Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Follicular adenoma (FA) is a benign encapsulated neoplasm of follicular epithelial origin and is the most common neoplasm of the thyroid gland. Autopsy studies show that 3% to 5% of adult thyroid glands harbor an FA. A subset may represent a precursor lesion to follicular carcinoma.
The most common tumor of the thyroid gland, FA is often discovered incidentally during routine physical exam as a solitary, painless, mobile mass or during radiographic studies for other reasons; sometimes patients present with a history of a slow-growing nodule present for months to years. Iodine deficiency, radiation, and inherited syndromes (PTEN–hamartoma tumor syndrome [Cowden, Proteus syndrome]; Carney complex; Werner, McCune–Albright, and Li-Fraumeni syndromes) may have an etiologic relationship to FA development, which is thought to be a monoclonal proliferation. Multiple FAs should raise clinical suspicion for an inherited syndrome. Women are affected more frequently than men (4 to 5 : 1). Patients present over a wide age range, but there is a peak in the 5th to 6th decade. Patients are typically euthyroid and only rarely develop hyperfunction (“toxic adenoma”) or hypofunction. Neck pain or pressure may be reported if bleeding into the tumor has occurred or due to compressive symptoms in large tumors. Initial management includes a fine needle aspiration (FNA) with or without ultrasonographic guidance.
Benign encapsulated tumor with evidence of follicular cell differentiation
About 3%-5% of the population
None (although hypoparathyroidism or recurrent laryngeal nerve damage may occur during surgery)
Females > > males (4-5 : 1)
Wide age range, but usually 5th to 6th decade
Painless neck mass, often present for years
Solitary nodule involving only one lobe
Ultrasound typically shows hypoechoic halo at the periphery of a solid mass
Imaging guides fine needle aspiration biopsy
Excellent
Surgery (lobectomy)
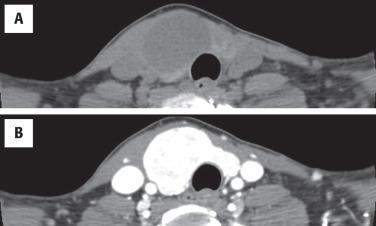
