Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
To a large extent, the distribution of benign smooth muscle tumors parallels the distribution of smooth muscle tissue in the body. The tumors tend to be relatively common in the genitourinary and gastrointestinal tracts, less frequent in the skin, and rare in deep soft tissue. In the experience of Farman, based on 7748 leiomyomas, approximately 95% occurred in the female genital tract, and the remainder were scattered over various sites, including the skin (230 cases), gastrointestinal tract (67 cases), and bladder (5 cases). This study, based on surgical material, probably underestimates the large number of asymptomatic gastrointestinal and genitourinary lesions documented in autopsy material only. In general, soft tissue leiomyomas cause little morbidity, so there are few studies on their presentation, diagnosis, and therapy. For purposes of classification, these tumors can be divided into several groups.
Cutaneous leiomyomas (leiomyoma cutis) are the most common group of benign smooth muscle tumors, with two types. Those arising from the pilar arrector muscles of the skin are often multiple and associated with significant pain. Multiple cutaneous leiomyomas are often associated with the hereditary leiomyomatosis and renal cell cancer syndrome (Reed syndrome), secondary to germline mutation in the fumarate hydratase gene. Those arising from the network of muscle fibers that lie in the deep dermis of the scrotum (dartoic muscles), labia majora, and nipple are almost always solitary and are collectively referred to as genital leiomyomas . The second group of benign smooth muscle tumors includes the angiomyomas (vascular leiomyomas) , which are distinctive, painful, subcutaneous tumors composed of a conglomerate of thick-walled vessels associated with smooth muscle tissue. They differ from cutaneous leiomyomas in their anatomic distribution, predominantly subcutaneous location, and predilection for women. The third group constitutes leiomyomas of deep soft tissue , lesions whose very existence has been questioned (see later section). Although recent studies provide reasonable evidence that soft tissue leiomyomas exist, they are rare and should be diagnosed using only the most stringent criteria. Leiomyomatosis peritonealis disseminata can be conceptualized as a diffuse metaplastic response of the peritoneal surfaces in which multiple smooth muscle nodules form and may be confused with metastatic leiomyosarcoma because of its unusual growth pattern. This chapter also discusses tumors of specialized genital stromal cell origin, including angiomyofibroblastoma, cellular angiofibroma, mammary-type myofibroblastoma, and deep (“aggressive”) angiomyxoma.
Smooth muscle cells are widely distributed throughout the body and contribute to the wall of the gastrointestinal, genitourinary, and respiratory tracts. They constitute the muscles of the skin, erectile muscles of the nipple and scrotum, and iris of the eye. Their characteristic arrangements in these organs determine the net effect of contraction. For instance, the circumferential arrangement in blood vessels results in a narrowing of the lumen during contraction, whereas contraction of the longitudinal and circumferential muscle layers in the gastrointestinal tract causes the propulsive peristaltic wave.
Smooth muscle cells are fusiform in shape and have centrally located cylindrical nuclei with round ends that develop deep indentations during contraction. The length of the muscle cell varies depending on the organ, achieving its greatest length in the gravid uterus, where it may measure as much as 0.5 mm. The cells are usually arranged in fascicles where the nuclei are staggered so that the tapered end of one cell lies in close association with the thick nuclear region of an adjacent cell. Typically, there are no connective tissue cells between individual muscle fibers, although a delicate basal lamina and small connective tissue fibers, presumably synthesized by the muscle cells, can be seen as a thin, periodic acid–Schiff (PAS)–positive rim around individual cells in light microscopy preparations.
Ultrastructurally, the cells are characterized by clusters of mitochondria, rough endoplasmic reticulum, and free ribosomes, which occupy the zone immediately adjacent to the nucleus. The remainder of the cytoplasm (sarcoplasm) is filled with myofilaments oriented parallel to the long axis of the cell. There are three types of filaments in the cell. Thick myosin filaments (12 nm) are surrounded by seven to nine thin actin filaments (6-8 nm). Thick and thin filaments are aggregated into larger groups, or units, which correspond to linear myofibrils on light microscopy. In addition to the contractile proteins, intermediate filaments, measuring 10 nm and forming part of the cytoskeleton, are centered around the dense bodies or plaques, which are believed to be the smooth muscle analogue of the Z band. The plasma membrane is dotted with tiny pinocytotic vesicles, and overlying the surface of the cell is a delicate basal lamina. Although the basal lamina separates individual cells, limited areas exist between cells where the substance is lacking and where the plasma membranes lie in close proximity, separated by a space of about 2 nm. This area, known as a gap junction or nexus , may allow the spread of electrical impulses between adjacent cells.
