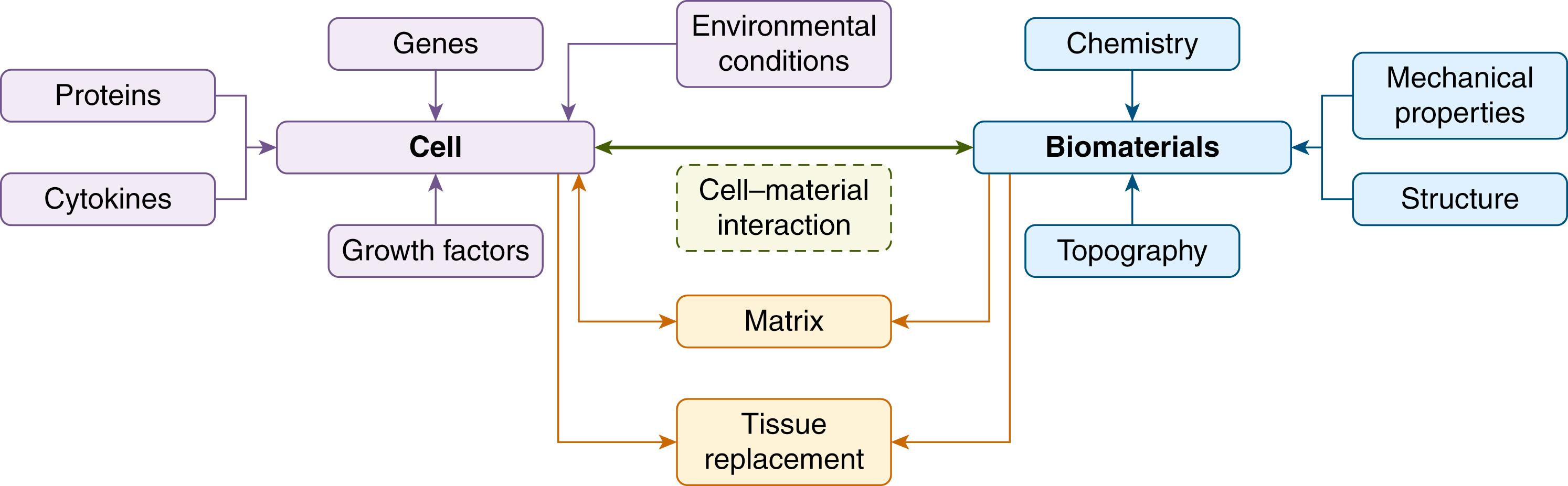
Ultimately, the scaffold should undergo nontoxic degradation as it is replaced by healthy host tissue, rendering a biodegradable scaffold ideal for use in tissue engineering. The cell–biomaterial interface also plays a significant role in the choice of scaffold due to the direct impact on cell adhesion, which is an important step in the survival of anchorage-dependent cells. Both cell proliferation and differentiation can be controlled by functionalization of the biomaterial surface; these interactions between cells and biomaterials have been shown to occur at the nanoscale.
With the advent of nanotechnology, nanoparticles are being merged with synthetic scaffolds to develop nanostructured biomaterials, enhance interaction of proteins that control cell adhesion and thus, tissue formation. Nanocomposites can be defined as multiphase solid materials where one of the phases has a dimension of less than 100 nm. Nanofibrous composite scaffolds are found to decrease immunogenicity, improve the capacity for cell interaction, and to harbor increased concentrations of fibronectin and vitronectin, the adsorption proteins that reduce apoptosis of transplanted cells. Biomaterials can be classified as bioinert, bioresorbable, or bioactive. A bioinert material does not induce a toxic response from the body on implantation and is usually associated with fibrous encapsulation, whereas a bioresorbable material undergoes degradation in the host by hydrolysis, enzymes or osteoclasts. Bioactive materials produce a biological response from the body that results in the formation of a bond between the material and host tissue. The type and site of biomaterial implantation governs a tissue response: hard tissues (bone, enamel, and dentin), soft tissues (breast and ocular implants), vascular implants (stents, heart valves, and vascular grafts) and connective tissue (cartilage, tendons, and ligaments).
A variety of possible scaffold biomaterials are available. Among these are biological polymers (e.g., collagen, elastin, chitosan, silk), biodegradable synthetic polymers (e.g., PGA, PLA, PDLA), synthetic biomimetic compounds (e.g., hydroxyapatite, calcium phosphates), allografts and xenografts or combinations of the above. Geometric variables such as porosity, pore size, and pore morphology are crucial. A porous scaffold provides a large surface area for neovascularization, nutrient and waste exchange, cell migration, and matrix deposition. Scaffolds can be made porous using several different methods. Porogens (i.e., salt or paraffin microspheres) can be incorporated in the polymer and subsequently leached out. Freeze drying of homogenized polymer solvents is another option, in addition to gel casting of organic scaffolds and sol-gel foaming. Surface properties, such as surface topography and chemistry, guide cellular behavior and control protein adhesion. If a biodegradable material is chosen for implantation, the rate of resorption should match the speed at which the body replaces the missing tissue and its degradation byproducts should be nontoxic. Finally, the biomaterial of choice ought to be economically viable and conform to the regulatory bodies.
