Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
We thank Dr. Stanley Kaplan for his valuable contributions to the previous edition of this chapter.
The human embryo begins as a single large cell, approximately 0.1 mm in diameter, just visible to the unaided eye. During the 266 days of gestation after fertilization, this cell increases in size, weight, and surface area in a rapid and markedly nonlinear fashion. From newly fertilized egg to newborn, length increases by a factor of 5000, surface area by a factor of 61 million, and weight by a factor of nearly 6 billion. During this process the fertilized egg divides and differentiates into more than 200 different morphologically recognizable cell types. Orchestration of the increase in size and specialization in cellular function is a complex process about which much remains unknown. However, it has been argued that the principles of development have been established and that details are missing only at the molecular level. This claim is undoubtedly an overstatement; nevertheless, during the past decade or so, understanding of the molecular control of development has increased substantially. That human embryonic development occurs normally in most pregnancies is a tribute to the design of the control mechanisms that are operating. This chapter presents a brief description of the growth and differentiation of the human embryo, along with a limited discussion of certain factors that play a part in control of these activities.
The human egg and sperm are two highly specialized cells that share little in common with the other cells of the adult body. They are different in both form and function. However, like other cells, they must achieve a degree of maturity before they can perform their function (i.e., combining to form the zygote). The steps and the chronology leading to this maturation are quite different in the male and in the female, and such differences reflect the diverse pathways of the two sexes beginning early in human development.
The human egg and sperm are derived from large, round primordial germ cells that can be identified in the wall of the yolk sac as early as 24 days after fertilization. As the yolk sac begins to be incorporated into the embryo, the germ cells migrate along the dorsal mesentery of the hindgut to the gonadal ridges, which they reach by the end of the fourth or early fifth week ( Fig. 3.1 ). This migration has been observed in vitro in pieces of hindgut, mesentery, and gonadal ridges of mouse embryos. It is facilitated in humans by a striking ameboid shape (which persists even after the cells have reached the gonad ) and pseudopodia typical of those found in ameboid cells. The pseudopodia disappear after the migration is complete. , In humans these cells contain glycogen stores that diminish over time and disappear when the cells have reached their destination in the gonad, suggesting that the glycogen may be the energy source for their journey.
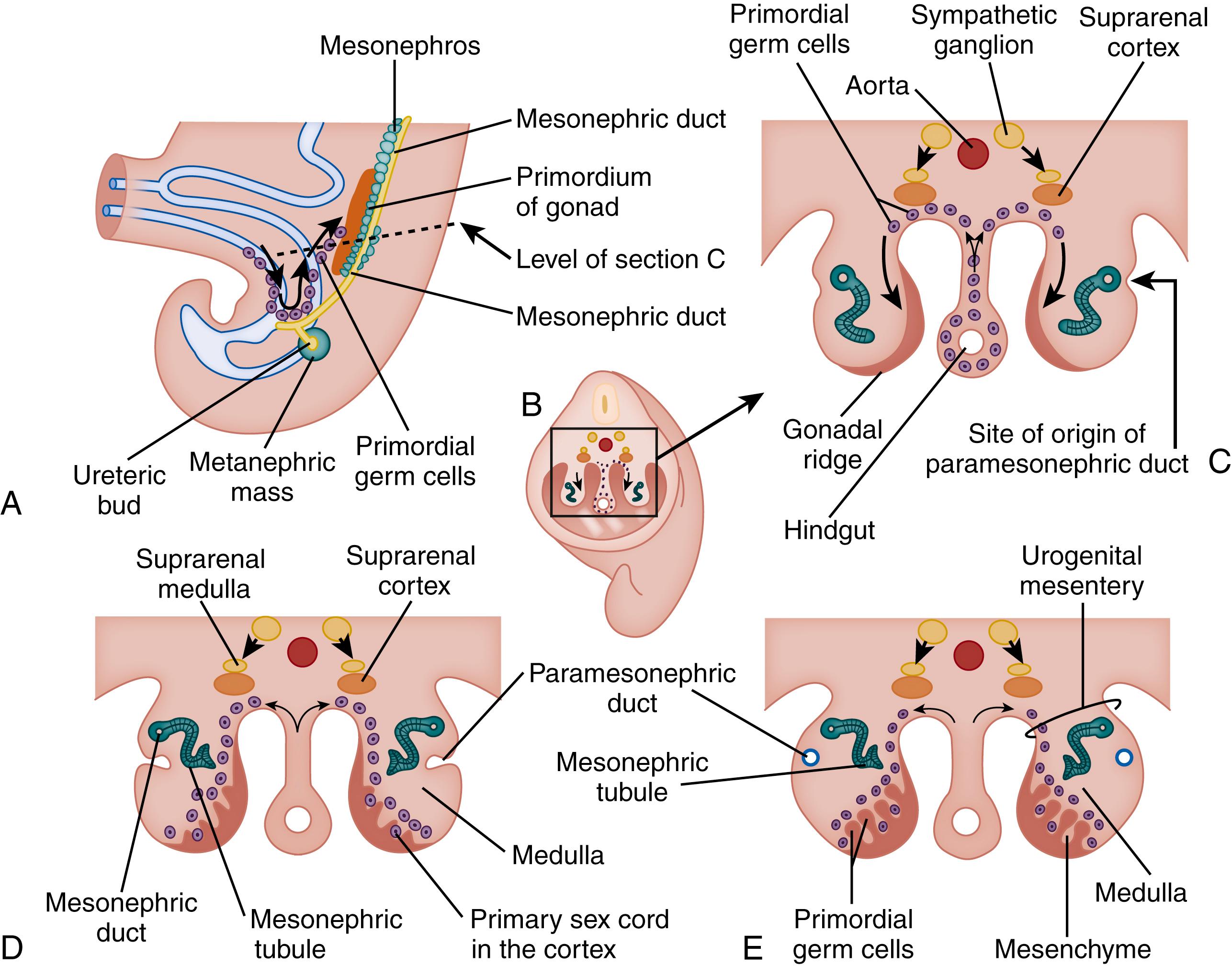
The coelomic epithelium covering the medial aspect of the gonadal ridges undergoes proliferation at approximately week 7 of gestation. As the epithelial cells multiply, they grow into the underlying mesenchyme in a series of fingerlike cords of cells called primitive sex cords. The primordial germ cells associate with these cords. If the embryo is to become a male, these cords continue to be prominent and eventually develop into the seminiferous tubules and rete testis. The early male gonad can also be recognized by the separation of the cords from their parent epithelial covering by a fibrous connective tissue layer, the tunica albuginea, which forms just under the epithelium. If the gonad is to become an ovary, the primitive sex cords remain rudimentary. The origin of the follicular cells of the ovary remains unclear, but likely candidates are cells from the coelomic epithelium and the mesonephros. The follicular cells associate with the primordial germ cells to form primordial ovarian follicles.