Smooth muscle cells display diversity in their content of contractile and intermediate filament proteins, depending on their location and function. It is useful to be aware of some of the regional variations when evaluating neoplasms. For example, the gamma isoform of muscle actin is present along with desmin in most smooth muscle cells, whereas in vascular smooth muscle the alpha isoform of muscle actin and vimentin predominates. Therefore, many smooth muscle tumors of vascular smooth origin may lack expression of desmin.
Superficial, or cutaneous, leiomyomas are of two types. Those arising from the pilar arrector muscles of the skin may be solitary or multifocal and are often associated with considerable pain and tenderness. The other form, the genital leiomyoma, arises from the diffuse network of muscle in the deep dermis of the genital zones (e.g., scrotum, nipple, areola, vulva). In the scrotum, leiomyomas arise from the dartoic muscles (dartoic leiomyoma) and, in the nipple, from the muscularis mamillae and areolae. This form is almost always solitary and rarely causes significant pain.
Although formerly believed to be the more common form of cutaneous leiomyoma, leiomyomas of pilar arrector origin are probably much less common than previously thought and outnumbered by those arising in genital sites. Solitary or multiple, most develop during adolescence or early adult life, although occasional cases appear at birth or during early childhood. Some occur on a familial basis.
Recent evidence suggests that the majority of patients presenting with multiple cutaneous leiomyomas have germline mutations of the fumarate hydratase gene, mapped to chromosome 1q43 and encoding an enzyme in the Krebs cycle. This disease also predisposes to early-onset uterine leiomyomas in women and to early-onset renal cell carcinoma of the collecting duct and papillary type in both men and women, termed hereditary leiomyomatosis and renal cell cancer (HLRCC). However, sporadic leiomyomas and leiomyosarcomas seemingly do not typically harbor somatic mutations of this gene. The prevalence of HLRCC is unknown, and it has been reported in approximately 200 families worldwide. The penetrance of HLRCC in affected families is almost 100%. HLRCC-associated cutaneous leiomyomas typically arise in young patients (mean age: 25 years), as do uterine leiomyomas (mean age: 30).
Typically, cutaneous leiomyomas develop as small, brown-red to pearly discrete papules that, in the incipient stage, can be palpated more readily than they can be seen ( Fig. 15.1 ). Eventually, they form nodules that coalesce into a fine linear pattern following a dermatome distribution. The extensor surfaces of the extremities are most often affected. The lesions often produce significant pain that can be triggered by exposure to cold. In one unusual case, the patient claimed that strong emotions evoked pain in the lesions. It is not clear whether the pain produced by these tumors is the result of contraction of the muscle tissue or compression of nearby nerves by the tumors. Pseudo-Darier sign, transient elevation of the nodule after rubbing, may be seen. Usually the tumors grow slowly over years, with new lesions forming as older lesions stabilize. The slowly progressive nature of the disease probably accounts for patients often seeking medical attention after a number of years.
