Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bile formation is essential for intestinal lipid digestion and absorption, cholesterol homeostasis, and hepatic excretion of lipid-soluble xenobiotics, drug metabolites, and heavy metals. The process of bile formation depends on hepatic synthesis and canalicular secretion of bile acids, the predominant organic anions in bile, and maintenance of hepatic bile formation is essential for normal liver function. Bile acids also undergo an efficient enterohepatic circulation, with most of the bile acids in bile having previously transited the small intestine and been returned to the liver for secretion by hepatocytes. As a result, disturbances in the synthesis, biliary secretion, or intestinal absorption of bile acids have profound effects on bile formation and hepatic and gastrointestinal physiology. Identification of the enzymes, transporters, and regulatory factors responsible for bile acid biosynthesis and enterohepatic cycling has advanced our understanding of genetic and acquired disorders of bile formation and secretion. In addition, the recognition that bile acids act as hormones that signal via nuclear and G-protein-coupled receptors has provided new insights into the pathogenesis of and potential treatments for bile acid–related hepatobiliary and intestinal disorders. This chapter reviews the current knowledge of bile acids and their function, synthesis, secretion, and enterohepatic circulation. Available bile acid-based therapies are also discussed.
Bile is a complex, lipid-rich micellar solution that is isosmotic with plasma and composed primarily of water, inorganic electrolytes, and organic solutes such as bile acids, phospholipids (mostly phosphatidylcholine [PC]), cholesterol, and bile pigments ( Table 64.1 ). Bile also contains extracellular vesicles, which carry hepatocyte or cholangiocyte-derived proteins, lipids, and RNA molecules. The relative proportion of the major organic solutes in bile is illustrated in Fig. 64.1 . The volume of hepatic bile secreted is estimated to range from 500 to 600 mL per day, and bile acids are the most abundant organic components. Active secretion of bile acids and other solutes across the hepatocyte canalicular membrane creates an osmotic gradient, allowing water and small solutes to enter the biliary space by solvent drag. In healthy humans, canalicular secretion of bile acids is efficient and remarkably concentrative; the intracellular monomeric concentration of bile acids is estimated to be in the low micromolar range in the hepatocyte and more than 1000 μmol/L in canalicular bile. After canalicular secretion, bile acids travel down the biliary tract and are stored and concentrated in the gallbladder. In response to a meal, the gallbladder contracts and empties its content into the duodenum. The bile acids then travel down the length of the small intestine, where they facilitate the digestion and absorption of fats, activate intestinal receptors, and stimulate the production of gut hormones. Bile acids undergo limited absorption in the proximal small intestine and are actively absorbed in the terminal ileum. The bile acids are then returned to the liver in the portal circulation, transported across the hepatocyte sinusoidal membrane, and resecreted into bile.
| Component | Concentration |
|---|---|
| Electrolytes and Minerals (mmol/L) | |
| Sodium | 140-160 |
| Potassium | 3-8 |
| Chloride | 70-120 |
| Bicarbonate | 20-50 |
| Calcium | 1-5 |
| Phosphate | 1-2 |
| Metals (μmol/L) | |
| Magnesium | 1-3 |
| Iron | 18-52 |
| Copper | 12-21 |
| Organic Constituents (mmol/L) | |
| Bile acids | 5-50 |
| Bilirubin (total) | 1-2 |
| Phospholipid (lecithin) | 0.5-20.0 |
| Cholesterol | 0.5-1.0 |
| Glutathione | 3-5 |
| Glucose | 0.2-1.0 |
| Urea | 2.2-6.5 |
| Protein (g/dL) | 0.2-3.0 |
The physiologic functions of bile acids in the liver and GI tract are multiple. First, bile acids induce bile flow and hepatic secretion of biliary lipids (phospholipid and cholesterol). The vectorial movement of bile acids from blood into the bile canaliculus generates an osmotic water flow and is a major determinant of bile formation. Second, bile acids facilitate the digestion of dietary fats and are essential for the intestinal absorption of cholesterol and fat-soluble vitamins. They also aid intestinal absorption by solubilizing dietary lipids and lipid digestion products as mixed micelles to promote their aqueous diffusion and delivery to the intestinal mucosa. Fat-soluble vitamins (A, D, E, and K) are poorly absorbed in the absence of bile acid micelles, and disturbances in the synthesis, hepatic secretion, or enterohepatic cycling of bile acids lead to fat-soluble vitamin deficiency. Along with their major role in dietary lipid absorption, bile acids may also facilitate intestinal assimilation of protein by accelerating protein denaturation and its subsequent digestion by pancreatic proteases. Third, bile acids play an integral role in maintaining cholesterol homeostasis. Bile acids are essential for the absorption of biliary and dietary cholesterol from the small intestine. Conversely, bile acids promote cholesterol elimination from the body by several different mechanisms. For example, cholesterol catabolism to bile acids balances fecal bile acid loss, and this pathway accounts for almost half of the cholesterol eliminated each day. Bile acids also facilitate biliary secretion of cholesterol, thereby promoting its passage into the intestinal lumen for excretion. Finally, bile acids have a role as cholesterol acceptors in the intestinal lumen to promote direct transintestinal cholesterol excretion. Bile acids contribute to intestinal antimicrobial defenses through direct bacteriostatic actions of bile acids or bile acid–fatty acid mixed micelles. Bile acids also signal, via receptors in the gut, induction of the production of antimicrobial factors and enhance mucosal barrier integrity, thereby reducing small bowel bacterial translocation and inflammation. Beyond their role in antimicrobial defense, bile acids function in the gut to shape the structure of the microbiome. Conversely, the intestinal microbiome is able to metabolize and biotransform bile acids, thereby significantly altering the physicochemical and signaling properties of the bile acid pool. These gut microbiota-bile acid interactions have been linked to the regulation of host metabolism as well as disease complications or disease progression in pathophysiologic conditions such as metabolic syndrome, NAFLD, and hepatic cirrhosis. Conjugated bile acids are fully soluble as calcium salts and prevent formation of insoluble calcium precipitates that promote development of gallstones in the biliary tract and gallbladder. Bile acids also protect against development of kidney stones by preventing enteric hyperoxaluria. Bile acids function as hormones that signal through nuclear and G-protein–coupled receptors to regulate their enterohepatic circulation and metabolism. In addition, bile acid signaling also contributes to the regulation of hepatic metabolism, gut motility, and fat, glucose, protein, and energy homeostasis.