If the gonad develops into an ovary, the primordial germ cells become oogonia, and mitotic division continues. Mitotic division of these cells has been observed in humans up to the seventh fetal month but ceases sometime shortly before birth. No oogonia form after the birth of the infant after a normal full-term pregnancy.
In both males and females the germ cells form a syncytium while dividing. , These intercellular connections permit communication and facilitate the high degree of synchrony that has been observed during both mitotic division and meiotic division.
By the eighth or ninth week after fertilization, some oogonia enter prophase of meiosis I and become primary oocytes. Meiosis begins first deep to the surface of the human ovary and then expands toward the surface. Thus, at an appropriate fetal stage, oogonia are found superficially, oocytes deep to the surface, and small follicles at the inner part of the ovarian cortex. It has been suggested that a diffusible meiosis-activating substance is secreted by rete cells (derived from the mesonephros), which lie in the center of the ovary, and good experimental evidence is available to support this hypothesis.
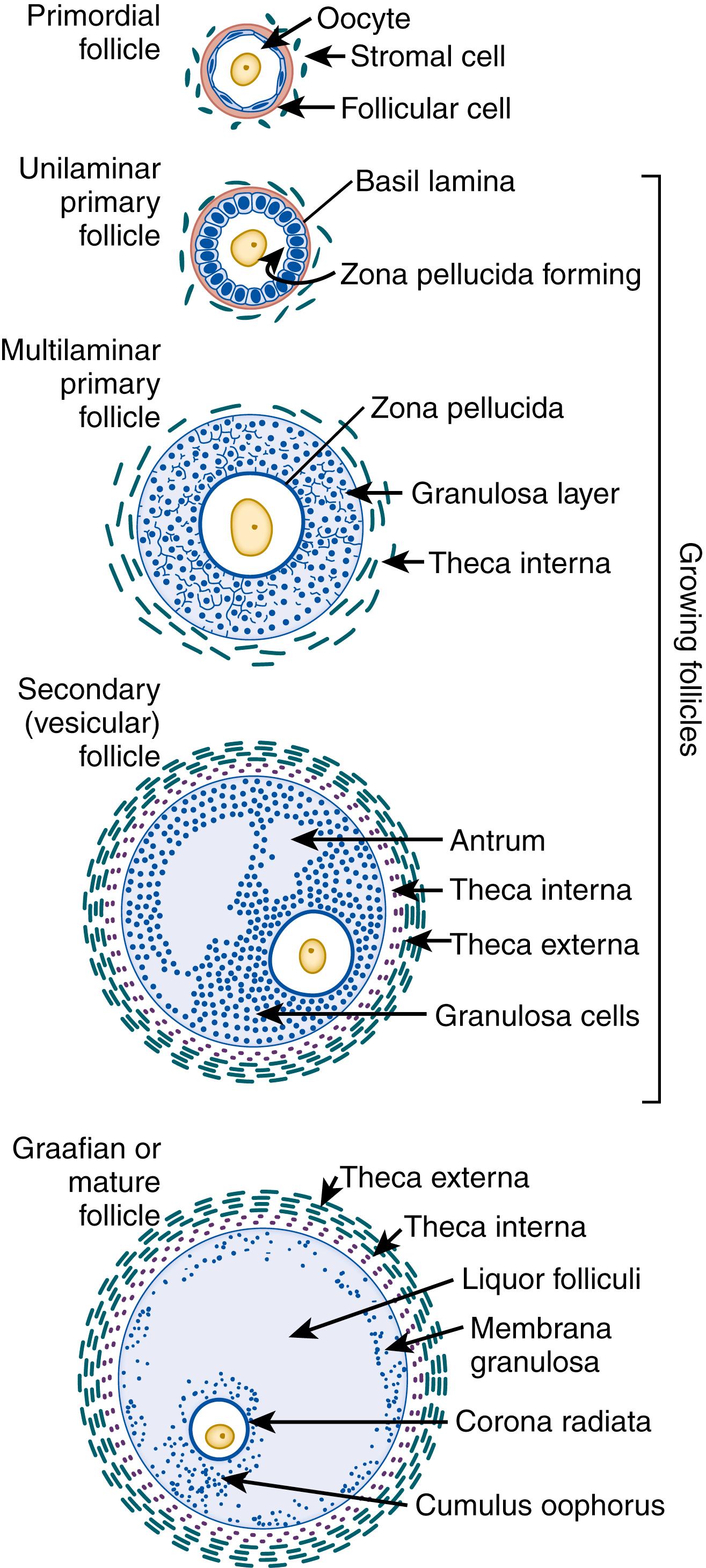
The oocyte goes through the leptotene, zygotene, and pachytene stages of meiosis I, and it then stops at the diplotene stage. At this point the oocyte becomes surrounded by a single, incomplete layer of flat follicular cells ; this unit is called a primordial follicle. The follicle’s large central nucleus is known as the germinal vesicle. A crescent-shaped assembly of cellular organelles containing mitochondria, endoplasmic reticulum, the Golgi complex, lysosomes, and annulate lamellae (stacked parallel membrane arrays with pores) remains clustered adjacent to the nucleus. , Once it has been incorporated into a primordial follicle, the oocyte enters a long period of quiescence, beginning before birth in humans and ending in either atresia or ovulation.