Imaging studies cannot reliably separate benign from malignant neoplasms, but ultrasound (US) is the best study, helping to identify the size, location, and character of the nodule ( Fig. 24.1 ). US may aid in guiding a FNA for deep-seated or nonpalpable masses. Since adenomas are encapsulated, they are easily identified by a well-defined hypoechoic halo at the periphery of an otherwise solid, homogeneous mass. Color Doppler gives a “spoke and wheel” appearance of peripheral blood vessels extending toward the center of the lesion. Nuclear imaging studies are “cold,” although a functional or “hot” nodule can rarely be seen ( Fig. 24.1 ). Computed tomography tends to be nonspecific, showing a hypodense intrathyroid mass and enhancement with contrast ( Fig. 24.2 ).
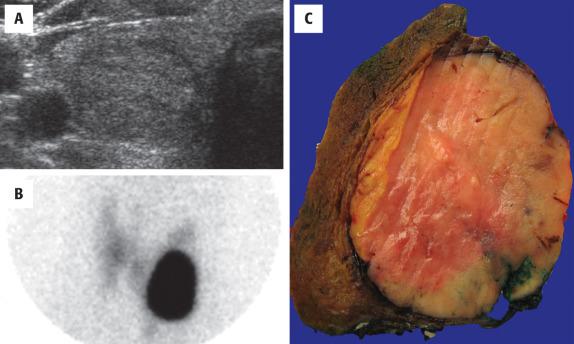
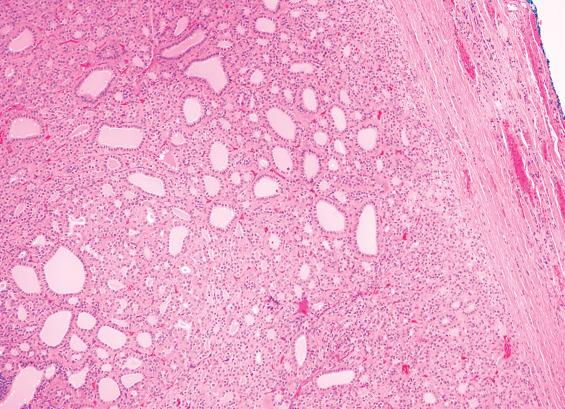
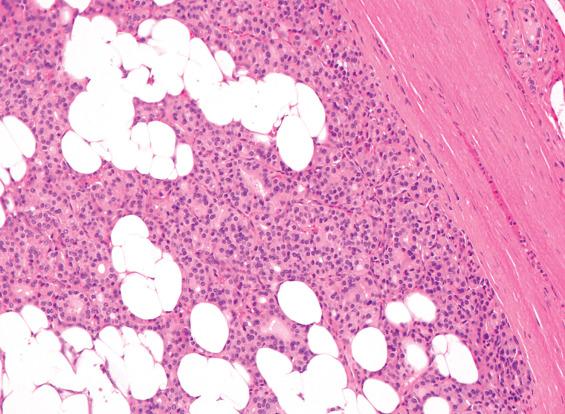
FAs are usually solitary, well demarcated, and encapsulated masses identified in one lobe or in the isthmus ( Fig. 24.1 ). The capsule is usually thin but distinct ( Fig. 24.3 ); a thick capsule should raise suspicion for a follicular carcinoma. Adenomas are usually round to spherical, measuring about 1 to 3 cm on average, although this may be quite variable depending on clinical presentation (palpable versus incidental radiographic feature). The cut surface is rubbery to firm with a homogeneous solid appearance. Gray-white lesions are usually more cellular, while brown-tan lesions tend to have more colloid. Cystic change, degeneration, calcification, and infarction are common and may alter the physical appearance. The oncocytic adenoma (10% to 15% of FAs) tends to have a mahogany-brown cut surface with central scarring.
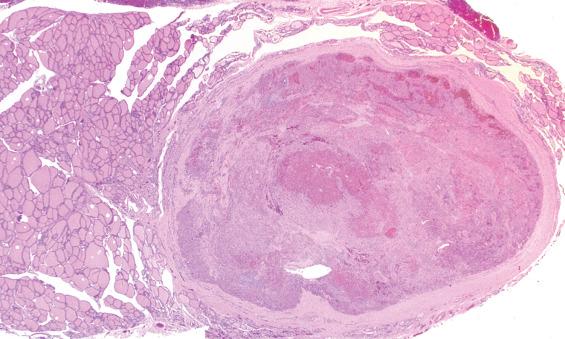
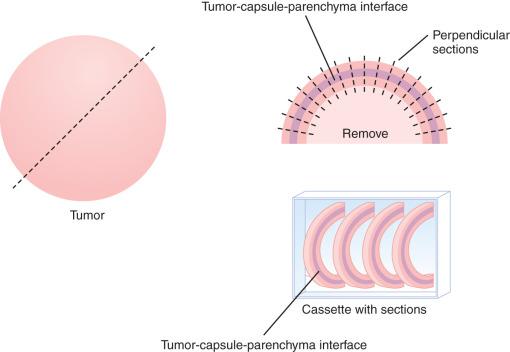
Thoroughly sampling the capsule for invasion (capsular or lymphovascular) is critical, as invasion leads to a diagnosis of follicular carcinoma rather than FA. It is our practice to submit the whole periphery. If this is not feasible, a minimum of three sections per 1 cm of tumor diameter should be submitted. If the tumor looks macroscopically homogeneous after being bivalved, then the center of the tumor is of less interest and should be sampled only representatively. The tumor is serially sectioned perpendicular to the capsule, making 2- to 3-mm thick sections. Then the very periphery of the tumor to capsule to parenchymal interface is embedded. As many as five sections, 3 mm thick and 2.5 cm long, can be placed side by side in the cassette. With this technique, most tumors can be placed in fewer than 10 blocks, allowing for complete evaluation of the capsule ( Fig. 24.4 ).