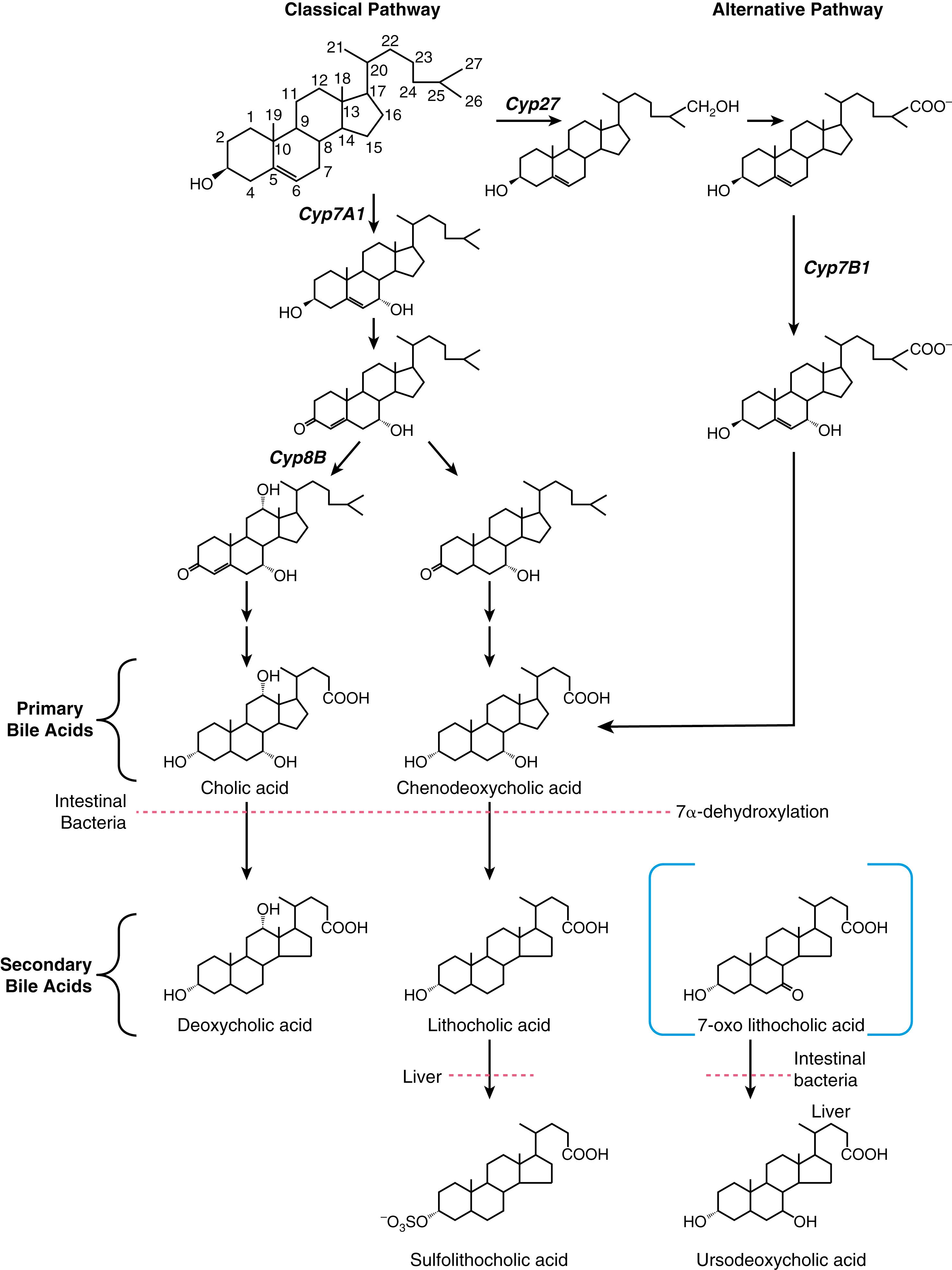
Bile acids are synthesized from cholesterol in pericentral hepatocytes of the hepatic acini. In this process, cholesterol, a lipophilic compound, is converted into a water-soluble product. In humans, the newly synthesized bile acids are cholic acid (CA), a trihydroxy-bile acid with hydroxy groups at the C-3, C-7, and C-12 positions, and chenodeoxycholic acid (CDCA), a dihydroxy-bile acid with hydroxyl groups at the C-3 and C-7 positions ( Fig. 64.2 ). The newly synthesized bile acids are termed primary bile acids to distinguish them from the products of bacterial metabolism, which are termed secondary bile acids. The kinetics of bile acid synthesis and turnover in humans is summarized in Table 64.2 . Under normal physiologic conditions, hepatic bile acid synthesis ranges from 0.2 to 0.6 g/day but can be induced to maximal levels of 4 to 6 g/day after small bowel resection. Hepatic bile acid synthesis involves 2 major pathways, the “classical” neutral pathway (cholesterol 7α-hydroxylase pathway) that favors CA biosynthesis and the “alternative” acidic pathway (oxysterol 7α-hydroxylase pathway) that favors CDCA biosynthesis. In the classical pathway, the enzyme cholesterol 7α-hydroxylase (cytochrome P450 7A1 [CYP7A1]) converts cholesterol directly into 7α-hydroxycholesterol. In the alternative pathway, cholesterol is first hydroxylated on the side chain by C-24, C-25, or C-27 sterol hydroxylases. The major oxysterol species is 27-hydroxycholesterol, which is then acted on by the oxysterol 7α-hydroxylase (CYP7B1).
| Bile acid | Pool size (mg) | Fractional turnover rate (days −1 ) | Hepatic synthesis (mg/day) | Daily input from primary bile acids (mg/day) |
|---|---|---|---|---|
| Cholic acid | 500-1500 | 0.2-0.5 | 120-400 | — |
| Deoxycholic acid | 250-800 | 0.1-0.4 | 0 | 40-200 |
| Chenodeoxycholic acid | 500-1200 | 0.2-0.4 | 100-250 | — |
| Lithocholic acid | 50-150 | 0.8-1.0 | 0 | 50-100 |
| Total | 1300-3650 | — | 220-650 | 90-300 |
The overall process of bile acid biosynthesis is complex, involving 17 different enzymes divided into 2 broad groups. The first group performs modifications to the sterol ring structure, whereas the second group modifies the sterol side chain. Sterol ring modifications precede side chain changes in the classical pathway, whereas side chain modifications occur before or during changes to the sterol ring structure in the alternative pathway. Of the 2 major biosynthetic pathways, the classical (CYP7A1) pathway is quantitatively more important in adult humans. This conclusion is supported by the finding that bile acid production is decreased by almost 90% in an adult patient with an inherited mutation in the CYP7A1 gene. The alternative pathway may be dominant in neonates, as evidenced by the low expression of CYP7A1 in newborns and the finding of severe cholestatic liver disease in children with inherited CYP7B1 mutations.