Once sexual maturity is attained, a small number of oocytes begin the process of folliculogenesis, or follicle maturation, during each menstrual cycle. The oocyte grows and eventually becomes one of the largest cells in the human body. The organelles disperse throughout the cytoplasm, and the germinal vesicle (nucleus) enlarges. It increases its complement of nuclear pores, facilitating transport of molecules between nucleoplasm and cytoplasm. The follicular cells resume mitosis and increase markedly in size, changing in shape from squamous to cuboidal, and the follicle becomes surrounded by a basement membrane. Those follicles containing an oocyte surrounded by a single layer of cuboidal follicular cells are known as unilaminar primary follicles, to distinguish them from cells of earlier or later stages.
During further growth of the primary follicle, a thick, acellular coat, the zona pellucida, begins to form between the oocyte and the follicular cells. Mitotic activity increases the number of follicular cell layers, and the follicle is now called a multilaminar primary follicle. The expanding follicle compresses the surrounding ovarian stoma, which organizes into a compact layer adjacent to the basement membrane of the follicle. This layer of stromal cells is called the theca interna, and its cells have the capacity to produce androgens when stimulated by luteinizing hormone activity ( Fig. 3.2 ). The theca interna is vascularized, but the epithelial layers of follicular cells remain avascular.
The zona pellucida is important in the process of fertilization because it contains sperm receptors, takes part in induction of the acrosome reaction, and becomes a block to polyspermy. It may also act after fertilization as a smooth, slippery envelope to contain the sticky ball of cells of the morula-stage embryo; these cells are free to adhere to the uterine endothelium when the zona breaks down, just before implantation.
The zona pellucida is made up of four separate filamentous glycoproteins, zona pellucida glycoprotein 1 (ZP1) through ZP4, which differ in molecular weight and isoelectric point and account for virtually all protein in the zona pellucida. ZP1 crosslinks these filaments, resulting in a three-dimensional matrix that is permeable to large macromolecules. ZP3 serves as a species-specific sperm receptor and also induces the acrosome reaction in sperm on contact. At or shortly after fertilization, these two characteristics are lost, reducing the likelihood of polyspermy. The ZP3 gene has been cloned; it is expressed only in oocytes, and then only during the growth phase of oogenesis. ZP1, ZP2, and ZP3 are located on chromosomes 19, 7, and 5, respectively, while ZP4 is located on chromosomes 11, 16, 7, and 1. The interesting story of these zona pellucida proteins has been the subject of a popularized account and several reviews. Radiolabeling studies in mice indicate that all three glycoproteins are synthesized by the oocyte itself, rather than by the follicular cells. Furthermore, immunofluorescence studies show that zona pellucida antigens are present within human oocytes but not in follicular cells. However, studies in species other than the mouse suggest that the granulosa cells that surround the oocyte also may play a role in the synthesis of zona pellucida components.
Numerous cytoplasmic projections of the follicular cells penetrate the zona pellucida to contact the cell membrane of the oocyte. In humans these filopodial extensions of the follicular cell may actually lie deeply buried in the oocyte, in straight invaginations or pits. These pits are lined by the oocyte cell membrane; however, no cytoplasmic continuity exists between the two cell types. Animal studies have demonstrated the presence of gap junctions along the association of these two cell membranes, permitting transfer of small molecules (molecular weight of approximately 1000) between them.
As the primary follicle enlarges, the follicular cells begin to produce follicular fluid, which collects within the intercellular spaces between follicular cells. These spaces coalesce to form a large fluid-filled cavity called the antrum, which is characteristic of the secondary (vesicular) follicle. The antrum expands, and the oocyte becomes located on one side of the follicle, where it is embedded within a mound of follicular cells known as the cumulus oophorus. The layers of follicular cells immediately surrounding the oocyte are termed the corona radiata. Because of its increased size, the follicle further compresses the surrounding ovarian stroma. A looser, less organized layer of flattened stromal cells encircles the follicle superficial to the theca interna. This is called the theca externa, and its cells have no steroid-secreting activity (see Fig. 3.2 ). A few days before ovulation, one secondary follicle becomes dominant and inhibits the growth of the remaining secondary follicles. The dominant follicle, now called a graafian follicle, can reach several centimeters in diameter. The oocyte is approximately 100 μm in diameter at this stage. Approximately 1 day before ovulation, its nuclear membrane breaks down, the nucleolus disappears, and the first polar body forms, containing one of the two sets of chromosomes. Meiosis I is completed, and the oocyte proceeds to meiosis II, but it again stops on reaching metaphase. In most mammalian species, including humans, meiosis II resumes only after the oocyte has been penetrated by a sperm. , Completion of meiosis in the fertilized oocyte results in production of the second polar body.
Follicles of any stage can undergo atresia. Atresia begins in the fetus and continues into menopause until all follicles have disappeared. At birth approximately 2 million primordial follicles are present within the two ovaries. It has been estimated that half of the 2 million follicles present at birth are atretic at that time. In humans, follicular growth starts before birth, and the newborn ovary contains multilaminar primary follicles and primordial follicles. Follicular growth and subsequent atresia are continuous during human childhood, and it has been clearly stated that “quiescent ovaries in which follicular growth is absent do not occur in normal children.”
Little is known about control of atresia. For example, it is not known whether atresia is initiated by action of the follicular cells, by that of the oocyte, or by both. However, the process of atresia can be manipulated experimentally. Approximately 40,000 follicles are present in the two ovaries of a young adult woman, indicating a reduction to 2% of the pool originally present at birth.