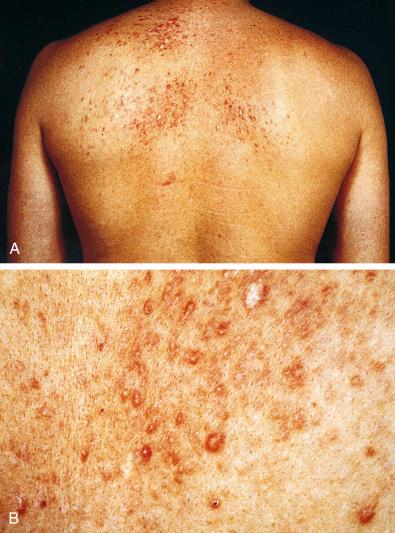
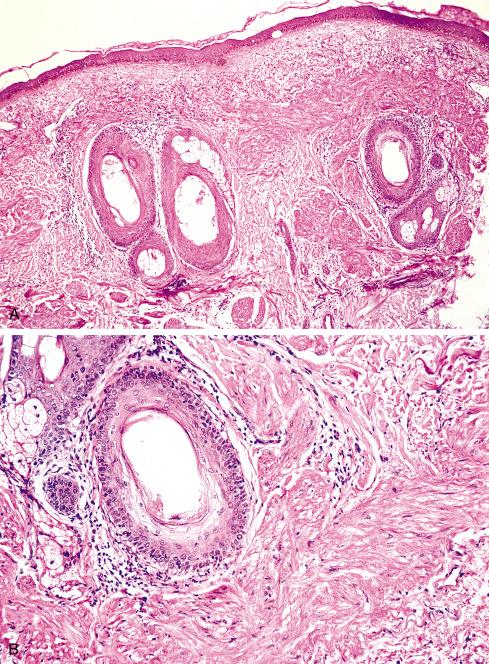
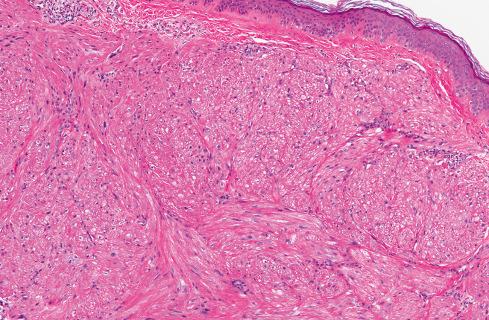
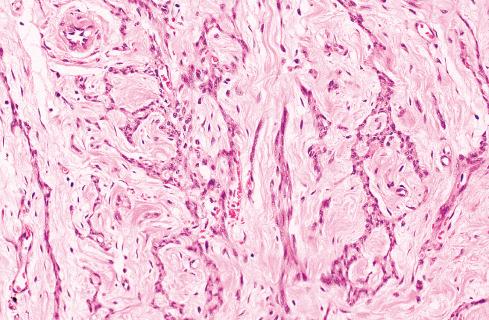
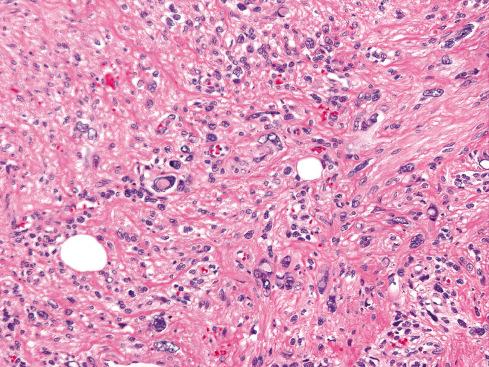
Most pilar leiomyomas are 1 to 2 cm in diameter. They lie in the dermal connective tissue and are separated from the overlying atrophic epidermis by a grenz zone. The lesions are less well defined than angiomyoma and blend irregularly with the surrounding dermal collagen and adjacent pilar muscle ( Figs. 15.2 and 15.3 ). The central portions of the lesions are usually devoid of connective tissue and consist exclusively of packets or bundles of smooth muscle fibers. They usually intersect in an orderly fashion and often create the impression of hyperplasia or overgrowth of the pilar arrector muscle. The cells resemble normal smooth muscle cells, and myofibrils can be easily demonstrated with special stains, such as the Masson trichrome stain, in which they appear as red linear streaks traversing the cytoplasm longitudinally. Leiomyomas associated with HLRCC often show somewhat greater nuclear atypia than do their nonsyndromic counterparts and may sometimes also show eosinophilic cytoplasmic inclusions and prominent nucleoli ( Figs. 15.4 and 15.5 ).
