Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Our two eyes view the world simultaneously from slightly different angles. The visual fields of the two eyes receive largely overlapping images on their two-dimensional retinal surfaces. The information contained in the separate and slightly dissimilar images arising in each eye is appreciated as a single image by the process of fusion, which blends sight from the two eyes to form a single percept. This process of normal binocular single vision depends on clear visual axes with reasonably good visual acuity in each eye, the ability of the visual cortical elements to appropriately combine the slightly dissimilar monocular inputs ( sensory fusion ), and the precise coordination of the two eyes for all directions of gaze, so that corresponding points on the two retinas are aligned ( motor fusion ). In addition to binocular single vision, our visual cortex has the ability to disambiguate the minute dissimilarities from the pair of two-dimensional retinal images and reconstruct the third dimension ( stereopsis ).
Milestones in the normal maturation of binocular vision are achieved rapidly during infancy. By the time they are 2 months old, nearly all infants have orthotropic alignment, except for “very infrequent” misalignment. While there is clear evidence that infants as young as 1 month old are capable of motor fusion, it is not until 2–4 months that consistent motor fusion responses are observed in nearly all infants.
Sensory fusion has been assessed using a wide variety of test paradigms, from the clinical 4 prism diopter (PD) test to forced-choice preferential looking fusion-rivalry discrimination and visual evoked potentials to dynamic random dot correlograms. Overall, the results are consistent. Few normal infants demonstrate sensory fusion before they are 2 months old, whereas most 4-month-olds and older infants demonstrate sensory fusion ( Fig. 73.1 ).
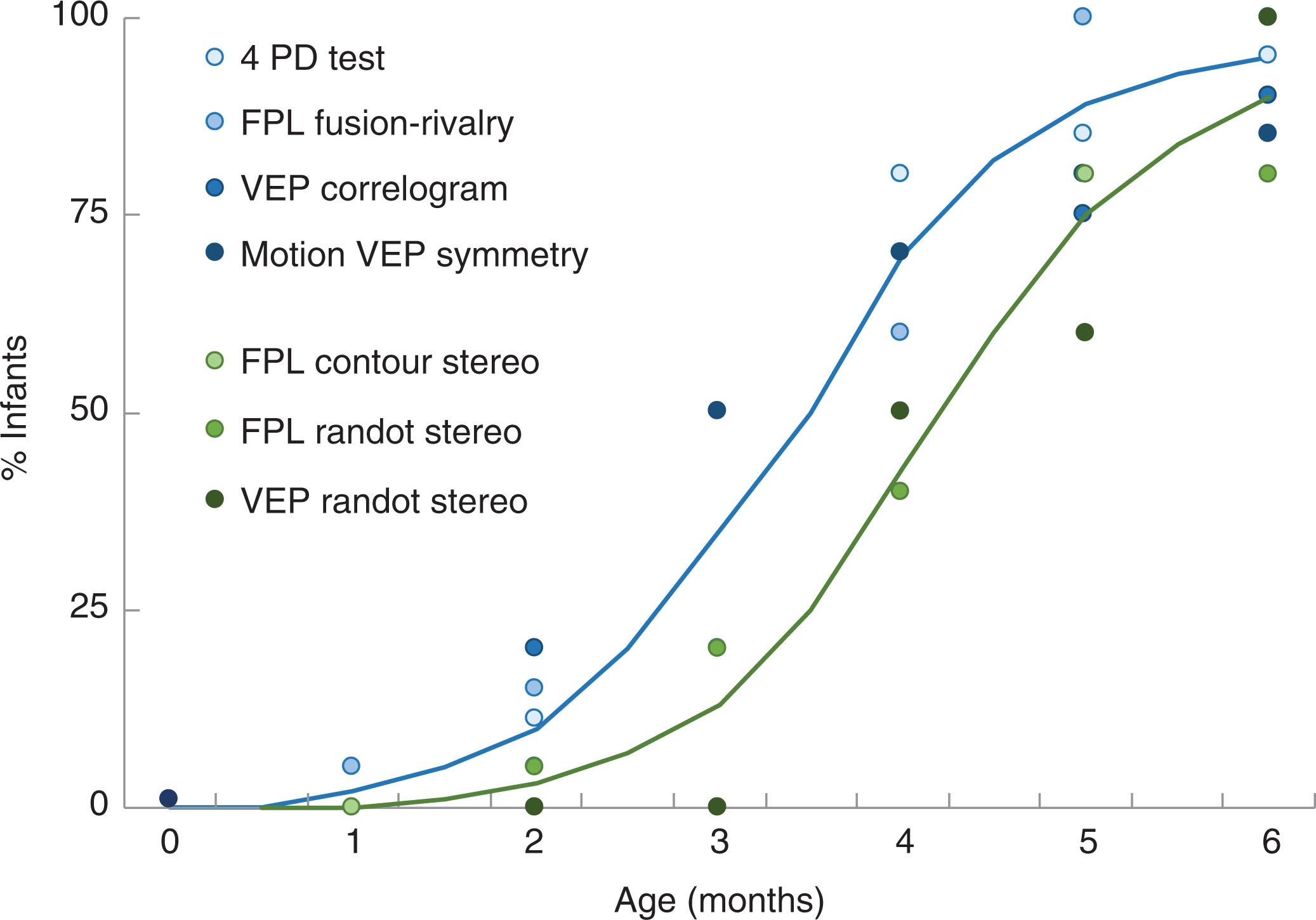
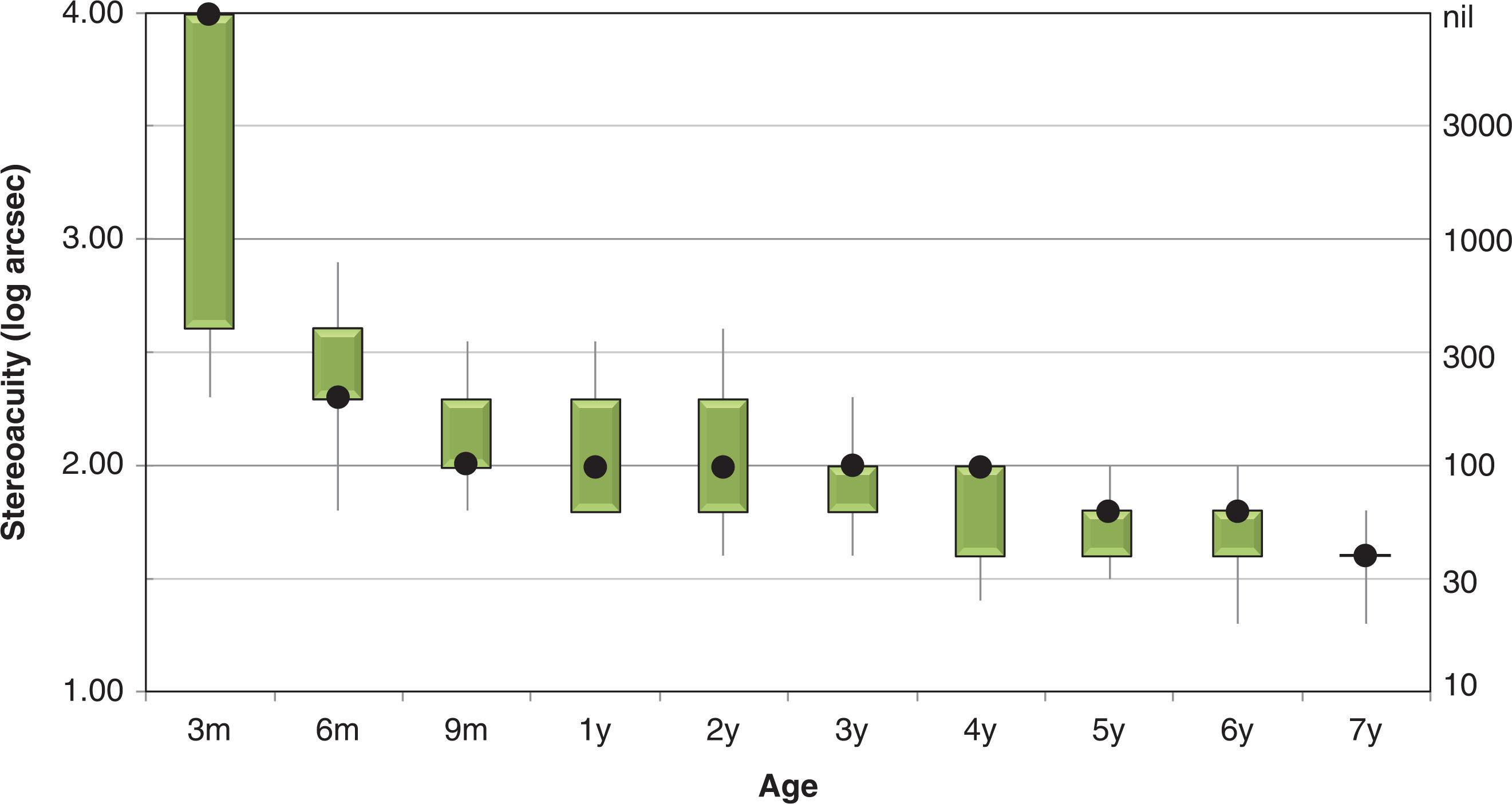
Likewise, the onset of stereopsis has been investigated using a number of different paradigms, including forced-choice preferential looking with contour stereograms and random dot stereograms, and visual evoked potentials to dynamic random dot stereograms. Again, despite the differences in stimuli and response measures used, there is general agreement across studies that the onset of stereopsis occurs at approximately 4 months (see Fig. 73.1 ). After the onset of stereopsis, the rate of stereoacuity maturation is rapid during infancy ; but further slow improvement of stereoacuity continues for several years ( Fig. 73.2 ).