These stages, up to and including the newly fertilized mature ovum, are summarized in Table 3.1 . Most or all of the RNA and protein found in a mature oocyte are synthesized during oocyte growth. Those macromolecules present in the oocyte of an atretic follicle are degraded, and the degradation products are subsequently used for new synthesis.
| Name | Approximate First Recognizable Time (After Fertilization) | Location | Approximate Total Number (Both Ovaries) | Size, Shape, Characteristics | Relevant Studies |
|---|---|---|---|---|---|
| Primordial germ cell | During wk 4 | Caudal yolk sac, among endoderm cells | 500 | Large, round, 15–20 μm in diameter | Witschi, 1948 ; Fujimoto et al., 1977 |
| During wk 5 | Dorsal mesentery of hindgut and gonadal ridges | 500 | Ameboid shape, migrating, with pseudopodia; >20–30 μm in long axis; alkaline phosphatase positive | Witschi, 1948 ; Fujimoto et al., 1977 | |
| Oogonium | During wk 6 | Sexually indifferent gonad | 100,000 | Rapid mitosis increases numbers (mitosis signals name change) | Witschi, 1948 ; Byskov, 1980 |
| During wk 7 | Gonad recognizable as ovary | 100,000 | Mitosis continues; almost all primordial germ cells are now in the gonad | Fujimoto et al., 1977 ; Moore and Persaud, 1998 | |
| Oocyte (in primordial follicle) | Wk 8–9 | Ovary | ? | Meiosis begins; ∼19 μm in diameter | Dvorak and Tesarik, 1980 |
| Quiescent oocyte in primordial follicle | Wk 16 | Ovary | ? | Arrest of meiosis I at diplotene; 50–70 μm; round to ovoid; vitelline body present | Baca and Zamboni, 1967 |
| 2 mo | Ovary | 600,000 | Baker, 1963 | ||
| 5 mo | Ovary | 6,800,000 | Baker, 1963 | ||
| 7 mo | Ovary | Mitosis of oogonia ceases | Baker, 1963 | ||
| Birth | Ovary | 2,000,000 (50% atretic) | Baker, 1963 ; Moore and Persaud, 1998 | ||
| 7 yr | Ovary | 300,000 | Baker, 1963 | ||
| Primary to mature (graafian) follicles | Puberty on | Ovary | 40,000 and declining | From oocyte to mature ovum: comes out of meiotic arrest and enters metaphase of meiosis II, then stops again | Moore and Persaud, 1998 |
| Mature ovum | Puberty on | Uterine tube | 1 per mo | Meiosis is completed and the second polar body is extruded when penetrated by a sperm | Moore and Persaud, 1998 |
As estimated from an assumed fertility span of 30 years, approximately 400 eggs are shed during a woman’s lifetime. Thus approximately 1 in every 100 of the eggs present in a young woman completes maturation and is ovulated; the rest degenerate. A human female has her full complement of eggs, albeit immature, on the day she is born. This is not the case for sperm development in males.
In male humans the primordial germ cells migrate into the gonadal ridges as outlined earlier (see Fig. 3.1 ). Once they have reached the gonad, they divide to form a pool of spermatogonia. Both spermatogonia and their supporting cells—the Sertoli cells—can be identified as early as 48 days after fertilization. The germ cells and the supporting cells combine to form seminiferous tubules.
Spermatogonia are located next to the basement membrane of the seminiferous tubule, where they lie quiescent until puberty. Experimental studies with mice have shown that male primordial germ cells are kept in that state by a meiosis-preventing substance, which also can arrest meiosis in female germ cells. Conversely, the female gonad secretes a meiosis-inducing substance, which can induce male germ cells to enter meiosis.
At puberty the spermatogonia begin to differentiate into sperm (spermatogenesis). Spermatogenesis occurs in three phases. In the first phase, the spermatogonia divide mitotically. In the second phase, some spermatogonia differentiate into primary spermatocytes and undergo meiosis. In the third phase, spermatids proceed through spermiogenesis to form spermatozoa. In contrast with women, the cycle of differentiation of gametes in men is essentially continuous throughout life. Studies in which tritiated thymidine was injected into healthy male volunteers indicate that the complete cycle takes approximately 74 days. However, the various stages of spermatogenesis are not synchronized along the length of the coiled seminiferous tubule in humans: different stages are found at different positions.
Development begins with the fusion of the male and female gametes at fertilization, which occurs in the distal third of the oviduct. Although fertilization is an “internal” process in humans and other mammals, the development of culture systems that support fertilization has made detailed study of the sperm-oocyte interaction possible, as well as providing a basis for in vitro fertilization for clinical ends. As a result, the precisely ordered events constituting a “fertilization pathway” have been identified ( Fig. 3.3 ). The mechanisms involved in the fertilization pathway have been the subject of investigation at the molecular level. , , Most studies on fertilization have been performed with mice, but comparative data suggest that the pathway is similar in all mammals, including humans.