The rate-limiting step for the classical pathway is the enzyme CYP7A1. Bile acid feedback inhibition of CYP7A1 is well established experimentally; bile acid synthesis is decreased after administration of hydrophobic bile acids and increased by interruption of the enterohepatic circulation following ileal resection or administration of bile acid sequestrants. The molecular mechanisms responsible for the negative feedback regulation of the CYP7A1 pathway involve the liver and small intestine and have been elucidated. For the major pathway, bile acids act as ligands for the farnesoid X receptor (FXR) in ileal enterocytes to induce synthesis of an endocrine polypeptide hormone, fibroblast growth factor-19 (FGF19; rodent ortholog is FGF15). FGF19 is secreted into the portal circulation and acts on hepatocytes through its cell surface receptor, a complex of the β-klotho protein and FGF4-receptor (FGFR4), to repress CYP7A1 expression and bile acid synthesis. In addition to regulation via FGF19, bile acids in the hepatocyte signal via FXR to repress CYP7A1 mRNA expression through indirect transcriptional and post-transcriptional mechanisms. At the transcriptional level, FXR increases expression of the orphan nuclear receptor small heterodimer partner (SHP), which interferes with the activity of hepatocyte nuclear factor 4α (HNF4α) and liver receptor homolog 1 (LRH-1), transcription factors required for CYP7A1 expression. At the post-transcriptional level, FXR increases expression of the RNA binding protein ZFP36L1, which promotes turnover of the CYP7A1 mRNA. These complex molecular titrations link bile acid synthesis to changes in intestinal as well as hepatic bile acid levels.
For the alternative pathway of bile acid synthesis, the primary mechanism for regulation appears to be post-transcriptional. This involves cholesterol delivery by members of the steroidogenic acute regulatory protein family to the inner mitochondrial membrane, the site of sterol 27-hydroxylation. However, the alternative pathway can also be regulated transcriptionally by bile acids. In that pathway, bile acids act via FXR to induce expression of MAP bZIP transcription factor G (formerly v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog G [MafG]), which represses transcription of genes important for the alternative bile acid biosynthetic pathway such as CYP27A1 and CYP7B1 but does not affect expression of CYP7A1 . The receptors and protein factors that regulate the biosynthesis and enterohepatic cycling of bile acids are summarized in Table 64.3 .
| Protein ( Gene ) | Description and function of protein in bile acid metabolism |
|---|---|
| FXR (NR1H4) | Bile acid–activated nuclear receptor; regulation of bile acid synthesis, transport, and metabolism |
| HNF4α (NR2A1) | Nuclear receptor; positive regulator of CYP7a1 expression and hepatic bile acid synthesis |
| LRH-1 (NR5A2) | Nuclear receptor; positive regulator of CYP7a1 expression and hepatic bile acid synthesis |
| SHP (NR0B2) | Nuclear receptor; negative feedback regulation of hepatic bile acid synthesis by antagonizing HNF4α and LRH-1; regulation of bile acid transport and metabolism |
| PXR (NR1I2) | Bile acid and xenobiotic-activated nuclear receptor involved in detoxification of secondary bile acids |
| VDR (NR1I1) | Vitamin D and bile acid–activated nuclear receptor; involved in detoxification of LCA |
| CAR (NR1I3) | Xenobiotic-activated nuclear receptor involved in detoxification of secondary bile acids |
| MafG (MAFG) | Transcription factor; negative regulator of bile acid synthesis and transport |
| FGFR4 (FGFR4) | Membrane receptor; negative feedback regulator of CYP7A1 and hepatic bile acid synthesis |
| ZFP36L1 (ZFP36L1) | RNA binding protein; negative regulator of CYP7a1 expression and hepatic bile acid synthesis |
| β-klotho (KLB) | Membrane co-receptor associated with FGFR4; confers liver specificity to FGFR4-FGF19 pathway; negative feedback regulator of CYP7A1 and hepatic bile acid synthesis |
| FGF19 (FGF19) | Protein growth factor; secreted by ileum, liver, and gallbladder in response to bile acids; regulator of hepatic bile acid synthesis via the FGFR4:β-klotho complex |
| TGR5 (GPBAR1) | Bile acid-activated G-protein coupled receptor; mediates the systemic actions of bile acids; regulates intestinal motility, metabolism |
Before secretion into the bile canaliculus, both CA and CDCA are N -acyl amidated by the addition of glycine or taurine on their side chain, a process commonly termed conjugation . This conjugation makes the bile acid more hydrophilic and increases the acidic strength of its side chain, in essence converting a weak acid (pK a ≈5.0 for the unconjugated bile acid) to a strong acid (pK a ≈3.9 for the glycine conjugate; pK a ≈2.0 for the taurine conjugate). The major function of conjugation to glycine or taurine is to decrease the passive diffusion of bile acids across cell membranes during their transit in the enterohepatic circulation. As a result, efficient uptake and export of conjugated bile acids requires the presence of specific membrane carriers. Compared with unconjugated bile acids, conjugated bile acids are also more soluble at acidic pH and more resistant to precipitation in the presence of high concentrations of calcium. The net effect of conjugation is to maintain high intraluminal concentrations of bile acids in the biliary tract, gallbladder and small intestine to facilitate lipid solubilization, digestion, and absorption. The physiologic significance of this bile acid modification is illustrated by the finding that patients with inherited defects in bile acid conjugation present with fat-soluble vitamin malabsorption and steatorrhea and respond favorably to administration of conjugated bile acids such as glycocholic acid.