Solitary, well-demarcated encapsulated spherical mass; mean size 3 cm
Interior of tumor distinct from remaining thyroid parenchyma
Surrounded by intact, easily identified capsule (no invasion)
Smooth muscle–walled vessels present in the fibrous tissue help confirm a true capsule
Appearance of tumor cells is distinct and separate from parenchyma
Colloid is usually present, although sometimes limited in oncocytic tumors
Cells are slightly enlarged with low nuclear:cytoplasmic ratio
Variants include oncocytic, trabecular, fetal, papillary, clear cell, signet-ring cell, spindle, among others
TTF1, thyroglobulin, pancytokeratin, PAX8
Cellular smears with ample colloid (usually)
Follicular groups without nuclear features of papillary carcinoma
Cannot reliably separate between adenomatoid nodule, follicular adenoma, and follicular carcinoma
Cellular/dominant adenomatoid nodule, follicular carcinoma, papillary thyroid carcinoma, noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), hyalinizing trabecular tumor, metastatic carcinoma
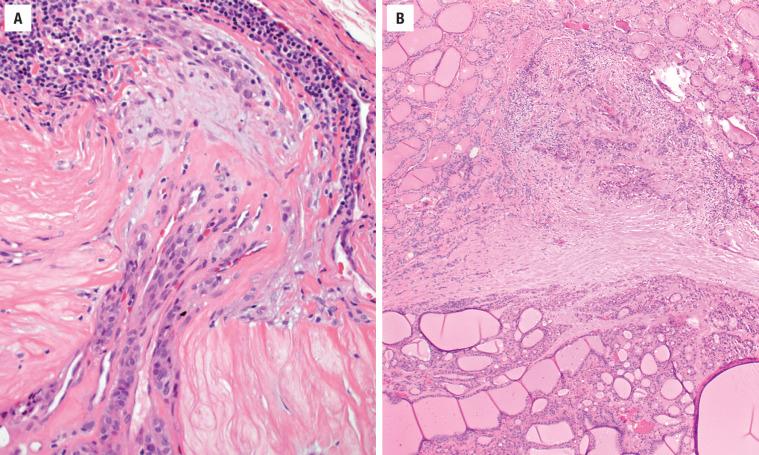
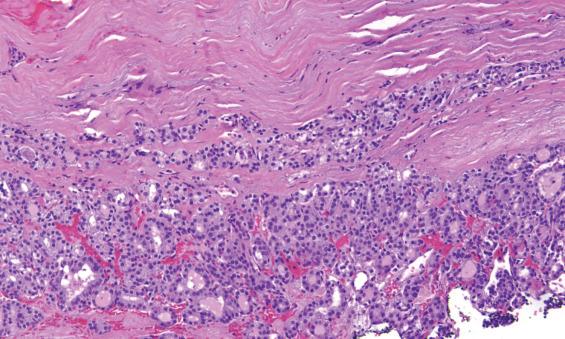
A well-defined, variably thick fibrous connective tissue capsule encloses the neoplastic cells, separating them from the compressed or atrophic thyroid parenchyma ( Figs. 24.5 and 24.6 ). If the capsule is very thick, additional sections should be evaluated to exclude a carcinoma. Small to medium-sized smooth muscle–walled vessels can usually be seen within the fibrous connective tissue ( Fig. 24.7 ). A reticulin or elastic stain can highlight fibers in a true capsule. Invasion is absent by definition, although entrapped epithelium can be seen. FNA may result in iatrogenic defects in the capsule, but these are usually associated with a linear tract, extravasated erythrocytes, hemosiderin-laden macrophages, “reactive” fibrosis, and endothelial hyperplasia ( Fig. 24.8 ). The site of puncture often has a “sharp edge” of transgression, suggesting a mechanical device rather than biologic aggression.