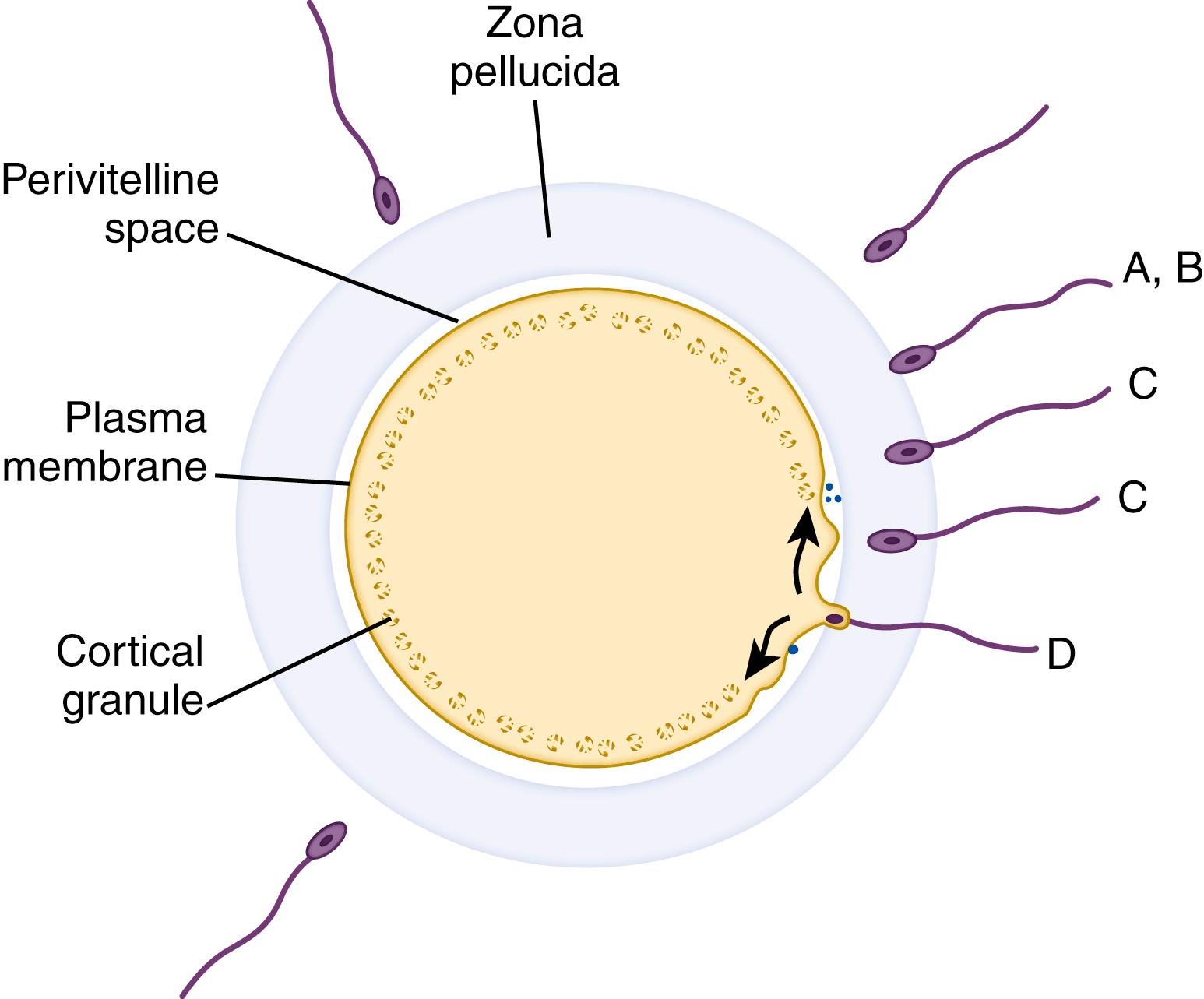
The fertilization pathway begins with binding of the sperm to the surface of the zona pellucida (see Fig. 3.3 ). On the surface of every sperm are thousands of copies of an egg-binding protein; these are recognized by thousands of copies of sperm receptors on the zona pellucida. Binding is relatively species-specific and requires a complete plasma membrane (i.e., an acrosome-intact sperm). , Once bound to the zona pellucida surface, the sperm undergoes a series of dynamic membrane fusions known as the acrosome reaction (see Fig. 3.3 ). During this phase the plasma membrane at the apical end of the sperm fuses with the outer membrane of the acrosome, forming a series of membrane-bound vesicles. These are eventually sloughed, which exposes the inner acrosomal membrane and its complement of enzymes. ,
As a result of enzyme modification of the zona pellucida, the sperm is able to tunnel its way through. The first sperm to penetrate the perivitelline space (between the zona pellucida and the oocyte plasma membrane) and fuse with the plasma membrane triggers activation of the egg (see Fig. 3.3 ). Oocyte activation is a dynamic, multistep process that includes mechanisms to prevent polyspermy, completion of meiosis by the oocyte, engulfment of the sperm, formation of male and female pronuclei, and initiation of the first mitotic division of the embryo. Prevention of fertilization by more than one sperm (polyspermy—a potentially lethal condition) is thought to be a biphasic reaction, although the first phase is not well documented in humans. The first phase is rapid and consists of hyperpolarization of the oocyte plasma membrane. The second phase may take several minutes and involves the release of enzymes from the cortical granules that alter the structure of the zona pellucida. As a result, the plasma membrane and the zona pellucida become refractory to further penetration by other sperm.
Studies at the molecular level have revealed that a component of the zona pellucida is a key substance in the fertilization pathway. As mentioned earlier, the zona pellucida is an acellular coat that surrounds the oocyte and consists of four glycoproteins—ZP1, ZP2, ZP3, and ZP4—arranged in an interlacing filamentous network. , ZP3 functions as the sperm receptor, initiates the acrosome reaction, and participates in the zona pellucida reaction. Sperm binding is mediated by a subset of the O -linked oligosaccharides associated with ZP3, whereas a segment of the polypeptide backbone is needed to induce the acrosome reaction. ,
Several important events necessary for development of the embryo—initially called a zygote after fertilization—are accomplished as a result of fertilization. First, the diploid chromosome number is restored by fusion of the two haploid gametes. Normally, half of the chromosomes come from each parent, and the new complement of chromosomes in the zygote promotes species variation. In addition, the genetic sex of the zygote is determined by the type of sperm that participates in fertilization. Sperm that bear a Y chromosome produce a genetically male zygote (XY), whereas an X-bearing sperm produces a female zygote (XX). Finally, fertilization initiates cleavage, the mitotic division of the zygote. Apposition of the male and female pronuclei results in the formation of a metaphase plate, and the first cleavage soon begins. In contrast with some animal species, the human male and female pronuclei never fuse (i.e., form a complete nucleus). Instead, they immediately enter mitotic metaphase.
