Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Traumatic rupture of the anterior cruciate ligament (ACL) can be a common source of knee instability and dysfunction in athletes. Reconstruction of a torn ACL, to restore function and limit injury to the menisci, has become a common orthopedic procedure. Despite advances in surgical techniques and the ability to implant an isometric graft that restores knee stability, ACL reconstruction is not always a universally efficacious procedure. Rates of recurrent laxity, 1 year postoperatively, have been reported to be as high as 17%.
Graft integration failure and inadequate tendon-to-bone healing may be an important cause of recurrent knee instability following ACL reconstruction. The healing of tendon to bone is the basic requirement for successful long-term survival of the graft. Whether an autograft or allograft tendon is used, biomechanical testing has shown that the initial strength of the graft material is superior to the intact ACL. Therefore, the weakest link after reconstruction is not the graft itself, but rather the fixation points until graft osteointegration occurs. The intraarticular portion of the graft must ultimately undergo remodeling and the process of ligamentization to form an organized structure that resembles a native ligament.
Current techniques of ACL reconstruction require tendon-to-bone healing in a surgically created bone tunnel. No native human tendons pass through an osseous tunnel; therefore, this type of healing environment is inherently unusual. When a bone-tendon-bone graft is used for ACL reconstruction, graft fixation initially depends on bone-to-bone healing. However, tendon-to-bone healing still remains critical regardless of whether an all soft-tissue graft or a graft with a bony plug is selected. The length of the tendinous portion of the bone-tendon-bone graft is greater than the intraarticular length of the native ACL, resulting in a substantial amount of tendon within the bony tunnel. Aperture healing to minimize graft micromotion and tunnel widening requires tendon-to-bone healing with any graft.
Because all grafts depend on tendon-to-bone healing, this chapter focuses on graft osteointegration and the process by which structural and functional continuity between the tendinous portion of the graft and bone is achieved. Both the biologic milieu and the biomechanical environment occurring at the bone tunnel result in formation of an attachment site that differs from the native ligament-bone insertion. The biology of this healing remains incompletely understood and is exposed to a number of biomechanical and physiologic influences. The current understanding of the biology of graft reconstruction is reviewed and potential strategies to enhance early graft integration are discussed.
The native ACL inserts via a direct type of insertion to bone, with distinct transition zones of ligament, unmineralized fibrocartilage, mineralized fibrocartilage, and bone.
Insertion site provides gradual transition in material properties, thereby minimizing stress concentration at the interface.
All ACL reconstruction procedures require tendon-to-bone healing in a surgically created bone tunnel, to which there is no analogous situation in the native human body.
Regardless of ACL graft choice, some degree of tendon-bone healing must take place for successful ligament reconstruction.
The ACL is an intraarticular, extrasynovial structure that acts to control anterior translation and rotational movements of the femur on a fixed tibia. It is composed of multiple fascicular collagen bundles enveloped in a sheath that contains vascular and neural elements. The ACL inserts to bone via a direct type of insertion, similar to the transition seen from tendon to bone. Microscopic examination of the sites of bony attachment shows interdigitation of the collagen fibers with bone though four distinct transition zones: tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone ( Fig. 5-1 ). This graduated change in stiffness allows for transmission of complex mechanical loads from soft tissue to bone while minimizing peak stresses at any single point along the ligament. Cartilage-specific collagens including types II, IX, X, and XI are found in the fibrocartilage insertion site. Collagen X plays a key role in maintaining the interface between the mineralized and unmineralized zones. Recently, it was noted that both the femoral origin and tibial insertion of the ACL contain discrete regions of both direct and indirect ligamentous insertions. The theoretical biomechanical advantages of recreating the direct fibers have been explored; however, few clinical data support targeted surgical reconstruction of these discrete tissue attachments.
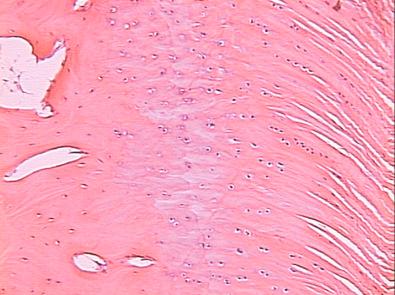
A native ACL insertion is not reproduced after ACL reconstruction and reflects an inability to recapitulate the events of embryonic development.
Graft-to-bone healing occurs via an interposed zone of vascular granulation tissue between the graft and tunnel wall that matures with time.
Attachment strength is proportional to the magnitude of bony ingrowth into the interface tissue and anchoring Sharpey-like collagen fibers that bridge the tendon to bone.