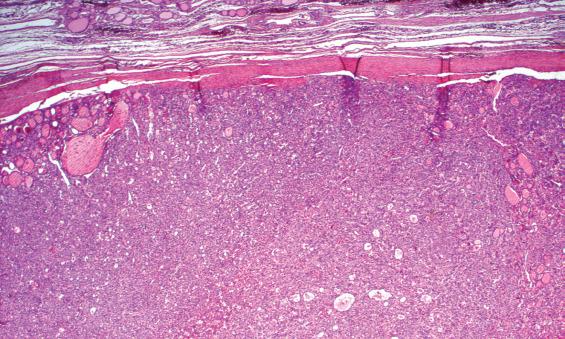
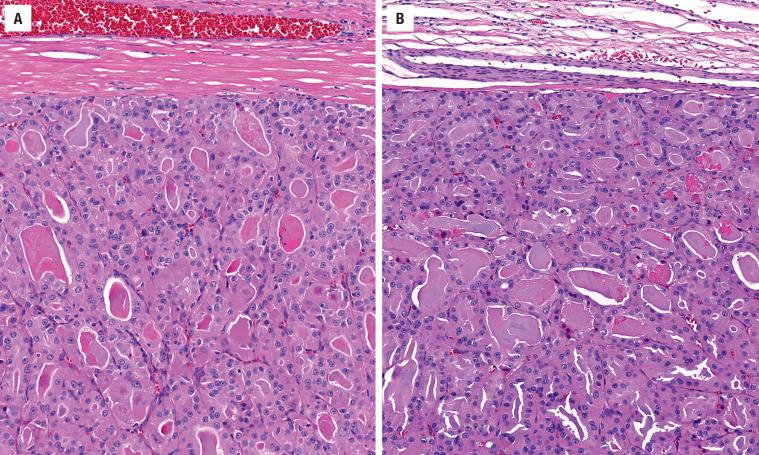
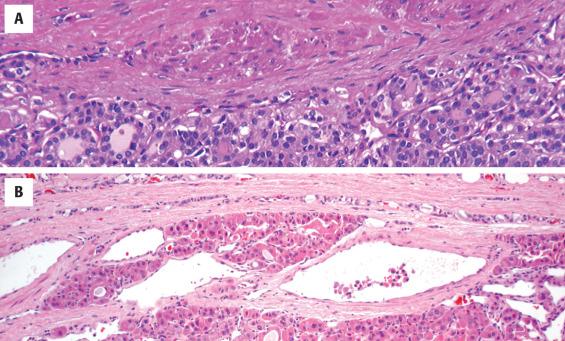
The cells are arranged in a variety of patterns with a variable amount of colloid, usually distinctive from the surrounding parenchyma. While the patterns of growth have been given “type” or “variant” designations (normofollicular [ Fig. 24.9 ], macrofollicular, microfollicular, fetal, embryonal, solid, trabecular [ Fig. 24.10 ], spindled [ Fig. 24.11 ], insular, organoid, papillary), these terms are of no clinical consequence. The follicles are usually uniform with a single architectural pattern predominating. Colloid may be highlighted by a periodic acid–Schiff (PAS) stain if it is limited. Delicate capillaries are easily identified, but intratumoral fibrosis is uncommon. The cells are monotonous and only slightly enlarged. There is a low nuclear:cytoplasmic ratio with well-defined cell borders in the cuboidal to columnar cells. The nuclei are usually aligned in an orderly arrangement along the basal aspect of the cell. Ample cytoplasm, ranging from eosinophilic, oncocytic ( Fig. 24.12 ), amphophilic, granular to clear, or signet-ring ( Fig. 24.13 ), surrounds round and regular nuclei with a coarse to heavy nuclear chromatin distribution. Nucleoli are inconspicuous, although they may be prominent and centrally placed in an oncocytic FA ( Fig. 24.12 ). Profound nuclear pleomorphism may be seen but is usually focal ( Fig. 24.12 ). Mitotic figures are generally inconspicuous but may be increased, particularly in the post-FNA setting. Degenerative and cystic changes include edema, fibrosis, hemorrhage, cyst formation, and calcification, but tumor necrosis is not identified. Infarction, especially in oncocytic tumors, may be associated with prior FNA or decreased blood supply. Viable tumor cells may be limited to the periphery. Fat is rarely noted (lipid-rich adenoma) within the tumor cells and presents as intracytoplasmic, oil red O–positive vesicles ( Fig. 24.14 ). This is distinct from lipoadenoma, in which mature fat is interspersed between the follicular epithelial cells ( Fig. 24.15 ). Squamous metaplasia may be seen but is more common in papillary carcinoma.