Parthenogenesis is activation of the unfertilized oocyte, leading thereafter to various degrees of successful development of the zygote and embryo. In some animal species this process is well known to occur and may even produce viable offspring. However, no verified human cases have been reported in the scientific literature.
The mechanisms mediating the transformation of a fertilized oocyte into a three-dimensional embryo are complex and still not completely understood. Studies on human embryos have been, for the most part, limited to observations of static images or serial reconstructions on preserved specimens of different developmental stages. Therefore most knowledge of the mechanisms controlling development has come from animal studies.
During development, cells of different genetic backgrounds are constantly interacting with each other and with a variety of molecules within their extracellular environment. The processes involved in these interactions consist of many well-recognized phenomena of cell biology, including cell division, adhesion, secretion, cytodifferentiation, motility, and cell death. Although the complex interactions that occur during morphogenesis may appear to be unorganized, they are recognized to occur not stochastically but rather in a precisely ordered sequence of events resulting in recognizable patterns of histogenesis and organogenesis. In the past decade an abundance of molecular studies has provided a much clearer picture of the complex signaling activity that controls embryonic development.
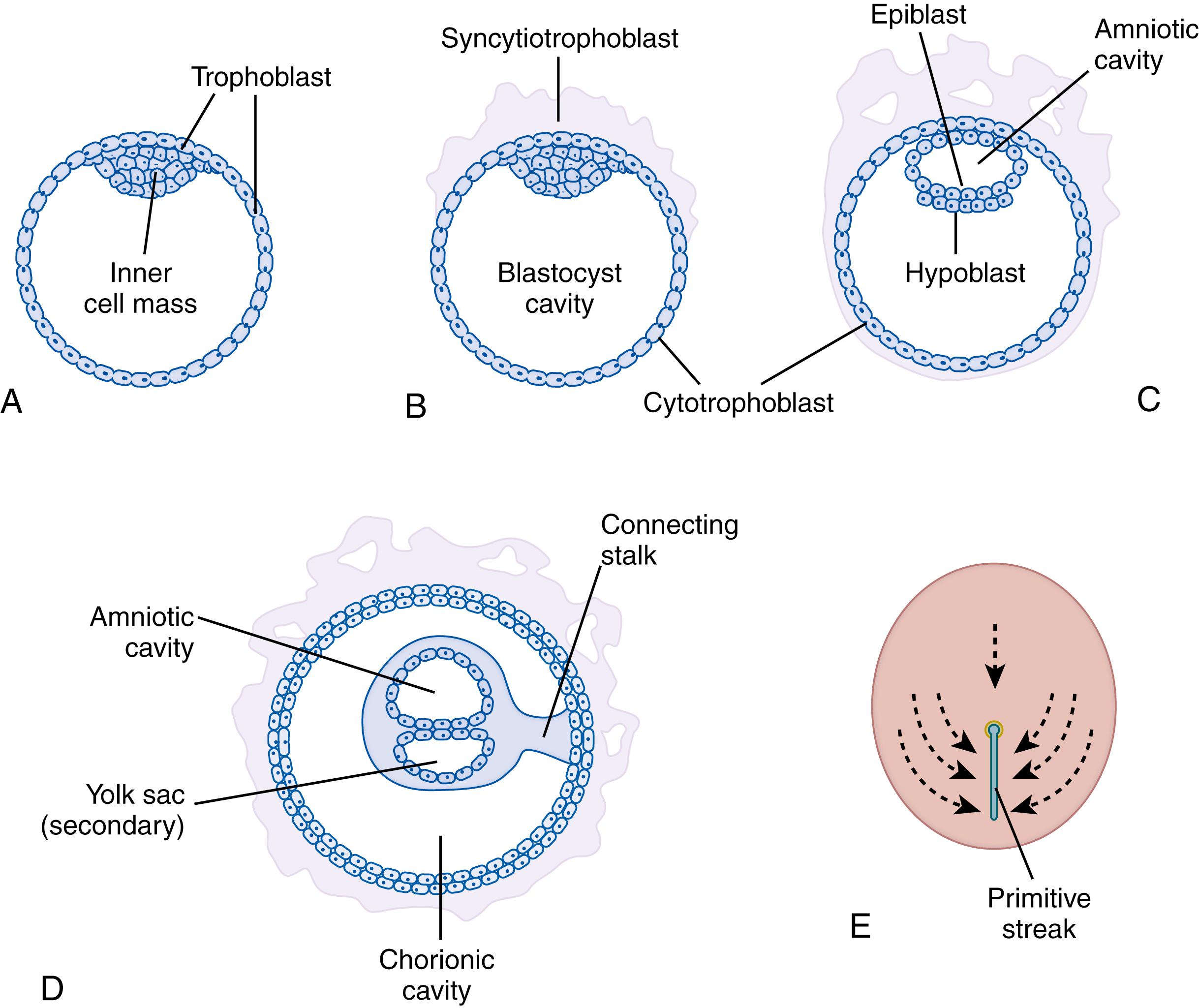
As a result of fertilization, the zygote undergoes a series of mitotic divisions termed cleavage. The cells derived from these repeated mitotic divisions are called blastomeres. The first divisions result in a solid mass of blastomeres that are still surrounded by the zona pellucida. Starting at the 8- to 16-cell stage, intercellular spaces between blastomeres coalesce to form a central cavity. The embryo, now termed a blastocyst, consists of a regionalized clump of cells termed the inner cell mass, which projects into the blastocyst cavity and is surrounded by an outer layer of trophoblast cells ( Fig. 3.4 A and B ). Initially the blastocyst floats freely within the uterine cavity. After shedding the zona pellucida, the blastocyst attaches to and implants within the uterine endometrium.