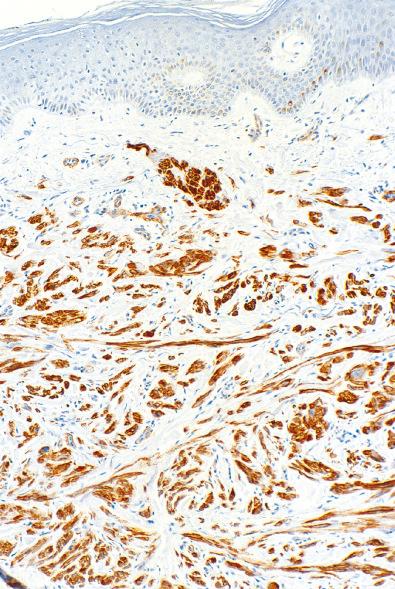
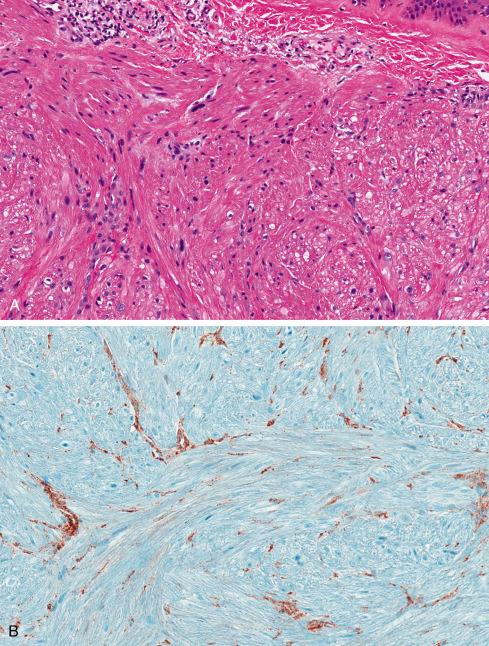
On immunohistochemistry (IHC), cutaneous leiomyomas show a “complete” smooth muscle phenotype, with coexpression of smooth muscle actins, desmin, and h-caldesmon (see Fig. 15.3 ). IHC for fumarate hydratase (FH) and 2-succinocysteine (2SC) have been shown to be extremely valuable in the identification of patients with possible HLRCC, and correlate well with the presence of FH gene mutations. In HLRCC, FH mutation results in loss of expression of FH and accumulation of 2SC. Buelow et al. recently showed that 11 cutaneous leiomyomas with known FH mutations were positive for 2SC, with 6 of 11 also showing loss of FH expression ( Fig. 15.5B ). They also identified FH mutations on IHC in rare patients presenting with solitary cutaneous leiomyomas and suggested that these be performed on all patients with cutaneous leiomyomas to identify potential HLRCC kindreds. Similar findings have also been reported in uterine leiomyomas and renal cell carcinomas from patients with FH mutation.
Diagnosis is rarely difficult in the typical case, particularly with a characteristic history. Occasionally, solitary forms of the disease are mistaken for other benign tumors, such as cutaneous fibrous histiocytoma (dermatofibroma). The cells in fibrous histiocytoma are slender, less well ordered, and lack myofibrils. Secondary elements such as inflammatory cells, giant cells, and xanthoma cells, common to cutaneous fibrous histiocytomas, are lacking in cutaneous leiomyomas. Distinction of cutaneous leiomyomas from lesions reported as smooth muscle hamartomas of the skin is less clear-cut and may relate more to differences in clinical presentation than histologic features. Smooth muscle hamartomas are typically described as a single lesion measuring several centimeters in diameter and occurring in the lumbar region during childhood or early adult life. Consisting of well-defined smooth muscle bundles in the dermis, these lesions are sometimes associated with hyperpigmentation and hypertrichosis (Becker nevus). Because atypical intradermal smooth muscle tumors ( cutaneous leiomyosarcomas ) also occur in the skin, care should be taken to ensure that neither atypia nor mitotic activity is encountered in a presumptive cutaneous leiomyoma. Cutaneous smooth muscle tumors with significant atypia (even in the absence of mitotic activity) recur, and when showing extensive involvement of the subcutis, they may develop some risk for metastasis (see Chapter 16 ). The possibility of a metastasis from a deeply situated leiomyosarcoma should also be considered for highly atypical smooth muscle tumors involving the skin, especially the scalp.
Cutaneous leiomyomas do not undergo malignant transformation; nonetheless, they may be difficult to treat. The lesions are often so numerous that total surgical excision is not possible. Laser therapy has been used with some success.
Early studies based on referred consultations suggested that genital leiomyomas were much less common than those of pilar arrector origin. Judging from more recent hospital-based series, genital leiomyomas may outnumber pilar ones by a 2:1 margin. Affected sites include the areola of the nipple, scrotum, labium, penis, and vulva. The tumors are small, seldom exceeding 2 cm, and pain is not a prominent symptom. Histologically, genital leiomyomas, with the exception of the nipple lesions, differ from pilar leiomyomas in that they tend to be more circumscribed and more cellular, and they display a greater range of histologic appearances. For example, Tavassoli and Norris, in a review of 32 vulvar leiomyomas, noted myxoid change and an epithelioid phenotype of the cells, features not encountered in pilar leiomyomas.