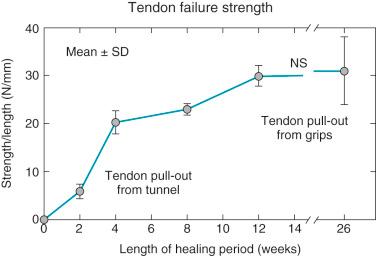
The overall structure, composition, and organization of the native ACL attachments sites are not reproduced after surgical reconstruction of the ACL. This disparity reflects an inability to recapitulate the events that occur during embryonic development with current surgical strategies. Rather than regenerating the four organized zones of direct insertion, the graft heals with an interposed zone of vascular, highly cellular granulation tissue between the graft and tunnel wall ( Fig. 5-2 ). The healing process of a tendon graft in a bone tunnel has been defined in rabbit and dog models. In the initial postoperative period, the graft-tunnel interface is filled with vascular granulation tissue containing an abundance of type III collagen. Vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) are expressed, stimulating an influx of macrophages and enlarged fibroblasts. Chondroid cells accumulate along the walls of the bone tunnel and deposit type II collagen as part of the endochondral process of new bone formation. After 3 to 4 weeks, this interface tissue undergoes a maturation process until its matrix consists of oriented Sharpey-like collagen fibers that bridge the bone to the graft ( Fig. 5-3 ). The chondroid cells are gradually replaced with lamellar bone in a process similar to endochondral ossification. The Sharpey-like fibers are composed of type III collagen and extend into the surrounding bone to resist shear stresses. The number and size of these collagen fibers positively correlate with the pull-out strength of the graft ( Fig. 5-4 ). The time interval for this process to occur has been variably reported in the literature, ranging from 8 to 30 weeks. Graft attachment strength further improves as bone grows into the interface tissue and outer portion of the graft.
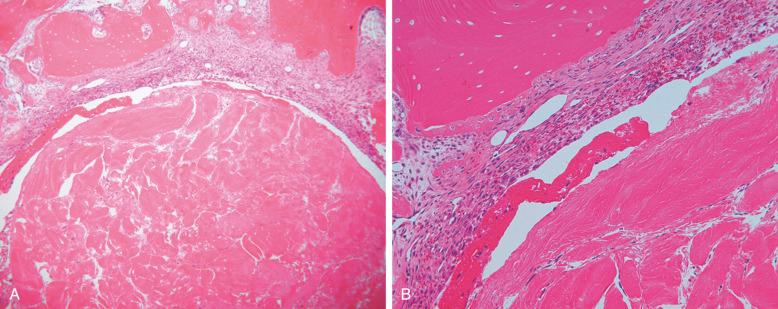
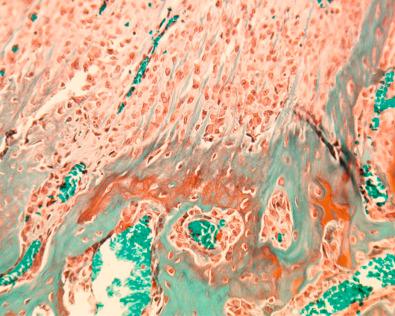
The biologic cascade of healing between a grafted tendon and bone tunnel remains incompletely understood. The biologic and biomechanic environments result in the formation of an inferior attachment site that is different from the organized, direct-type ACL insertion. Current work suggests a number of fundamental challenges responsible for the suboptimal healing response between tendon and bone instead of regeneration of the insertion site. These factors include:
The presence of inflammatory cells at the graft-tunnel interface that precipitates scar formation.
Slow and limited bony ingrowth into the graft from the tunnel walls, resulting in a biomechanically weaker attachment.
Insufficient number of undifferentiated stem cells at the healing tendon-bone interface.
Graft-tunnel micromotion that precludes the formation of a firm attachment at the tunnel aperture.
Lack of a coordinated gene-signaling cascade that directs healing toward regeneration rather than scar tissue formation in the postnatal organism.
Strategies to promote tendon-to-bone healing in ACL reconstruction focus on overcoming these challenges through modification of the biologic and biomechanical environment.
Maximizing the surface area of the tendon-bone interface by minimizing graft tunnel mismatch and using long bone tunnels can help to improve the chances of secure graft-bone healing.
ACL graft-tunnel micromotion, often seen with suspensory fixation techniques, can preclude the formation of a secure attachment to the tunnel wall and is associated with osteoclast-mediated bone resorption and tunnel widening.