Studies on embryos suggest that the earliest cleavage divisions appear to be driven by maternal messages stored within the oocyte cytoplasm. In mammals (including humans), the embryonic genome is activated by the two- to four-cell stage and begins to synthesize proteins on its own. This functional maturation is reflected in the steady rise in the synthesis of many intracellular proteins, such as actin.
During cleavage, several changes fundamental to embryonic development occur at the molecular level. One of the most important processes is the generation of cell diversity. Initially all blastomeres express a specific transcription factor, oct-4, which reflects the undifferentiated state of these cells. If separated from the others at this stage, each of the blastomeres has the capacity to form a complete embryo. By the 8- and 16-cell stage the embryo is a solid mass of cells called a morula. At this time the outer cells of the morula are distinguishable from the inner cells because the outer cells no longer express oct-4. The outer cells, now designated the trophoblast, also begin to exhibit epithelial polarity. As a result, the first embryonic tissue (trophoblast epithelium) is formed. Subsequent cytodifferentiation of the trophoblast results in a double-layered membrane, which is a progenitor tissue of the chorion, the fetal portion of the placenta (see Fig. 3.4C ). The inner cellular layer is called the cytotrophoblast, and the outer layer the syncytiotrophoblast. The latter structure, which secretes human chorionic gonadotropin and proteolytic enzymes, is critical to implantation.
During the second week of development, the cells of the inner cell mass that face the blastocyst cavity become flattened, forming a second layer of epithelium. The upper layer, located next to the trophoblast, is now designated the epiblast, whereas the bottom layer of flattened cells is called the primary endoderm or hypoblast (see Fig. 3.4C ). Cells of the epiblast become organized into an epithelial disk, the progenitor of all embryonic tissues, as well as the extraembryonic mesoderm, amnion, and yolk sac. The cells forming the extraembryonic mesoderm apparently arise from the presumptive caudal end of the epiblast and coat the internal surface of the cytotrophoblast. The extraembryonic mesoderm combined with the trophoblast constitutes the chorion. Even at this early stage of development it is possible to determine that the surface of the epiblast adjacent to the trophoblast represents the dorsal side of the embryo.
Rearrangement of some of the epiblast cells results in the formation of a small amniotic cavity. It is unclear whether the amnion is derived from epiblast cells adjacent to the newly formed cavity or from the cytotrophoblast. Primary endoderm cells of the hypoblast proliferate and migrate onto the inner surface of the cytotrophoblast, forming the yolk sac or umbilical vesicle. Therefore, by the end of the second week of development, the embryo consists of a circular bilaminar disk located between two fluid-filled cavities (see Fig. 3.4C and D ). At this time no axial features are visible within the embryonic disk.
At the outset of the third week of development, dynamic cell movements result in extensive rearrangement of the epiblast cells. In most species this period, called gastrulation, is characterized by morphogenetic movements and the changes resulting from them. A midline thickening of the now elongated epiblast becomes visible, designating the future posterior end of the embryo. This thickening is the primitive streak (see Fig. 3.4E ). Cellular activity at the streak results in another fundamental process of morphogenesis, epithelial-mesenchymal transformation. This process begins when some epiblast cells enter the streak while others remain within the epiblast to become the embryonic ectoderm. The transformation from epithelium to mesenchyme consists of a cascade of cellular dynamics, including loss of intercellular connections, cell shape changes, and eventual freedom from the confines of the epiblast. Thus, at the primitive streak, subsets of polarized epithelial cells within the epiblast transform into nonpolarized free cells termed mesenchyme, the second embryonic tissue. These events are thought to be mediated by modulation of adhesive molecules located on the cell surface, as well as by cytoskeletal rearrangements. In addition, variable expression of homeobox genes and many other signaling molecules occurs during gastrulation, leading to patterning of axial and nonaxial structures.
The primitive streak provides a means by which subsets of epiblast cells can ingress and be distributed to more ventral regions of the embryo as the endoderm and the mesoderm. The first cells through the streak probably represent the definitive embryonic endoderm. These are followed by a solid cord of cells, the notochordal process, which extends cranially from the streak. These cells form the notochord, which defines the axis of the embryo and plays a significant role in the induction of the nervous system. Studies suggest that the notochord is an important signaling center for organizing the embryo. It secretes several important morphogenetic signaling molecules, such as retinoic acid and Sonic hedgehog. , Another important signaling center, the prechordal plate, forms just cranial to the notochord. The prechordal plate is an important organizing center for the head of the embryo. Just cranial to the prechordal plate, the endoderm fuses to the overlying ectoderm. This region of fused ectoderm and endoderm is the site of the future mouth. The remainder of the cells that pass through the streak become the intraembryonic mesoderm and come to lie between the endoderm and the ectoderm. Thus the primitive streak provides the embryo with a means to organize epiblast cells, perhaps already partially fate specified, into three primary germ layers—ectoderm, mesoderm, and endoderm.
As a consequence of cleavage and gastrulation, subpopulations of cells in various states of determination are brought together in new spatial relationships, which permits new tissue interactions. Subsequent histogenesis and organogenesis are driven by these tissue interactions, defined as the action of one dissimilar group of cells on another, resulting in the alteration of cell behavior of one of the component groups in a developmentally significant direction. Tissue interactions often result in induction, in which signals from one cell group mediate the change in developmental direction of another group of cells that are competent to respond to the inductive signals. These interactions are mediated by a variety of signaling molecules, such as growth factors, secreted factors, and transcription factors, produced by cells and often concentrated in the extracellular matrix.
The following brief account of the development of some of the major organs provides an overview of some of the complex processes that occur as the embryo is built from raw materials. It is an amazing and precisely timed process. That it happens properly in most conceptions is even more remarkable. For a much more complete account, several other excellent texts are recommended, , , as are appropriate chapters elsewhere in this book.