Metabolism of bile acids by the gut microbiota begins in the small intestine. Although most of the conjugated bile acids secreted into the small intestine are efficiently absorbed intact, gut bacteria-derived bile salt hydrolases will remove the glycine or taurine group from ≈15% of the bile acids in the small intestine. These deconjugated bile acids can pass into the colon or be absorbed by passive or active mechanisms from the small intestine and returned to the liver, where they are reconjugated to glycine or taurine and resecreted into bile along with newly synthesized bile acids. This process of intestinal deconjugation and hepatic reconjugation is a normal part of bile acid metabolism. An additional modification of bile acids carried out by the gut microbiota is epimerization of the α-hydroxyl groups to generate their corresponding β-hydroxy derivatives (“iso” bile acids), such as isolithocholic acid and isodeoxycholic acid. Although potentially abundant in the cecal or colonic contents of some individuals, these gut-derived iso-bile acids have not been the subject of extensive investigation and their physiologic or clinical significance are still unclear. The 7α-hydroxy group of CDCA is also epimerized by the gut microbiota to form the 3α,7β-dihydroxy bile acid UDCA. After being absorbed from the intestine, UDCA is conjugated to glycine or taurine in the liver and circulates as a minor component, normally less than 5%, of the bile acid pool. Note that in addition to being generated by the gut microbiota as a minor secondary bile acid, UDCA is also administered therapeutically for various forms of cholestatic liver disease (see Chapter 91 ).
A fraction of the circulating bile acid pool escapes absorption from the small intestine and passes into the colon, where deconjugation continues almost to completion. In the colon, a small subset of the gut bacteria is able to efficiently remove the hydroxyl group at the C-7-position (7α-dehydroxylation), thereby converting CA to deoxycholic acid (DCA), a dihydroxy bile acid with hydroxyl groups at the C-3 and C-12 positions, and converting CDCA to lithocholic acid (LCA), a monohydroxy bile acid with a hydroxyl group at position C-3 (see Fig. 64.2 ). Although other gut microbiome-catalyzed bile acid modifications such as deconjugation, epimerization, and oxidation can be reversed by host enzymes in the liver, bile acids are not rehydroxylated at the C-7-position in humans. Therefore this bacterial reaction is particularly important for shaping the composition and properties of the bile acid pool. The colon absorbs about 50% of the DCA as well as a portion of the LCA formed. After returning to the liver, DCA is reconjugated to glycine or taurine and circulates with the primary bile acids, whereas LCA is reconjugated and sulfated. Hepatic reconjugation of bile acids returning in the enterohepatic circulation is extremely efficient, so virtually all the endogenous biliary bile acids (primarily CA, CDCA, DCA, and UDCA) are in conjugated form. Bacterial deconjugation and dehydroxylation of bile acids in the colon is also efficient, so the feces contain primarily unconjugated secondary bile acids and only small amounts (<15%) of other bile acid species (see Fig. 64.1 ).
Several pathways exist for host metabolism of secondary bile acids, including hepatic re-epimerization of β-hydroxy iso-bile acids, hepatic reduction of 7-oxo-lithocholate to form CDCA or UDCA, and hydroxylation, glucuronidation, or sulfation of bile acids (see Fig. 64.2 ). The modification of bile acids by sulfation or glucuronidation blocks their intestinal and renal reabsorption, and these sulfated or glucuronidated species are rapidly lost from the circulating pool of bile acids. Sulfation is particularly significant for metabolism of LCA. Unmodified LCA is intrinsically hepatotoxic, and sulfation of LCA at the 3-hydroxy position hastens its elimination from the body, thereby playing an important hepatoprotective role. In addition to sulfation or glucuronidation, bile acids can be hydroxylated at other sites on their sterol rings, including the C-1, C-2, C-4, or C-6 positions. Under normal physiologic conditions in adults, these polyhydroxylated bile acids are typically present at only very low levels. However, these unusual bile acid species are present at higher levels in newborns, in patients after bariatric surgery, and in patients with cholestatic liver disease. Expression of the hepatic phase 1 and phase 2 enzymes responsible for the hydroxylation and sulfation of bile acids is induced by LCA or agents such as rifampin by a transcriptional mechanism involving the xenobiotic-sensing nuclear receptors pregnane X receptor (PXR) and constitutive androsterone receptor (CAR) (see also Chapter 88 ).
The anatomic components of the enterohepatic circulation are the liver, biliary tract, gallbladder, small intestine, portal venous circulation, and, to a lesser extent, colon and systemic circulation ( Fig. 64.3 ). At a fundamental level, the enterohepatic circulation of bile acids can be considered to consist of a series of storage chambers (gallbladder and small intestine), valves (sphincter of Oddi and ileocecal valve), mechanical pumps (small intestine), and chemical pumps (hepatocyte, cholangiocyte, and ileocyte).