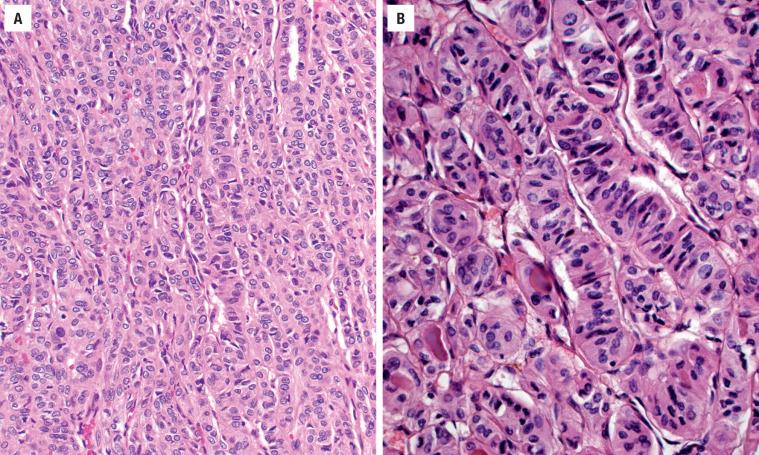
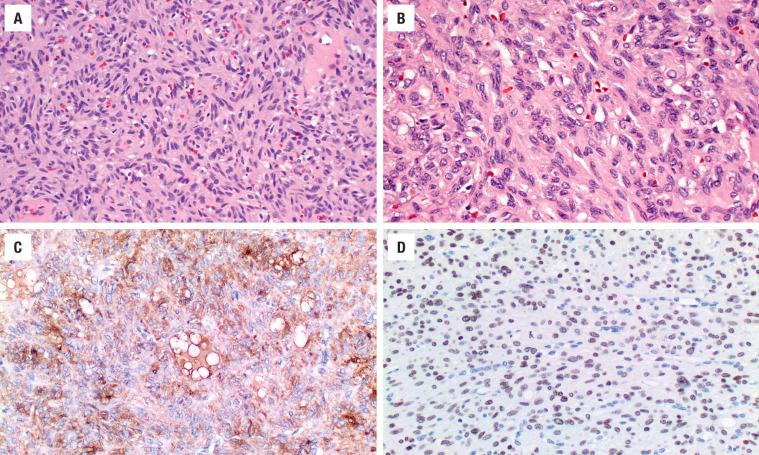
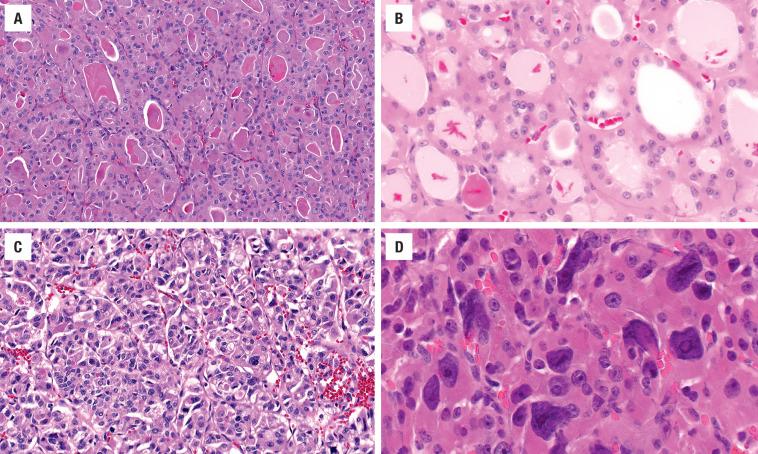
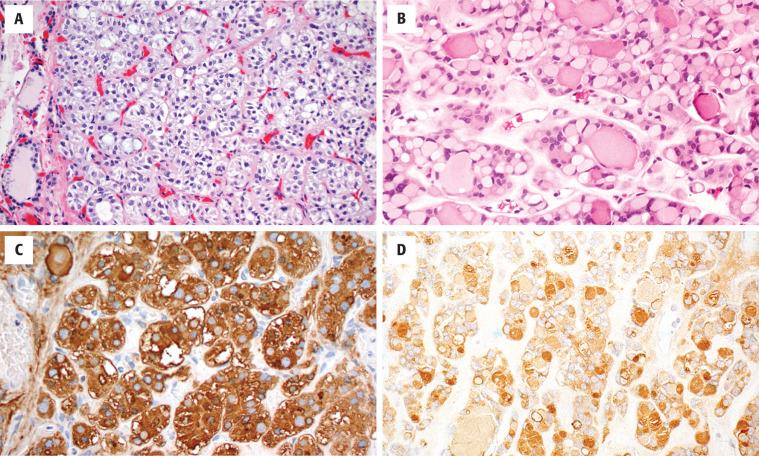
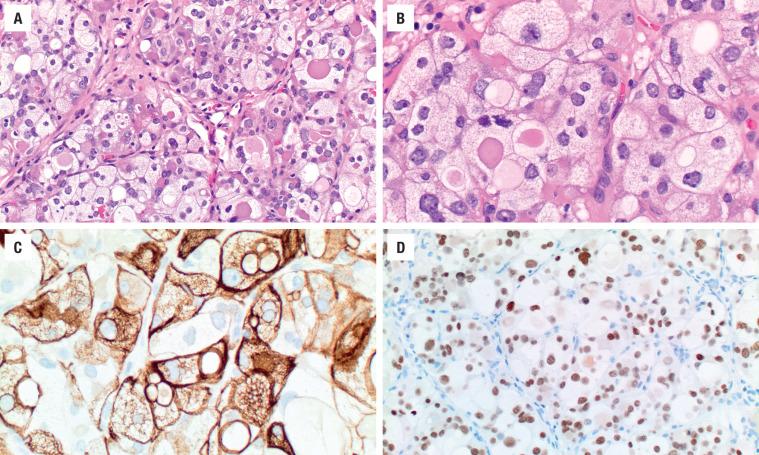
Oncocytic (oxyphilic, Hürthle cell, Ashkenazi cell) adenoma typically has less colloid production, frequently resulting in inspissated colloid that can mimic psammoma bodies ( Figs. 24.12 and 24.16 , left). Tumor cells tend to have a greater degree of nuclear atypia ( Fig. 24.12 ), with vesicular nuclear chromatin, nuclear contour irregularity, and prominent nucleoli. The cytoplasm is eosinophilic and granular. However, oncocytic adenoma is a histologic description only and does not imply a different biologic potential. To be qualified as a clear cell variant , the tumor should be predominantly or exclusively composed of cells with clear cytoplasm ( Fig. 24.13 ). The nuclei are usually centrally situated and are hyperchromatic. Signet-ring cell adenoma is extremely rare but has cells with large intracytoplasmic vacuoles, which compress the nucleus to the side ( Fig. 24.13 ). These vacuoles contain diastase-resistant PAS-positive material, which is strongly thyroglobulin-immunoreactive, suggesting an abnormal thyroglobulin accumulation. Metastatic adenocarcinoma may rarely give a similar histologic appearance.
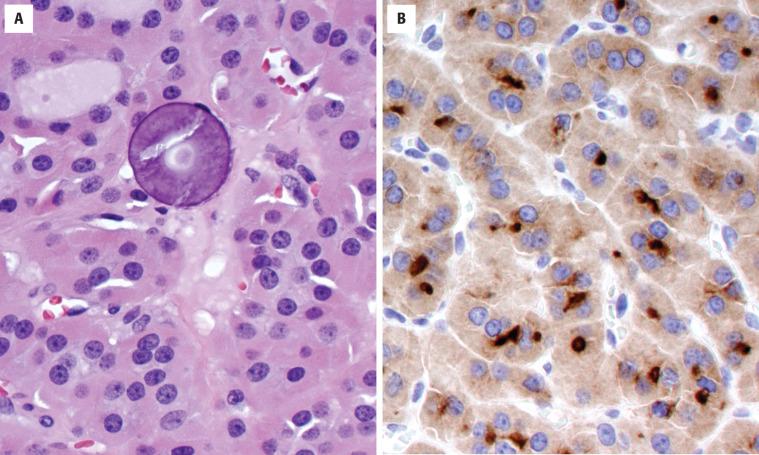
Immunohistochemistry is seldom necessary for the diagnosis, but in cases where the diagnosis is in question, the neoplastic cells will be immunoreactive to thyroglobulin ( Figs. 24.11, 24.13, and 24.16 ), TTF1 ( Figs. 24.11 and 24.14 ), PAX8, and keratin (CAM5.2, AE1/AE3, CK7). Oncocytic tumors must be interpreted with caution owing to high background and nonspecific staining.