Angiomyoma, a solitary form of leiomyoma that usually occurs in the subcutis, is composed of numerous thick-walled vessels. In the early literature, little attempt was made to distinguish these lesions from cutaneous leiomyomas, and the two were collectively termed tuberculum dolorosum because of their pain-producing properties. Stout later designated them “vascular leiomyomas” to contrast them with cutaneous leiomyoma, which has inconspicuous thin-walled vessels. These lesions account for about 5% of all benign soft tissue tumors and one-fourth to one-half of all superficial leiomyomas. They occur more frequently in women, except for those in the oral cavity. Unlike cutaneous leiomyomas, these tumors develop later in life, usually between the fourth and sixth decades, as solitary lesions. They occur preferentially on the extremities, particularly the lower leg. In the Hachisuga et al. series, 375 of 562 occurred in the lower extremity, 125 on the upper extremity, 48 on the head, and 14 on the trunk. Most were less than 2 cm in diameter.
Affected patients complain most often of a small, slowly enlarging mass usually of several years’ duration. Pain is a prominent feature in about half the patients, and in some cases it is exacerbated by pressure, change in temperature, pregnancy, or menses. The prevalence of pain has led some to suggest that these tumors are probably derived from arteriovenous anastomoses, similar to the glomus tumor . However, they differ in appearance from the glomus tumor and are almost never encountered in a subungual location. The tumors are usually located in the subcutis and less often in the deep dermis, where they produce overlying elevations of the skin but without surface changes of the epidermis. Grossly, the tumors are circumscribed, glistening, white-gray nodules. Occasionally, they are blue or red, and rarely, calcium flecks are visible grossly. The leiomyomas that visibly contract or writhe when touched or surgically manipulated are probably of this type.
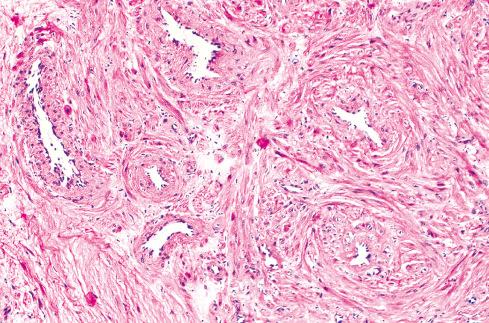
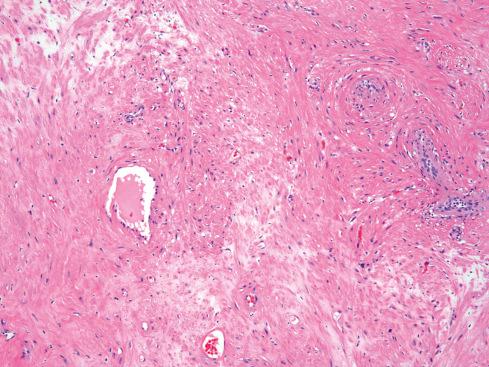
Microscopically, the tumors have a characteristic appearance that varies little from case to case. The usual appearance is a well-demarcated nodule of smooth muscle tissue punctuated with thick-walled vessels with partially patent lumens ( Figs. 15.6 to Fig 15.9 ). Typically, the inner layers of smooth muscle of the vessel are arranged in an orderly circumferential fashion, and the outer layers spin or swirl away from the vessel, merging with the less well-ordered peripheral muscle fibers. The morphologic features of angiomyoma overlap to a degree with those of myopericytoma , and the distinction between these two entities may be quite subjective (see Chapter 24 ). Areas of myxoid change ( Fig. 15.8 ), hyalinization, calcification, and fat may be seen. The vessels in these tumors are difficult to classify because they are not typical of veins or arteries. Their thick walls and small lumens are reminiscent of arteries, but they consistently lack internal and external elastic laminae. In the experience of Hachisuga, a small number of angiomyomas are composed of predominantly cavernous-type vessels. Nerve fibers are usually difficult to demonstrate but undoubtedly are present, accounting for the exquisite sensitivity of these lesions to manipulation. Rarely, angiomyomas display degenerative nuclear atypia similar to that seen in symplastic leiomyomas. Angiomyoma is a benign tumor, causing few problems apart from pain. Simple excision is adequate. None of the patients reported by Duhig and Ayer developed recurrence after excision. In the Hachisuga series, only two patients had a recurrence, although their follow-up data were incomplete.