The rapid inflammatory response after ACL reconstruction is highly complex and may trigger a cascade of events that favors fibrosis and scar formation over tissue regeneration, resulting in an inferior tendon-bone attachment site.
Ingrowth of bone from the surrounding tunnel wall into the interface zone and graft is ultimately responsible for the improved biomechanical properties of the attachment site after healing is complete.
Use of undifferentiated stem cells and/or cytokines at the tendon-bone interface may have a future role in promoting tissue regeneration and the restoration of native insertion site morphology after ACL reconstruction.
Adjustments can be made in the surgical technique of reconstruction to optimize tendon-to-bone healing. The fundamental principle is to maximize the surface area of the tendon-bone interface. Animal studies have shown that increasing the length of the bone tunnel positively correlates with the quality and strength of the reconstruction. Minimizing graft tunnel mismatch by effectively achieving a press fit of the graft into the bony tunnel improves healing. Additionally, maximizing circumferential contact area of the graft and tunnel (i.e., avoiding use of an interference screw) may improve healing. However, this final factor must be ultimately balanced with the increased stiffness from decreased fixation-to-fixation construct length that aperture fixation provides to the healing graft environment.
Relative graft-tunnel micromotion may preclude the formation of a firm attachment to the tunnel wall. Yu and Paessler compared “aggressive” versus “conservative” rehabilitation protocols in patients after ACL reconstruction with quadrupled hamstring grafts. Significantly greater tunnel widening was reported in the aggressive rehabilitation group, supporting the view that graft-tunnel micromotion is a cause of tunnel widening and that the mechanical environment in the bone tunnel influences tendon-bone healing.
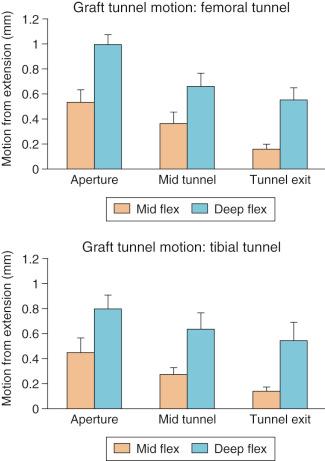
Animal studies have corroborated these clinical observations. Sakai and associates compared immediate motion with varying periods of immobilization for up to 6 weeks after ACL reconstruction in a rabbit model. Biomechanical analysis demonstrated a greater load to failure of the graft in immobilized animals, and histologic studies demonstrated closer tendon-bone apposition with less fibrovascular interface scar tissue. Rodeo and colleagues evaluated the effect of graft-tunnel motion on tendon-to-bone healing in a rabbit ACL reconstruction model. ACL reconstruction was performed in five cadaveric rabbit limbs with aluminum beads fixed to the tendon and bone tunnel. Three-dimensional graft-tunnel motion was quantified using microcomputed tomography (microCT) analysis. Motion was observed to be greatest at the tunnel apertures and least at the tunnel exits, adjacent to the graft fixation at the tunnel exit ( Fig. 5-5 ). Histomorphometric analysis demonstrated healing to be slowest at the apertures, with a wider fibrovascular interface tissue present between tendon and bone. An inverse relationship between graft-tunnel motion and healing in the femoral tunnel was demonstrated. In addition, osteoclasts were preferentially observed at the tunnel aperture, supporting the hypothesis that graft-tunnel motion may stimulate osteoclast-mediated bone resorption and secondary tunnel widening.
These findings have been further investigated in rodent models. We developed a rat ACL reconstruction model that permits application of controlled cyclic axial loading postsurgery. We found that low-magnitude cyclic axial loading of the ACL graft for a short period of time each day, postoperatively, was associated with greater inflammation and less bone formation in the tunnel when compared with animals that underwent continuous immobilization; however, this loading regimen was not detrimental to the strength of the healing tendon-bone interface. Delayed application of cyclic axial load after ACL reconstruction in the same model resulted in improved mechanical and biologic parameters of tendon-to-bone healing compared with those associated with immediate loading or prolonged postoperative immobilization of the knee. Higher strain levels applied to the healing ACL graft in this model had deleterious effects during the early time periods of graft healing. However, stress deprivation to the graft can also have negative consequences on the biomechanical properties of the healing graft.
Modulation of the mechanical environment may have profound effects on the cellular and molecular events at the tendon-bone interface. An improved understanding of these mechanisms may help to develop a postoperative rehabilitation program that provides an optimal environment for tissue regeneration and tendon-bone healing. The delicate balance that must be achieved will need to be explored with larger animal models, along with well-designed clinical trials to improve our understanding of the mechanobiology of healing.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here