FNA is the first-line evaluation technique. Unfortunately FNA does not reliably distinguish between a cellular and a dominant adenomatoid nodule, FA, or follicular carcinoma with any degree of certainty or reproducibility. After adequacy is assessed (five or six follicular epithelial groups of at least 10 epithelial cells per group), a follicular neoplasm may be favored over an adenomatoid nodule (colloid goiter). Features that favor a neoplasm are syncytial groups with a microfollicular arrangement in a cellular smear in which there is increased cellularity compared with the amount of colloid and uniform, monotonous cells that vary little from one another ( Fig. 24.17 ). The epithelial groups are arranged as small spherical aggregates surrounding a colloid droplet. Oncocytic adenomas have large polygonal cells with granular cytoplasm. The nucleoli tend to be more prominent. Adenomatoid nodules tend to have cellular variability and often show extensive degenerative changes. The diagnosis of “follicular neoplasm” or “suspicious for a follicular neoplasm” will result in appropriate surgery for a solitary thyroid mass (equivalent to Bethesda System for Reporting Thyroid Cytopathology category IV).
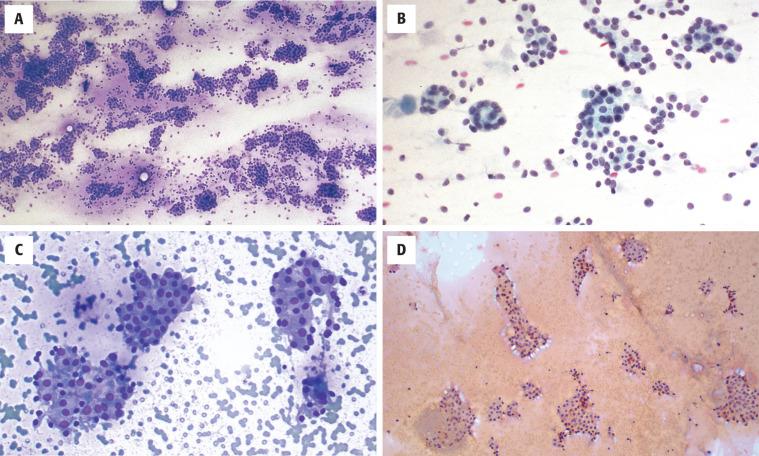
Intraoperative consultation cannot reliably separate FA from follicular carcinoma because extensive sampling is needed to exclude invasion; therefore it is not helpful, and should be deferred.
Numerical chromosome changes can be seen, with trisomy 7 seen in some 15% of FAs, while tetrasomy is seen in about 45% of oncocytic adenomas. Translocations involving 2p21 ( THADA ) and 19q13 ( ZNF331-RITA ) are seen in approximately 10% and 20%, respectively, of FAs. The PAX8-PRARγ rearrangement, characteristic of follicular carcinoma, is also found in less than 10% of FAs. PAX8-PRARγ -rearranged tumors often have thickened capsules, a feature worrisome for malignancy. Infrequent TERT promoter mutations have been associated with atypia and aggressive behavior. Thus, PAX8-PRARγ -rearranged and TERT -mutated FAs may represent follicular carcinoma precursors or, in some cases, undersampled follicular carcinomas. In these cases, more extensive sampling to identify invasion is advocated.
Activating point mutations of RAS genes (specifically NRAS and HRAS ) are most prevalent and detected in about 20% to 30% of cases overall, but they are less common in oncocytic adenomas. RAS mutations are not restricted to FA and occur in all types of thyroid neoplasms, including follicular, papillary, and medullary carcinomas and even occasionally in nodular hyperplasia. Somatic mutations in mitochondrial DNA (mtDNA) are found in oncocytic tumors. Thyroid-stimulating hormone (TSH) receptor gene mutations have been described in hyperfunctioning “toxic” adenomas.
The differential diagnosis includes cellular or dominant adenomatoid nodules, follicular or papillary thyroid carcinoma (PTC), noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP), medullary carcinoma, hyalinizing trabecular tumor, and metastatic carcinomas. Definitive separation between FA and adenomatoid nodule is arbitrary and sometimes semantic, as both are benign lesions calling for no difference in management. With that said, adenomatoid nodules are usually multiple, lack a capsule with smooth muscle–walled vessels, and usually have much more abundant colloid and degenerative changes than an adenoma. Oncocytic cells can be seen in either.
Without definitive capsular or vascular invasion, a diagnosis of carcinoma cannot be rendered in the case of a follicular neoplasm. However, features that raise the suspicion of carcinoma include a remarkably thickened fibrous capsule, increased cellularity (especially at the periphery of the tumor), increased mitotic activity, atypical mitotic figures, and tumor necrosis. Still, invasion must be present to call the tumor a follicular carcinoma. The designation atypical adenoma may be used in circumstances where a definitive separation is impossible ( Fig. 24.18 ). To date, cytogenetic and molecular studies cannot reliably separate follicular tumors.