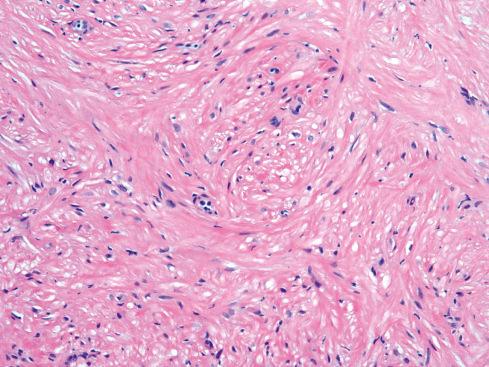
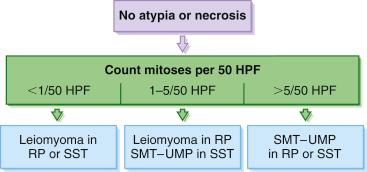
Unequivocal leiomyomas of deep soft tissue are exceptionally rare compared to their malignant counterpart, and until recently there has been no consensus as to how to separate soft tissue leiomyomas from leiomyosarcomas. In fact, the observation that some tumors initially labeled “leiomyoma” ultimately proved to be malignant enhanced the impression that it was nearly impossible to establish a minimum threshold for malignancy, leading inevitably to the conclusion that all smooth muscle tumors of deep soft tissue should be considered malignant. Recent studies have presented convincing evidence that leiomyomas of deep soft tissue exist but are rare and should be diagnosed using strict criteria derived empirically from the evaluation of soft tissue smooth muscle tumors. Soft tissue leiomyomas are of two distinct types, somatic and gynecologic, that differ in their clinical presentation and in the criteria of malignancy.
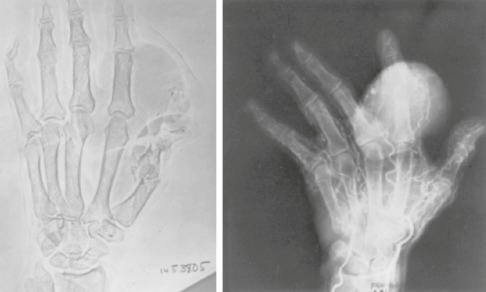
The less common somatic leiomyoma arises in the deep somatic soft tissue of the extremities and affects the sexes equally. Measuring several centimeters at presentation, about one-third also contain calcification ( Fig. 15.10 ), probably a reflection of long duration, and a feature that occasionally leads to such radiologic diagnoses as “calcifying schwannoma,” “synovial sarcoma,” or “myositis ossificans.” Histologically, these lesions are composed of fascicles of well-differentiated smooth muscle cells with abundant eosinophilic cytoplasm similar to vascular smooth muscle ( Figs. 15.11 and 15.12 ). Rarely, somatic leiomyomas may have a predominantly clear cell appearance or display psammoma bodies ( Figs. 15.13 and 15.14 ).