The word autologous means “related to self” and refers to tissue that has been derived or transferred from the same organism. Autologous grafts or “autografts” can be harvested and transferred from several different tissues in the body, e.g., skin, bone, tendon, cartilage. The focus of this section is on the autologous transfer of skin, adipose tissue, bone, and cartilage.
From superficial to deep, skin is composed of epidermis, dermis, and skin appendages. It has several functions, ranging from physical protection, prevention of fluid loss, protection against ultraviolet radiation, regulation of body temperature, sensation, immunological surveillance, to protection from microbiological organisms. Injured skin can heal itself through different phases of wound healing and epithelialization, whereby epithelium is reestablished across a wound. The different phases of wound healing consist of hemostasis, inflammation, proliferation, and remodeling, whereas epithelial repair consists of mobilization and migration of epithelial cells, followed by mitosis and differentiation into stratified squamous epithelial cells. If the injury to the skin is grave or the area of skin loss large, this process may take several months and result in significant scarring. In this instance the use of a skin autograft can aid the healing process. Skin grafts can either be split- or full-thickness. Split-thickness skin grafts (STSG) contain a variable amount of dermis and can be harvested from several sites (i.e., thigh, buttocks, and scalp). The donor site heals through epithelial repair and does not require closure. Full-thickness skin grafts (FTSG) contain the entire dermis and are usually taken from areas of the body with sufficient skin laxity to allow direct closure (i.e., groin, forearm, arm, postauricular and supraclavicular areas).
Skin grafts heal in four phases:
Adherence of the graft to its bed via immediately formed fibrin bonds.
Serum imbibition. Skin grafts swell in the first 2–4 days after application due to absorption of fluid.
Revascularization commences around the 4th day and is a consequence of new vessel formation along new and existing vascular channels in the graft.
Remodeling is the process whereby the histological structure of the graft returns to that of the normal skin.
Skin grafts can fail to “take” due to several reasons, e.g., hematoma, infection, excessive shearing, an inappropriate bed, or technical errors such as placing the graft upside down on its bed or allowing it to dry before application.
The main limitation of skin grafts is their tendency to contract. Contraction of grafts is more pronounced with split-thickness skin grafts compared to full-thickness skin grafts.
Although autologous fat transfer has gained popularity over the past two decades, Neuber first described the technique in the medical literature in 1890s. The injectable fat transfer was first described in the 1920s. In the 1950s Peer reported that fat grafts lose approximately 45% of their weight and mass per year. He also emphasized the importance of a good vascular recipient bed combined with meticulous hemostasis to promote optimal graft survival. Autologous fat transfer can be regarded as the ideal filler. It induces neither host reaction nor a carcinogenic response. Being minimally invasive renders the procedure safe, and fat is also readily available and inexpensive. Limitations include the unpredictable and variable rate of absorption, the need for overcorrection, and the requirement for a surgical procedure.
The most common site is the abdomen due to availability and ease of access when the patient lies supine. Other sites include the flanks, extremities (inner thighs, inner knees, inner arms, trochanteric area) and gluteal area. A study investigating the viability of lipoaspirates in vitro using flow cytometry from three different anatomical sites – abdomen, flank, and thighs – found no difference in fat viability 12 weeks post transplantation. Currently there is no consensus on the best donor site for autologous fat harvest.
Vacuum extraction, syringe aspiration, and direct fat excision are well-described techniques in harvesting fat tissue. Although syringe aspiration is the most popular technique, there exists no current objective evidence to corroborate the benefit of this technique relative to other methods in terms of weight or volume of the fat grafts isolated. However, high negative pressure exerted on adipocytes in conventional liposuction has been reported to cause up to 90% adipocyte rupture. Coleman popularized autologous fat transfer employing the syringe aspiration technique. A 3-mm cannula is connected to a 10-mL syringe and aspiration is achieved through manual suction by withdrawing the plunger.
Some clinicians do not use local anesthetic during fat harvest but the majority inject the donor site with short-acting local anesthetic agents with or without epinephrine. The most commonly used agent is lignocaine with concentration doses ranging from 0.5% to 2% and doses of epinephrine ranging from 1:80,000 to 1:200,000. Coleman recommends using 0.5% lignocaine with 1:200,000 adrenaline. The main clinical advantage of infiltration with local anesthetic combined with epinephrine are reduction of potential donor site pain, blood loss and bruising.
It is uncertain whether the use of lidocaine adversely impacts fat graft survival. In a study by Moore et al an inhibitory effect of lidocaine on adipocyte growth in cell culture was observed. In contrast, Keck et al demonstrated that exposure to a variety of anesthetic solutions increased adipocyte viability in contrast to a dry technique. Furthermore, animal models pretreated with saline or lidocaine and epinephrine have demonstrated no significant effects on fat graft volumes or histological architecture.