The first building block of the binocular vision system is sensory fusion, frequently assessed with the Worth 4-dot test, requiring the child to wear anaglyphic glasses. For young children who have difficulty with color-naming and counting there is an alternative Worth 4-shape test. When assessing sensory fusion, the angle of deviation should be neutralized with prisms, although this can be challenging in some patients. As the anaglyphic glasses required for the Worth 4-dot could be dissociative, potentially providing an impediment to binocular vision, demonstrating fusion on the Worth 4-dot test can be interpreted as strong evidence that fusion is present but a test failure may be inconclusive. The Worth 4-dot test may also be used to estimate the size of a suppression scotoma by presenting the flashlight targets at different distances from the child. The size of the scotoma can be calculated in visual angle (degrees), and degree of fusion may be classified as peripheral, macular, or foveal based on the size of the scotoma. A new prototype app-based version of the Worth 4-dot is available, with the ability to quantify the size and depth of suppression scotomas. The 4 PD base-out test is commonly used to detect small suppressions scotomas associated with monofixation syndrome or presence/absence of bifoveal fixation.
Motor fusion is the ability to maintain alignment of the eyes through a range of movements and consists of tonic (smooth movement when tracking an object) and phasic (quick changes in fixation from one object to another) components. The stimulus for motor fusion is peripheral retinal disparity outside Panum’s area and the motor response is vergence. While the presence of motor and sensory fusion are linked, the amplitude of motor fusion is not related to the amount of sensory fusion. Assessment in young children primarily consists of identifying the ability to maintain motor fusion in the presence of a 20 or 15 diopter base-out prism (prism reflex test). While this does not quantify the fusional ability, it may identify a lack of motor fusion, or confirm the presence of binocular vision in a young child with suspected strabismus. In older children, quantification of motor fusion can be accomplished by measurement of a break point , at which vergence demand exceeds fusional reserve and a deviation becomes manifest, and a recovery point , where motor fusion can be recovered as vergence demand is lessened. In practice, the child is asked to observe a target at a fixed distance (typically 1/3 and 6 m) through a prism of increasing strength, while attempting to maintain a single image. The phasic range is assessed using a prism bar, with discrete steps, and tonic range is assessed using a Risley rotary prism. There is variation in the reported normative values, likely attributable to imprecision of test distance, differences in target size, prism placement and speed of prism change. Total fusion range (convergence and divergence combined) is normally about 25 diopters for phasic and 20 diopters for tonic measurements.
The highest grade of BV is stereoacuity with two types of stereoacuity tests widely available, contour tests and random dot tests. Contour tests have the disadvantage that some children with nil stereoacuity can pass the initial 2–4 levels on the basis of monocular or nonstereoscopic binocular cues. Despite the difficulties associated with interpretation of results from contour stereoacuity tests, they are commonly reported as outcome measures in pediatric ophthalmology. Passing the Titmus Fly test with nil stereopsis may be possible because the child knows it is a fly and expects the wings to be elevated, because the child has been repeatedly exposed to the same test with only two possible responses, or because the child alternates fixation and observes image jump in disparate portions of the test. For these reasons, a modified test protocol has been proposed, repeating the test with glasses that eliminate the disparity cues, and has been shown to improve accuracy in identifying nil stereopsis.