Oxyphilia often results in nuclear contour irregularities and intranuclear cytoplasmic inclusions (any cell with abundant cytoplasm is prone to intranuclear cytoplasmic invaginations), both features seen in PTC. However, additional architectural and cytomorphologic features of papillary carcinoma must be present before making the diagnosis: invasive growth, intratumoral fibrosis, thick eosinophilic colloid, and the characteristic nuclear features of enlargement, crowding, irregular placement around the follicle, grooves, folds, and contour irregularities, with chromatin clearing and margination. Nuclear features of papillary carcinoma alone in a follicular neoplasm are no longer sufficient to render a diagnosis of papillary carcinoma; architecture, pattern of growth, cytoplasmic qualities, and invasion must be taken into consideration. Otherwise, a diagnosis of NIFTP must be considered (see next section for discussion of NIFTP). The most significant distinguishing feature between FA and NIFTP is the presence of nuclear features of PTC in the latter, as both are noninvasive.
Medullary thyroid carcinoma should be considered in any FA that lacks well-developed colloid, especially if spindled or clear cells are present; it should be assessed with immunohistochemistry (calcitonin, synaptophysin, chromogranin). Other clear cell tumors , such as some parathyroid neoplasms or metastatic renal cell carcinoma (CAIX, CD10, RCC), can usually be separated on immunohistochemical grounds as well. Hyalinizing trabecular tumor shows a trabecular growth pattern with a perpendicular arrangement of nuclei, grooves, and pseudoinclusions; it demonstrates a distinctive membranous staining pattern with Dako Ki-67 antibody (MIB-1).
There is an excellent long-term clinical prognosis without recurrences or metastasis when the lesion is removed by conservative surgery (lobectomy alone). Hypoparathyroidism and recurrent laryngeal nerve damage may result from surgery, but they are uncommon complications.
The diagnostic term NIFTP represents a reclassification of the entity previously known as noninvasive encapsulated follicular variant of PTC. It is a noninvasive, follicular-patterned tumor with nuclei resembling those seen in papillary carcinoma. The terminology change reflects growing evidence that these tumors have extremely low malignant potential when completely excised and therefore should not be classified as malignant. NIFTP, like FA, is a noninvasive tumor, so it is equally important to exclude invasive growth with extensive sampling of the periphery of the tumor (i.e., complete submission of periphery). The absence of invasion in NIFTP distinguishes it from papillary carcinoma. A set of inclusion and exclusion criteria must be applied to diagnose NIFTP ( Fig. 24.19 ), described in the following paragraphs.
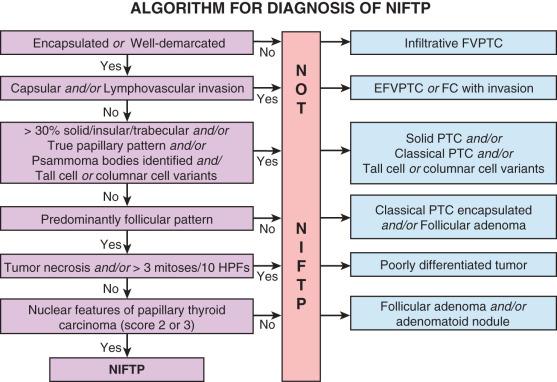
Similar to FA, NIFTP typically presents as a painless, mobile thyroid nodule. The nodule may be palpable or incidentally discovered on imaging performed for another indication. Large tumors may cause compressive symptoms. Patients are euthyroid. Although NIFTP is a new diagnostic category, the incidence of NIFTP can be estimated from that of encapsulated follicular variant of PTC, which was about 10% to 20% of all PTCs. Thus, the incidence in the general population is estimated to be roughly 1 in 100,000. NIFTP is more common in women (female-to-male ratio of 3 to 4 : 1), who most often present in the 5th decade of life.
Noninvasive follicular neoplasm with nuclear features resembling PTC with an extremely low malignant potential
Incidence of ~1 per 100,000
Recurrence is possible with incomplete excision
Females > males (3-4 : 1)
Wide age range (20-80); mean, 46 years
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here