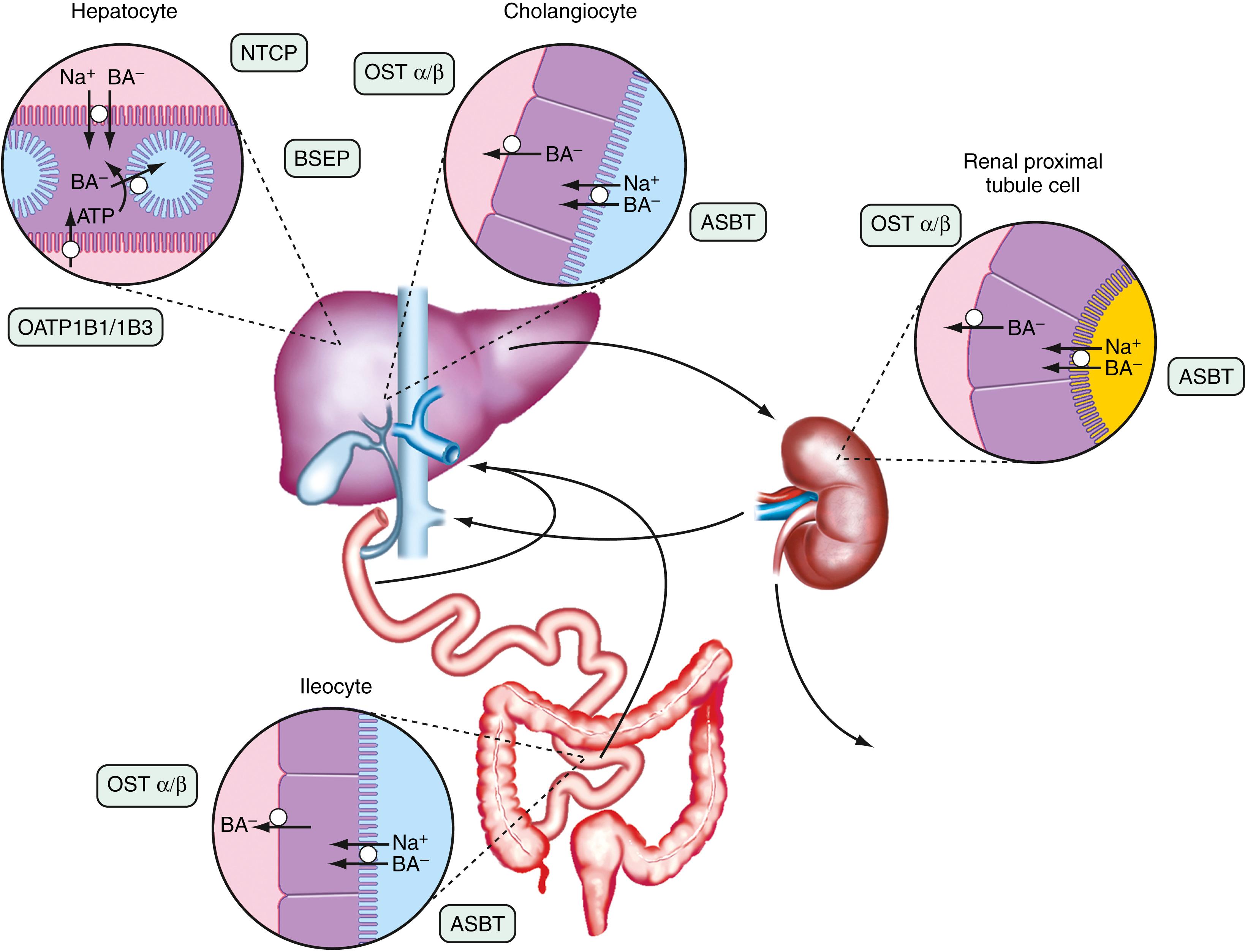
Efficient intestinal reabsorption and hepatic extraction of bile acids enable an effective recycling and conservation mechanism that largely restricts bile acids to the intestinal and hepatobiliary compartments. During fasting, bile acids move down the biliary tract and are concentrated approximately 10-fold in the gallbladder, resulting in lower levels of bile acids in the small intestine, portal vein, systemic circulation, and liver. However, basal rates of hepatic bile acid secretion are maintained, and enterohepatic cycling continues for that portion of the bile acid pool that is not sequestered in the gallbladder. In response to a meal, cholecystokinin is released from the intestinal mucosa and acts on the biliary tract to relax the sphincter of Oddi and stimulate gallbladder contraction. A concentrated solution of mixed micelles (bile acids, phospholipids, and cholesterol) then passes via the bile duct from the gallbladder bile into the small intestine. In the intestinal lumen, these micelles facilitate fat digestion and absorption by stimulating the action of pancreatic lipase on triglyceride, solubilizing the hydrolytic products such as long chain saturated fatty acids and shuttling these hydrophobic lipids across the unstirred water layer to the mucosal surface. During digestion of a large meal, the gallbladder remains contracted, and bile acids secreted by the liver bypass the gallbladder and empty directly into the duodenum. During this period, the intraluminal bile acid concentration in the small intestine is 5 to 10 mmol/L, well above the threshold concentration of approximately 1.5 mmol/L that is required for micelle formation. During the interdigestive period, the sphincter of Oddi contracts and the gallbladder relaxes, causing a larger fraction of the bile acids secreted into bile to enter the gallbladder for storage. This is controlled in part by bile acids, which act directly by activating TGR5, a G-protein-coupled receptor and indirectly by stimulating the ileal synthesis and release of FGF19, a polypeptide hormone that induces gallbladder relaxation. In general, the enterohepatic cycling of bile acids accelerates during digestion and slows between meals and during overnight fasting. This pulsatile rhythm of bile acid secretion is maintained even after cholecystectomy. When the gallbladder is absent, bile acids are stored in the proximal small intestine during fasting, with the intestinal migrating motor complex driving the distal movement of the intraluminal bile acid pool (see Chapter 99 ). Following ingestion of a meal, small intestinal contractions accelerate and propel the stored bile acids to the distal ileum, where they are actively reabsorbed and carried back to the liver for resecretion into bile.
The enterohepatic cycling of bile acids is extremely efficient; less than 10% of the intestinal bile acids escape reabsorption and are eliminated in the feces. Bile acids are absorbed from the small intestine by passive absorption down the length of the intestine and by active transport in the terminal ileum. In adult humans, the enterohepatic circulation maintains a bile acid pool size of approximately 2 to 4 g. The bile acid pool cycles 2 to 3 times per meal, and the intestine may reabsorb between 10 and 30 g of bile acid per day. Approximately 0.2 to 0.6 g of bile acid escapes reabsorption and is eliminated in the stool each day. Hepatic conversion of cholesterol to bile acid balances fecal excretion to maintain the bile acid pool size. The kinetics of bile acid turnover in humans is summarized in Table 64.2 .
An enterohepatic circulation of bile acids is advantageous because it results in the accumulation of a large mass of detergent molecules that can be used repeatedly during digestion of a single meal or multiple meals throughout the day. The presence of an ileal active transport system and enterohepatic circulation dissociates hepatic bile acid secretion from bile acid synthesis, thereby improving the efficiency of intestinal nutrient digestion and absorption. Because bile acid secretion induces hepatic bile flow, maintenance of the enterohepatic circulation also promotes continuous secretion of bile. The dissociation of bile acid biosynthesis from intestinal delivery is also aided by the presence of a gallbladder because the availability of a concentrative storage reservoir permits bile acids to be delivered in a controlled fashion at high concentrations to the duodenum. The ileal bile acid transporter and gallbladder are complementary rather than redundant, and they function together to conserve bile acids. In the presence of a gallbladder but absence of an active ileal bile acid transporter, the bile acids secreted into the intestine would not be efficiently reabsorbed. Emptying of the gallbladder contents would necessarily be followed by a refractory period during which the supply of bile acids is insufficient to promote efficient lipid digestion and absorption. The refractory period would last until hepatic synthesis restores the bile acid pool.