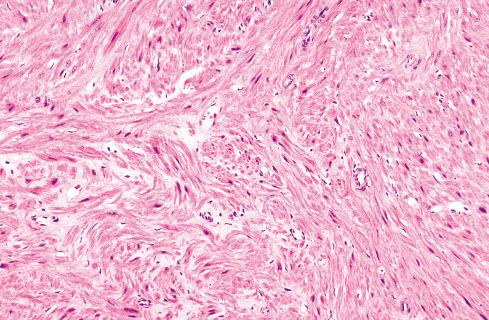
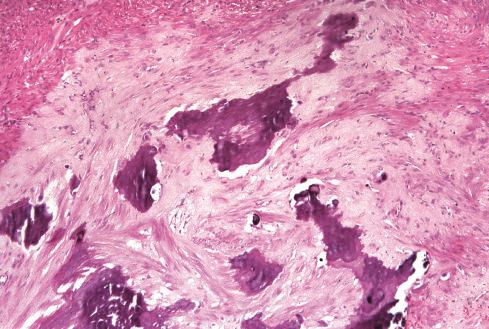
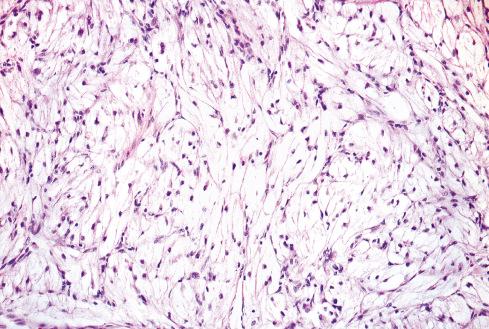
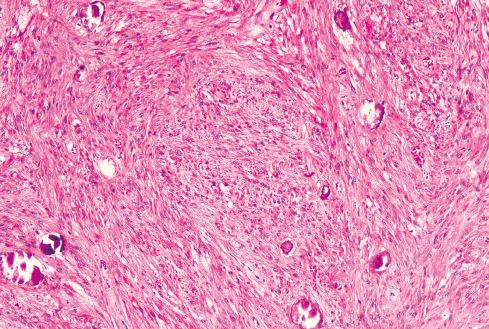
By definition, somatic leiomyomas should harbor no necrosis, at most mild atypia, and virtually no mitotic activity (<1 figure/50 high-power fields [hpf]). The number of somatic leiomyomas with extended follow-up information is still quite small, but in the largest series reported by Billings et al., all 11 patients were alive and well from 5 to 97 months (median: 67 months) after diagnosis.
The more common leiomyoma of gynecologic (or uterine ) type occurs almost exclusively in women, usually in the perimenopausal period. These tumors are situated predominantly in the pelvic retroperitoneum, although other peritoneal sites may be affected. Benign-appearing inguinal smooth muscle tumors in women also appear to be of gynecologic (müllerian) origin. Although in the past some of these lesions have undoubtedly been interpreted as autoamputated uterine leiomyomas, recent experience showing origin at sites clearly distinct from the uterus or in women without uterine fibroids suggests that they are more likely de novo soft tissue lesions.
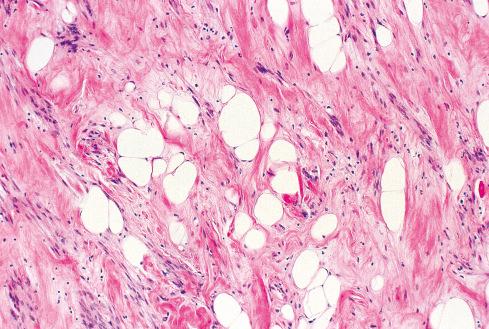
Grossly and histologically, these tumors bear an unmistakable similarity to uterine leiomyomas, thereby accounting for the term leiomyoma of gynecologic type . Grossly, they are well circumscribed, gray-white lesions that range greatly in size from a few centimeters to more than 10 cm and consist of intersecting fascicles of slender-tapered smooth muscle cells with less cytoplasm than their somatic counterparts. The stroma contains vessels often with striking mural hyalinization ( Fig. 15.15 ). Other features of uterine leiomyomas include hydropic change ( Fig. 15.16 ), myxoid change, hyaline necrosis, and an epithelioid or cordlike arrangement of the cells ( Fig. 15.17 ). Fatty change is quite common ( Figs. 15.18 and 15.19 ) and has led to the use of alternative terms (e.g., lipoleiomyoma, myolipoma).