The human nervous system begins to form approximately 18 days after fertilization, , making it the first of the organ systems to initiate development. It begins as a thickening of the ectodermal layer along the craniocaudal axis of the embryo in the area destined to become the cervical region ( Fig. 3.5B ). This thickening is the result of an increase in the height of the ectodermal cells as they change shape from cuboidal to tall columnar, as well as intercalatory movements within the local population of cells. The result is an oval or keyhole-shaped area of thickened ectoderm known as the neural plate. Two ridges of this neural plate on each side of the midline undergo accelerated growth, giving rise to two longitudinal neural folds with a neural groove between. Before this folding, a mesencephalic flexure forms in the cranial portion of the neural plate. This flexure demarcates the future prosencephalon, mesencephalon, and rhombencephalon. These neural folds increase in height, curve toward each other, touch, and fuse to form the rudiment of the neural tube midway along the embryonic axis (see Fig. 3.5B ). This fusion then proceeds both cranially and caudally, as if two zipper fasteners were operating simultaneously but in different directions.
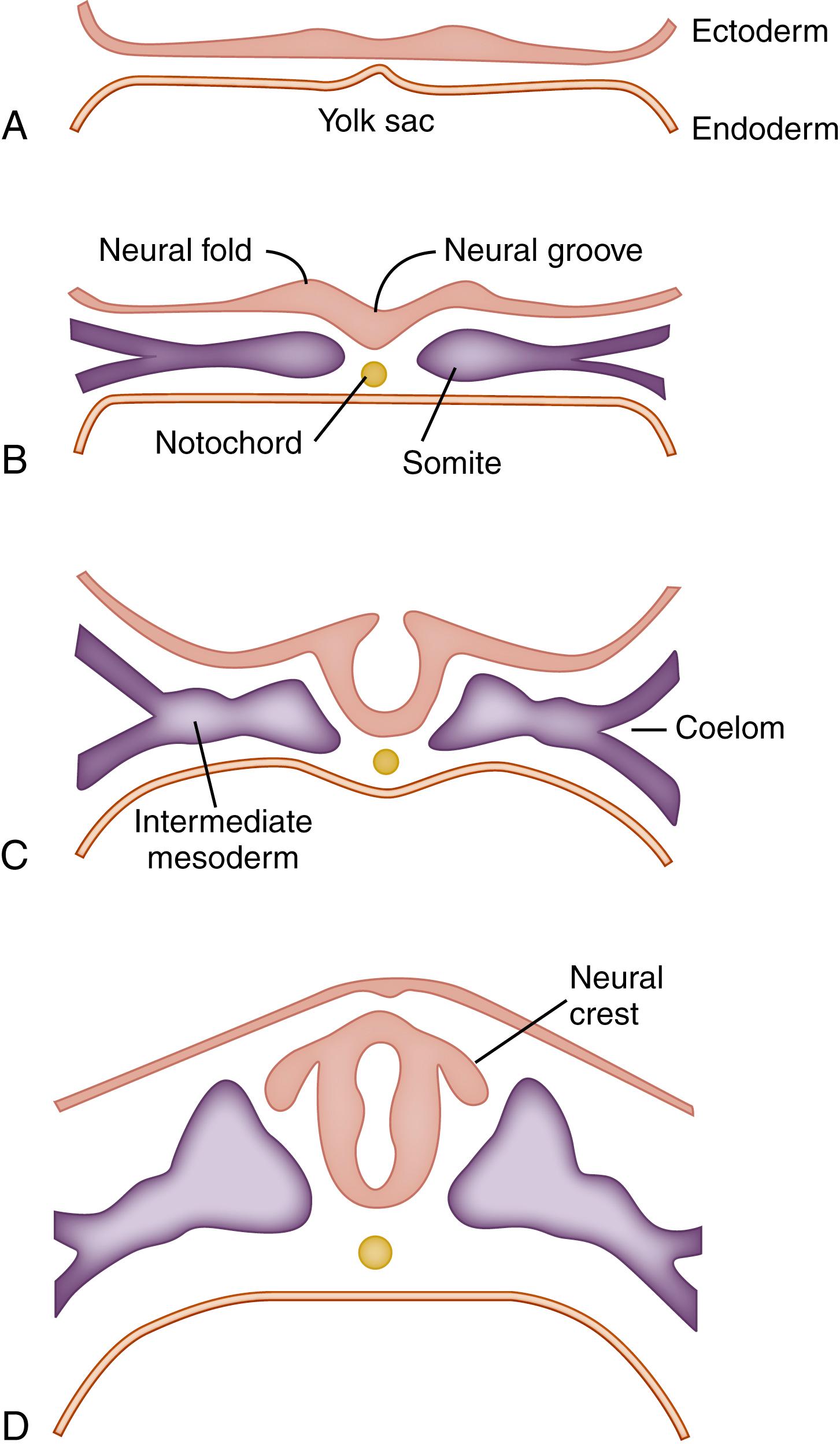
The remaining unfused ends of the neural folds at each end of the embryo are called the cranial and caudal neuropores because the neural tube is open at these sites. The cranial neuropore closes on day 25 and the caudal neuropore on day 27 of development. , This folding and shaping of the neural tube occur through both intrinsic (cell cycle, cell shape) and extrinsic (proliferation of adjacent tissue) mechanisms. , Shortly after fusion of the neural folds in a given region of the embryo, the neural tube separates from the ectoderm and becomes buried in the mesenchyme below the surface.
During this process of neural tube formation, an epithelial-mesenchymal transformation occurs, resulting in formation of a group of cells derived from the crests of the neural folds. These neural crest cells come to lie on the superolateral margins of the tube. The neural tube proper goes on to form the central nervous system, which consists of the brain and spinal cord, whereas the neural crest forms much of the peripheral nervous system, consisting of portions of autonomic, cranial, and spinal ganglia and nerves. The lumen of the neural tube becomes the central canal of the spinal cord and the ventricles of the brain.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here