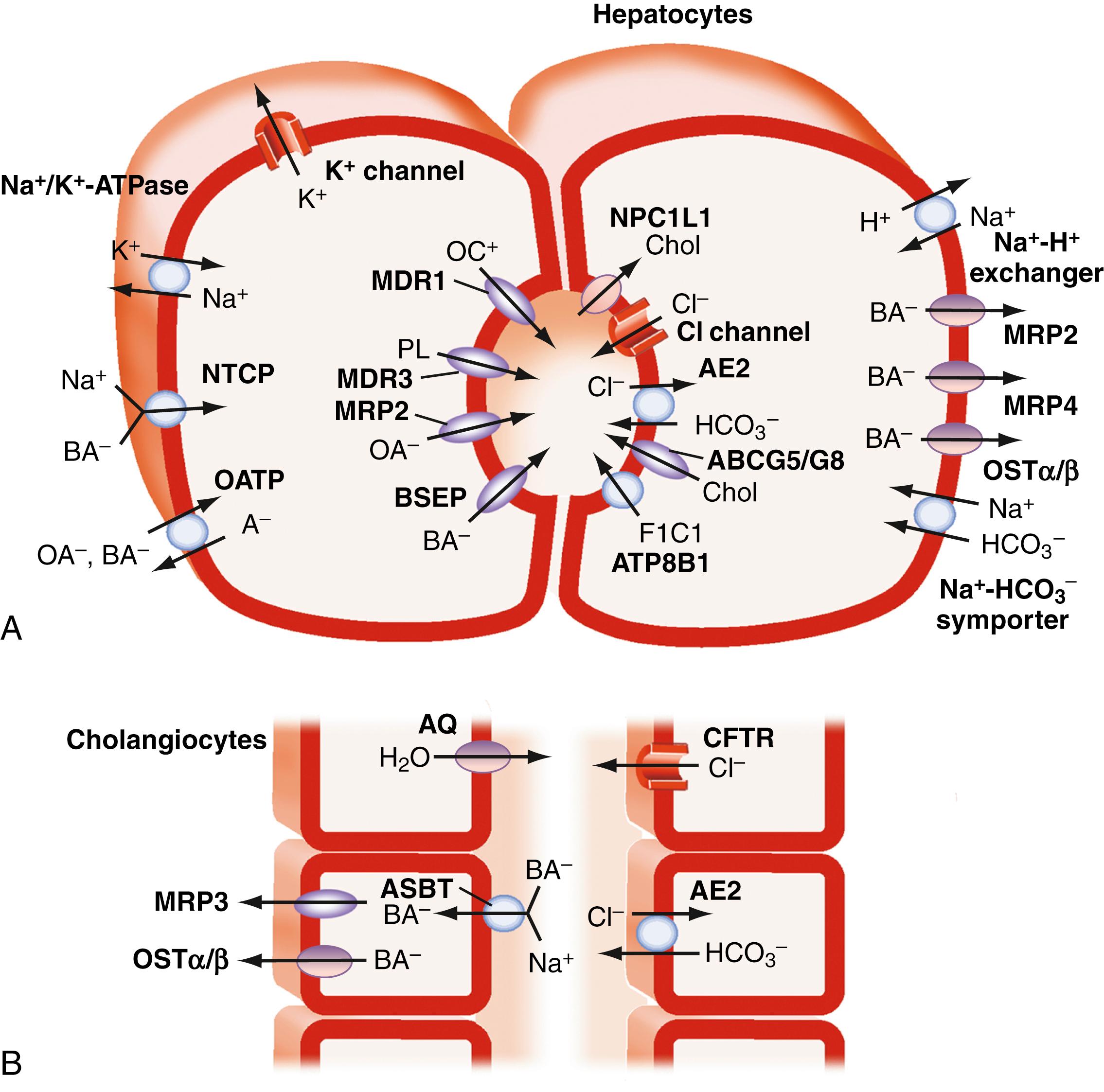
Bile formation involves the secretion of osmotically active inorganic and organic solutes into the canalicular space and biliary tract lumen by hepatocytes and the biliary epithelial cells (cholangiocytes) and has been studied using metabolically inert markers such as mannitol or erythritol. Hepatocyte canalicular bile formation is traditionally divided into 2 components: bile acid – dependent bile flow (bile flow relating to bile acid secretion) and bile acid – independent flow (bile flow attributed to active secretion of inorganic electrolytes and other solutes), which account respectively for approximately 60% and 40% of spontaneous basal bile flow. Hepatic ATP – dependent carriers actively secrete bile acids into the canalicular lumen, where they form aggregates and mixed micelles that are too large to diffuse back across the paracellular junctions that line the canaliculi. Compounds such as the conjugated bile acids that are actively pumped across the canalicular membrane generate bile flow and are termed primary solutes . Besides bile acids, primary solutes include glutathione, conjugated bilirubin, heavy metals, and conjugates of various metabolites and xenobiotics. Water, plasma electrolytes, calcium, glucose, amino acids, bicarbonate, and other low-molecular-weight solutes that flow into the canaliculus in response to the osmotic gradient are termed secondary solutes . The choleretic activity of each primary solute is defined as the volume of bile flow induced per amount of solute secreted. For natural bile acid species, the choleretic activity ranges from 8 to 40 μL of bile flow induced per micromole of bile acid secreted. The secretion of other primary solutes besides bile acids such as glutathione and bicarbonate by the hepatocyte and biliary epithelium also contributes to bile formation. Newly secreted hepatic canalicular bile is modified during its transit through the biliary tract via the action of cholangiocytes. The ductular modifications to canalicular bile include (1) the absorption of solutes such as glucose, amino acids , and bile acids; (2) the movement of water through specific channels (aquaporins) and paracellularly; and (3) the secretion of solutes such as bicarbonate and chloride. The contribution of this ductular secretion varies among species, with estimates suggesting that it may account for up to 30% of bile flow in humans but less than 10% of bile flow in animals such as rats.