Centrifugation is a popular method of fat graft preparation. The process separates the nonviable components, including blood, oil, and lidocaine, leaving behind the adipocyte-rich segment for transfer. Coleman advocates 3 minutes of centrifugation of aspirated fat at 3000 rpm. This process separates the fat into a bottom layer of tumescent lidocaine and blood, a middle layer of usable fat graft and a top layer of oil from ruptured adipocytes. Other clinicians advocate the use of centrifugation at different spinning rates and duration.
Rohrich et al have recently challenged the process of centrifugation and found no quantitative difference between the viability of processed and unprocessed fat graft viability. Similarly, Ramon et al utilized a mouse model to demonstrate that fat cell survival after 16 weeks between centrifuged and noncentrifuged fat grafts in terms of weight and volume were comparable, whilst histological analysis showed noncentrifuged fat grafts exhibited less fibrosis.
Washing harvested fat grafts as a form of preparation has also been described, using lactate Ringer’s solution to improve viability. The rational is to decrease levels of inflammatory mediators from the graft and minimize the immune response at the recipient site. However, there is a paucity of high-level evidence in the literature to affirm that washing harvested fat graft improves overall graft survival.
To maximize graft survival the adipocytes must be in close vicinity to a blood supply. Studies have suggested that fat placed within 2 mm of an arterial supply will survive and greater distances risk necrosis and fibrosis. Thus the surface area of the fat graft in contact with a vascular recipient bed is essential to optimize graft survival. The “fanning out” technique is one method of injection that ensures small aliquots of fat graft are spread over a large surface area. Other commonly used injection methods, such as linear threading, serial puncture, and cross-hatching, can also be used to achieve the desired outcome. Slower injection speeds of 0.5–1 mL/s have resulted in greater fat graft survival rates compared to faster injection speeds of 3–5 mL/s. A curved microcannula 2–3 mm in diameter or blunt-tip needle 14–19 gauge is commonly used for autologous fat transfer.
In general, autologous fat transfer is a safe procedure with a low risk profile. However, patients must be warned about bruising, swelling, contour irregularity, and infection, in addition to fat necrosis and calcification. Rare cases of severe complications such as fat embolism of cerebral artery and retinal artery leading to stroke and blindness, respectively, have been reported in the literature during soft tissue augmentation of nasolabial fold and periorbital area. ,
During the consent process it is important to ensure that the common and rare risks of fat transfer are made evident. Moreover, it important to discuss the limitations of the procedure, with emphasis on the unpredictability of the graft take in regard to partial and complete absorption over time.
The rise in popularity of autologous fat transfer in addressing contour deformity and volume restoration over the past few decades has propelled this technique to the forefront of reconstructive and aesthetic surgery. Despite its widespread adoption, there remains no consensus on the optimal donor site or most effective method of harvest and preparation. Further objective randomized clinical trials are required to guide current variations in practice. However, autologous fat transfer functions as a powerful tool in the armamentarium of plastic surgeons and practitioners must keep abreast of developments in order to ensure optimal graft outcome.
The success of bone autografts in the treatment of nonunions is well described. In the tibia, union rates of more than 90% have been reported using iliac crest bone graft at a mechanically stable site. Bone grafting has also been effective in treating recalcitrant and infected nonunions as well as aiding the healing at the docking site of nonunions treated with distraction osteogenesis. In addition to the mechanical properties and volume effect, the biological properties of bone autografts are also advantageous.
Autologous bone grafting has been considered as the gold standard in management of bone defects and nonunions. However, the introduction of a new generation of bone substitutes has challenged this notion, largely due to the morbidity associated with the harvesting procedure with reports of donor site pain, infection, and hematoma of varying incidences between 9% and 55%.
Bone grafts heal by the following mechanisms: (1) adherence to the surrounding tissue, i.e., incorporation, (2) creeping substitution, also referred to as osseoconduction, a process whereby the bone graft acts as a scaffold along which angiogenesis occurs and new bone is formed by progenitor cells, and (3) osseoinduction, the differentiation of mesenchymal stem cells into osteocytes.
Autologous bone grafts possess biological advantages over allografts and synthetic bone grafts as they confer greater histocompatibility as well as an excellent combination of osteogenic, osteoinductive, and osteoconductive properties.
The trabecular structure of cancellous bone generates a large surface area. This permits a greater incorporation of cellular components (mesenchymal stem cells and osteoblasts) and provides excellent osteogenic and osteoinductive properties. Additionally, the sizable trabecular surface area facilitates revascularization and integration of the graft at the host site. Conversely, a significant limitation of cancellous bone is its poor mechanical strength during the initial phase. However, increased stability is achieved within months due to the biological capacity of trabecular bone graft to induce new bone formation, once incorporated.
Cortical bone autografts have a different biological profile compared to cancellous bone autografts. In contrast to the low density of cancellous bone, the dense and highly organized structure of cortical bone provides initial mechanical strength and stability. However this structural integrity compromises the available surface area and constitutes a barrier to vascular in-growth and remodeling during graft incorporation. Furthermore, the subsequent lack of cellularity and growth factors diminishes the osteogenic potential of cortical bone grafts. The integration of cortical bone is mediated through osteoclastic activity resulting in resorption of the cortices and loss of bone. This results in transient weakness with reduction of mechanical strength of up to 75%.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here