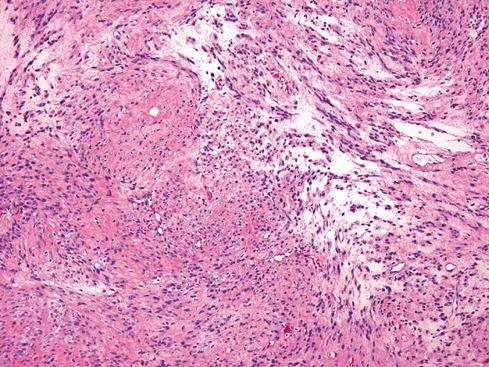
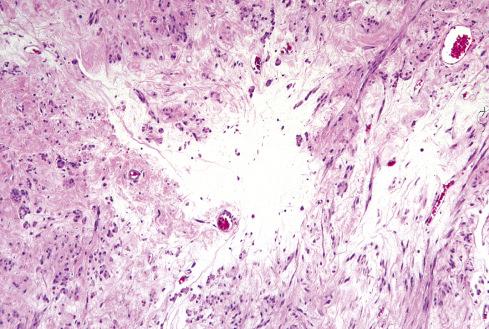
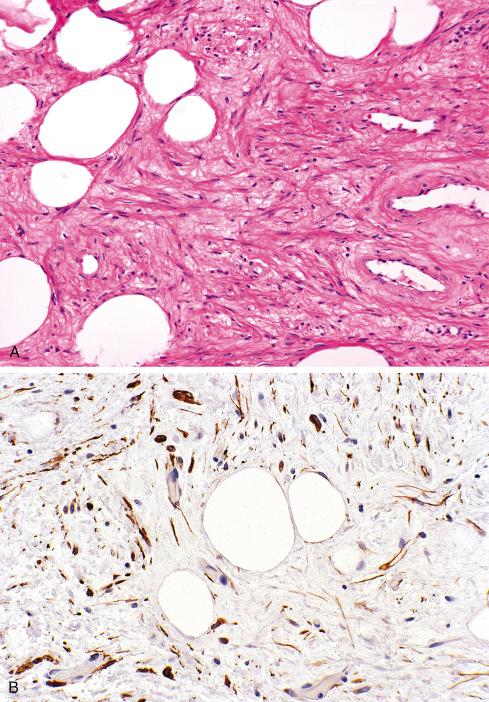
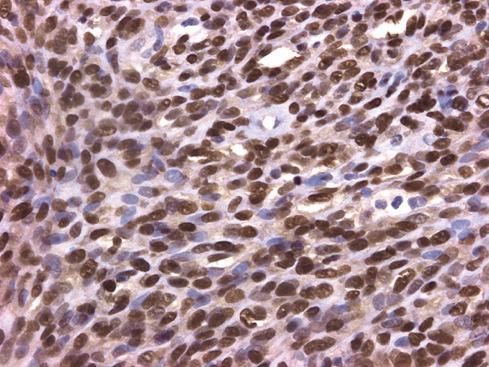
Unlike somatic leiomyomas, gynecologic leiomyomas typically display both nuclear estrogen and progesterone receptor protein ( Fig. 15.20 ), suggesting that they arise from hormonally sensitive smooth muscle of the retroperitoneum. Not surprisingly, the criteria of malignancy for evaluating this group of lesions seem to approximate those used for uterine lesions (see later section). Although, by definition, neither necrosis nor more than mild atypia should be allowed in making this diagnosis, mitotic activity occurs frequently and does not seem to imply any adverse outcome. In two large studies, mitotic activity of 5 figures/10 hpf was encountered in about one-quarter of cases with no observed adverse event. Although higher levels of mitotic activity may still be compatible with a benign diagnosis, the experience with such lesions is limited, and they are best labeled as “uncertain malignant potential” until further data are accrued ( Fig. 15.21 ). Given the similarity to uterine lesions, one might question whether there is a soft tissue analogue to a symplastic leiomyoma of the uterus ( Fig. 15.22 ). Until there is published information on this point, again it is recommended that such lesions be considered of uncertain malignant potential. These criteria of benignancy have been validated in two recent large studies. Of the 54 patients reported collectively by Billings et al. and Paal and Miettinen, 20% experienced local recurrence, but no metastasis within an average follow-up of 42 and 142 months, respectively. Lastly, even though hormone receptor proteins are characteristic of soft tissue leiomyomas of the gynecologic type, they may be expressed in leiomyosarcomas from women. Therefore, identification of positive receptor status in a deep smooth muscle tumor does not identify it as a leiomyoma; that feature should be assessed in the context of traditional histologic features.
The currently available genetic evidence also supports that retroperitoneal/inguinal leiomyomas in women are derived from gynecologic-type smooth muscle. As in uterine leiomyomas, mutations in the MED12 gene typically are present in pelvic and retroperitoneal leiomyomas. Rearrangements involving KAT6B , HMGA2, and PLAG1 have also been reported in both uterine and extrauterine leiomyomas of the gynecologic type. A retroperitoneal leiomyoma showing t(9;22)(q33;q12) ( EWSR1-PBX3 ) has also been reported.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here