Hepatocyte canalicular bile flow is also generated by active secretion of primary solutes besides bile acids. Reduced glutathione (GSH) and bicarbonate (HCO 3 − ) constitute major components of the bile acid–independent fraction of bile flow. The ATP-dependent canalicular secretion of GSH and GSH conjugates via the multidrug resistance–associated protein 2 (MRP2) is important at several levels. In addition to being secreted at high concentrations into bile, intraluminal catabolism of GSH by GGTP and dipeptidases raises the solute concentration and contributes to the osmotic driving force for canalicular bile formation. Besides the ATP-dependent secretion of organic anions into bile, hepatocyte and cholangiocyte ATP-independent secretion of bicarbonate via the HCO 3 − /Cl − anion exchanger AE2 is important for the bile acid–independent bile flow. The majority of this HCO 3 – secretion occurs at the level of the bile duct epithelial cells in response to stimulation by a variety of hormones and neuropeptides, such as secretin, vasoactive intestinal peptide, and bile acids.
The term cholehepatic shunt was coined by Alan Hofmann to describe the cycle whereby unconjugated dihydroxy bile acids in bile are passively absorbed by cholangiocytes and returned via the venous drainage directly to the hepatocyte for uptake and resecretion into bile. Absorption of the protonated unconjugated bile acid molecule generates a bicarbonate anion, which together with hepatic resecretion of the bile acid produces a bicarbonate-rich choleresis. This cycle was proposed to explain the increased bile flow observed after administration of therapeutic doses of unconjugated C-24 dihydroxy bile acids such as UDCA or unconjugated side chain–shortened C-23 bile acid analogs such as norUDCA. Although the proposal is conceptually sound and in general agreement with existing data, the intrahepatic location and passive nature of the cholehepatic shunt cycle have made it difficult to study or prove. In addition, because ductal bile normally contains almost all conjugated bile acids under normal physiologic conditions, the contribution of cholehepatic shunting of unconjugated bile acids to bile flow was originally thought to be negligible. The subsequent discovery that the biliary epithelium expresses the apical sodium-dependent bile acid transporter (ASBT; gene symbol SLC10A2 ) and the organic solute transporter OSTα-OSTβ (gene symbols SLC51A and SLC51B ) offered a potential mechanism for active cholehepatic shunting of conjugated bile acids. However, the significance of this pathway in humans still remains to be determined. Indeed, the role of ASBT in the biliary epithelium may be to permit cholangiocytes to sample biliary bile acid concentrations to activate cellular signaling pathways rather than to transport significant quantities of bile acids.
More than 90% of the bile acids secreted into bile are derived from the recirculating pool. To maintain this process, hepatocytes must transport bile acids efficiently from the portal blood into bile. This vectorial trans-hepatocellular movement of bile acids is a concentrative transport process that is driven by a distinct set of primary (ATP-dependent), secondary (Na + gradient dependent), and tertiary (OH − - or HCO 3 − -dependent anion exchange) transport systems at the sinusoidal and canalicular plasma membranes. Bile acid flux through the liver and the number of participating hepatocytes vary. In the fasting state, uptake of bile acids is highest in the periportal hepatocytes (closest to the portal venules), whereas during feeding, more distal hepatocytes in the liver acinus are recruited to participate. Conversely, production and secretion of newly synthesized bile acids is highest in pericentral hepatocytes (closest to the central vein). In this fashion, the periportal hepatocytes transport a larger fraction of the bile acid pool and are thought to be major drivers of bile acid-dependent bile flow.
The concentration of bile acids in the portal blood of healthy humans is 20 to 50 μmol/L. Uptake by the liver is typically expressed as fractional extraction, or first-pass extraction, and represents the percentage of bile acids removed during a single passage through the hepatic acinus. The fractional extraction of bile acids from sinusoidal blood ranges from 50% to 90% and remains constant irrespective of systemic bile acid concentrations. The hepatic fractional extraction is related to bile acid structure and albumin binding and is highest (80% to 90%) for hydrophilic conjugated bile acids such as conjugated CA and lowest (50% to 60%) for unconjugated hydrophobic protein-bound bile acids such as CDCA. Due to the rapid differential hepatic clearance, the concentration of total bile acids in the systemic circulation is low, averaging 2 to 5 μmol/L and 5 to 15 μmol/L in the fasting and fed states, respectively, and the bile acid composition in the systemic circulation does not strictly mirror that of the other compartments in the body.
The major transport proteins that function to maintain the enterohepatic circulation of bile acids have been identified and are shown in Fig. 64.3 and 64.4 . The general properties of these carriers are listed in Table 64.4 . Because of their importance for bile secretion, the bile acid transporters are highlighted; however, the hepatocyte sinusoidal and canalicular membranes also express specialized transport proteins for a wide spectrum of endogenous and exogenous compounds.