Random dot tests contain no monocular or nonstereoscopic binocular cues; depth can only be appreciated by global binocular evaluation of corresponding points and disparate points. Most stereoacuity tests are conducted at a near viewing distance, usually at 40 cm, performed with optical correction as stereoacuity is sensitive to blur, especially anisometropic blur. Where possible, stereoacuity testing is performed early in the examination, before the child’s eyes are dissociated from other testing. For infants and pre-verbal children, preferential looking can be used with the PASS Stereotest. For older children, stereoacuity can be tested with the matching, pointing, or naming tasks available in the PASS, Randot Preschool, TNO, Lang and Frisby Stereoacuity tests. Normative data for the infant and pediatric stereoacuity tests have been published ( Table 73.1 ).
| Near stereoacuity | Distance stereoacuity | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yr) | PASS | Frisby | RPST | TNO | PDI | Distance Randot | FD2 | |||||||
| Norm | Lower limit | Norm | Lower limit | Norm | Lower limit | Norm | Lower limit | Norm | Lower limit | Mean | Lower limit | |||
| 0.5-0.9 | 480 | 960 | ||||||||||||
| 1 | 240 | 960 | ||||||||||||
| 2 | 120 | 480 | 400 | 800 | 200 | 400 | ||||||||
| 3 | 60 | 240 | 100 | 400 | 30 | 60 | ||||||||
| 4 | 60 | 240 | 100 | 200 | 100 | 200 | ||||||||
| 5 | 60 | 240 | 60 | 200 | 50 | 60 | ||||||||
| 6 | 25 | 75 | 60 | 100 | 60 | 120 | 80 | 650 | ||||||
| 7 | 40 | 60 | ||||||||||||
| 8 | 60 | 100 | ||||||||||||
| 9 | 20 | 85 | 40 | 60 | 60 | 120 | ||||||||
| 10 | ||||||||||||||
| 11 | 40 | 60 | 60 | 100 | ||||||||||
| 12 | ||||||||||||||
| Adult | 20 | 40 | 30 | 70 | 60 | 120 | 40 | 325 | 60 | 160 | 10 | 60 | ||
One limitation of currently available random dot stereoacuity tests for children is the maximum disparity available, e.g. 800 seconds of arc (arcsecs) for the Randot Preschool Stereoacuity Test and 960 arcsecs for the PASS. Thus, it is possible that some children who appear to have nil stereoacuity are simply not within the measurable range of disparities available. A second factor that may limit performance on current random dot stereoacuity tests is dot size; most random dot tests require visual acuity of 0.4 logMAR or better in order to be able to appreciate the dot texture sufficiently to extract disparity information. In addition, the clinical tests consist of relatively small images that are static; there is some evidence to suggest that larger, dynamic stimuli provide additional information that may allow children who appear to have nil stereoacuity on standardized tests to appreciate depth.
Recent developments in the assessment of near stereoacuity include the redesigned Lang Stereopad, where an increased range of disparities are available (50–800′′) and the ability to randomize the presentation of the stereoscopic stimuli. In addition, tests are now available in game format on digital devices. The Asteroid test utilizes a column-interleaved digital stereoscopic display, and tracks the patient’s distance from the device, automatically adjusting the disparity based on distance. Responses have shown good test–retest reliability and a wide range of stereo thresholds. The PDI check presents stimuli on a Nintendo® 3DS™ hand-held console as part of a package which also assesses near visual acuity and color vision. Potential advantages of digital devices are random presentation of the stimuli, automated staircases, and game format, which may prove more engaging and more reliable for children, but further research is required to confirm these benefits.
There are also distance stereoacuity tests available, including the FD2, which has moveable real-depth targets, and the Distance Randot; normative data are available for both of these tests (see Table 73.1 ). Children and adults with normal binocular vision generally have poorer stereoacuity at distance than at near. In addition, many electronic visual acuity test systems have implemented optional distance stereoacuity tests with LCD shutter glasses, polarized filter glasses, or anaglyphic filter glasses; few have been formally evaluated and most lack normative data. Distance stereoacuity may be especially useful for detecting deterioration of control of intermittent exotropia.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here