| Transporter ( Gene ) | Location | Function |
|---|---|---|
| Hepatocyte | Bile Acid–Dependent Bile Flow | |
| NTCP (SLC10A1) | Basolateral membrane | Na + -dependent bile acid and xenobiotic uptake |
| OATP1B1 (SLCO1B1) | Basolateral membrane | Na + -independent bile acid and xenobiotic uptake |
| OATP1B3 (SLCO1B3) | Basolateral membrane | Na + -independent bile acid and xenobiotic uptake |
| Na + ,K + -ATPase | Basolateral membrane | Secretion of 2 Na + in exchange for 3 K + |
| BSEP (ABCB11) | Canalicular membrane | ATP-dependent bile acid export |
| MDR3 (ABCB4) | Canalicular membrane | ATP-dependent phosphatidylcholine export |
| ABCG5/ABCG8 | Canalicular membrane | ATP-dependent sterol export |
| NPC1L1 | Canalicular membrane | Sterol import |
| FIC1 (ATP8B1) | Canalicular membrane | ATP-dependent aminophospholipid flipping |
| Bile Acid–Independent Bile Flow | ||
| OATP1B1, 1B3, 2B1 | Basolateral membrane | Na + -independent transport of organic anions, cations, and neutral steroids |
| MRP2 (ABCC2) | Canalicular membrane | ATP-dependent transport of glucuronide, glutathione, and sulfate conjugates |
| Sinusoidal Bile Acid Export | ||
| MRP3 (ABCC3) | Basolateral membrane | ATP-dependent export of bile acids and glucuronide conjugates |
| MRP4 (ABCC4) | Basolateral membrane | ATP-dependent export of glutathione and bile acids |
| OSTα-OSTβ( SLC51A, SLC51B ) | Basolateral membrane | Bile acid export |
| Cholangiocyte | Ductular Secretion | |
| Aquaporin 1 (AQP1) | Apical membrane | Water transport |
| Aquaporin 4 (AQP4) | Basolateral membrane | Water transport |
| AE2 (SLC4A2) | Apical membrane | HCO 3 − secretion in exchange for Cl − |
| CFTR (ABCC7) | Apical membrane | Cl − secretion |
| ASBT (SLC10A2) | Apical membrane | Bile acid uptake (cholehepatic shunt) |
| Ileal Enterocyte | ||
| ASBT (SLC10A2) | Apical membrane | Na + -dependent bile acid uptake |
| NPC1L1 | Apical membrane | Sterol import |
| OSTα-OSTβ( SLC51A, SLC51B ) | Basolateral membrane | Bile acid export |
| MRP3 (ABCC3) | Basolateral membrane | Bile acid export |
The uptake of conjugated bile acids at the sinusoidal (basolateral) membrane is predominantly mediated (>80%) by a secondary active Na + -dependent transport system, which is driven by the basolateral Na + ,K + -ATPase that maintains the prevailing out-to-in Na + gradient. Although important for conjugated bile acids, Na + -dependent transport accounts for less than half of the uptake of unconjugated bile acids such as CA and UDCA. The major transporter responsible for hepatocytic Na + -coupled uptake of bile acids is the Na + -taurocholate cotransporting polypeptide (NTCP; gene symbol SLC10A1 ). Inherited defects in the NTCP gene are associated with greatly elevated (25 to 100-fold) plasma levels of conjugated bile acids in the absence of jaundice, pruritus, or other signs of liver disease. In addition to being the major hepatic bile acid uptake transporter, NTCP has been identified as a cell surface receptor on hepatocytes for the binding and entry of HBV and HDV (see Chapters 79 and 81 ). A naturally-occurring variant in NTCP, which converts a serine at position 267 to a phenylalanine (c.800C>T; rs2296651; p.Ser267Phe), leads to nearly complete loss of bile acid transport activity. This variant, which is prevalent in Asian populations (minor allele frequency ranging from 3.1% to 9.2%) has an attenuated ability to support viral entry. The variant is associated with increased resistance to HBV infection. These findings have stimulated efforts to target NTCP for the development of viral entry inhibitors to block de novo HBV infection or reduce viral load in chronic HBV-infected patients.
Unconjugated bile acids such as CA are taken up predominantly in a Na + -independent fashion by members of the organic anion-transporting polypeptide (OATP) gene family (gene symbol SLCO , in which “SLC” and “O” are designations for solute carrier and OATP, respectively). Unlike the Na + -cotransporters such as NTCP and ASBT, the driving force responsible for OATP-mediated solute uptake is not well understood, and solute transport may be bidirectional. Potential transport mechanisms include facilitative diffusion and electroneutral exchange that couples solute uptake to bicarbonate or GSH efflux. Human OATP1B1 (gene symbol SLCO1B1 ; original protein name OATP-C) and OATP1B3 (gene symbol SLCO1B3 ; original protein name OATP8) are expressed primarily in liver and account for the majority of hepatic Na + -independent bile acid clearance. However, in addition to bile acids, OATP1B1 and OATP1B3 also transport a wide variety of organic anions and solutes, including bilirubin glucuronides (see Chapter 21 ), steroid metabolites (estradiol-17β-glucuronide, dehydroepiandrosterone-3-sulfate, and estrone-3-sulfate), arachidonic acid products (prostaglandin E 2 , thromboxane B 2 , and leukotriene C 4 ), diagnostic dyes (such as bromosulfophthalein and indocyanine green [ICG]), and drugs (such as rifampin, statins, digoxin, and fexofenadine). Notably, combined loss of SLCO1B1 and SLCO1B3 , which are adjacent genes on human chromosome 12, causes Rotor syndrome, a rare and benign hereditary conjugated hyperbilirubinemia distinct from Dubin-Johnson syndrome. Although the plasma clearance of unconjugated bile acids has not been measured in human subjects deficient in OATP1B1/OATP1B3, mice deficient in the orthologous Oatp1a/1b genes show defective hepatic clearance of unconjugated but not conjugated bile acids. Moreover, loss of OATP1B3 alone is sufficient to delay ICG clearance, thereby yielding an abnormal ICG retention test result in subjects with otherwise normal laboratory values and normal liver biopsy specimens. Because the bile acid pool is composed mostly of conjugated bile acids, which are taken up by hepatocytes in a sodium-dependent fashion, these broad-specificity OATPs in humans may be more important for hepatic clearance of non–bile acid metabolites and xenobiotics. Indeed, inherited polymorphisms in the OATP genes influence drug metabolism and contribute to some forms of drug toxicity.
Unconjugated, conjugated, or modified (sulfated, glucuronidated, and polyhydroxylated) bile acids are also effluxed from across the hepatocyte basolateral (sinusoidal) membrane into the space of Disse by the heteromeric organic solute transporter, OSTα-OSTβ, members of the MRP family including MRP3 (gene symbol ABCC3 ) and MRP4 (gene symbol ABCC4 ), and possibly other carriers. After their efflux, the conjugated or unconjugated bile acids are carried in sinusoidal blood to more pericentral hepatocytes for reuptake and secretion into bile. This process dynamically recruits additional hepatocytes from zones 2 and 3 within the liver lobule to maintain the clearance and secretion of bile acids into bile, thereby safeguarding vulnerable zone 1 periportal hepatocytes. In addition, the modified bile acids generated by hepatocyte phase 1 or phase 2 metabolism are also effluxed across the sinusoidal membrane and pass into the systemic circulation, where they can be filtered by the kidney and excreted in urine. These hepatoprotective mechanisms, which also include down-regulation of the major liver bile acid uptake transporters, are an important part of the adaptive response to conditions of bile acid overload.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here