Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Q15.1 What are the pros and cons of ordering baseline testing for thiopurine methyltransferase (TPMT) enzyme activity (phenotype) before initiating azathioprine therapy? (Pg. 170x2)
Q15.2 What is a general guide for interpretation of the laboratory testing for the genotype for TPMT activity? (Pg. 170)
Q15.3 Concerning azathioprine drug interactions, (1) what is the most important drug interaction and its mechanism, and (2) what are several other important interactions? (Pgs. 171, 176)
Q15.4 Based on TPMT enzyme levels (phenotype testing), what is the appropriate azathioprine dose in mg/kg/day for a patient with (1) a normal enzyme level, (2) an intermediate enzyme level, and (3) a low enzyme level? (Pg. 172)
Q15.5 Concerning the azathioprine mechanisms of action, (1) what is the most general theory on the mechanism, and (2) why is the drug likely effective treating immunobullous diseases? (Pg. 172)
Q15.6 How does the incidence of lymphoproliferative malignancies and cutaneous squamous cell carcinomas differ in patients receiving azathioprine for dermatologic diseases, rheumatoid arthritis, and solid organ transplantation? (Pg. 173)
Q15.7 What is a reasonable lower limit for a white blood cell count during azathioprine therapy and what are some measures clinicians can use when this lower limit is passed? (Pg. 174)
Q15.8 What are the important clinical features of the hypersensitivity syndrome occasionally induced by azathioprine? (Pg. 175)
Q15.9 Concerning azathioprine gastrointestinal adverse effects, (1) what are the most common symptoms, and (2) what are several simple measures to decrease these symptoms? (Pg. 175)
Q15.10 Why is hepatic monitoring of importance in following patients receiving azathioprine therapy? (Pg. 176)
6-Mercaptopurine
6-Thioguanine
Complete blood count
US Food and Drug Administration
Gastrointestinal
Hypoxanthine-guanine phosphoribosyl transferase
Inflammatory bowel disease
Leukocytoclastic vasculitis
Liver function test
Red blood cell
Randomized controlled trial
Squamous cell carcinoma
Systemic lupus erythematosus
Tumor necrosis factor
Thiopurine methyltransferase
White blood cell
Xanthine oxidase
Azathioprine (Imuran, Azasan) ( Table 15.1 ) was synthesized in 1959 from its parent drug 6-mercaptopurine (6-MP), and became drug of choice for organ transplantation during the 1960s and 1970s. During this time, it became apparent that azathioprine had not only immunosuppressant, but also anti-inflammatory properties. Rheumatologists, gastroenterologists, neurologists, and dermatologists began using azathioprine for a wide variety of inflammatory and autoimmune diseases. Dermatologists use azathioprine for the treatment of immunobullous diseases, atopic dermatitis, and actinic dermatitis as well as other inflammatory and autoimmune dermatoses.
| Generic Name | Azathioprine |
|---|---|
| Trade names | Imuran, Azasan |
| Date released | 1959 |
| Drug formulations | 50 mg, 75 mg, 100 mg 50 mg tablets are scored 100 mg vials |
| Drug dosing—empiric | Up to 2–2.5 mg/kg daily |
| Drug dosing by TPMT level and genotype High TPMT >15.1–26.4 U/mL or homozygous wild type Medium TPMT 6.3–15 U/mL or heterozygous wild type Low TPMT <6.3 U/mL or homozygous mutant type |
Up to 2–2.5 mg/kg daily Up to 1.0 mg/kg daily Do not use azathioprine |
Paramount to the safe and efficacious use of azathioprine is an understanding of the metabolism of this drug, specifically, awareness of the genetic polymorphism of an enzyme, thiopurine methyltransferase (TPMT) and pretreatment phenotypic testing of this enzyme. Pretreatment TPMT enzyme testing may avoid catastrophic myelosuppression and guide proper dosing, but regular complete blood counts (CBC) and liver function tests (LFT) are still needed to monitor for myelosuppression and hepatotoxicity throughout the course of treatment. Adverse effects (AE) of azathioprine include myelosuppression, gastrointestinal (GI) symptoms, hypersensitivity reaction, infections, pancreatitis, lymphoproliferative malignancy, and cutaneous squamous cell carcinomas (SCC).
Table 15.2 contains the key pharmacologic concepts for azathioprine. Figure 15.1 shows the structure of azathioprine.
| Drug Name | Absorption and bioavailability | Elimination | ||||
|---|---|---|---|---|---|---|
| Peak Levels | Bioavailable (%) | Protein Binding (%) | Half-Life | Metabolism | Excretion | |
| Azathioprine | 1–2 h | 88 | 30 | 5 h | Thiopurine methyltransferase Xanthine oxidase HGPRT (active 6-TG metabolites) |
Negligible unmetabolized azathioprine is excreted; virtually completely metabolized |
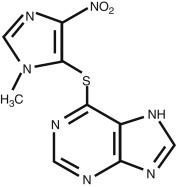
After oral administration, more than 88% of azathioprine is absorbed through the GI tract. Azathioprine does not cross the blood–brain barrier, but easily crosses the placenta and into mammary glands. The peak plasma levels occur in less than 2 hours. Azathioprine is rapidly and extensively metabolized. The active metabolite 6-thioguanine (6-TG) slowly accumulates in tissues and eventually provides maximal clinical immunosuppression at around 8 to 12 weeks, using traditional, somewhat conservative, dosage schemes.
| Enzyme Pathway | End Product | ‘Inhibition’ of Pathway |
|---|---|---|
| Thiopurine methyltransferase (TPMT) | Inactive metabolites | Genetic predisposition |
| Xanthine oxidase (XO) | Inactive metabolites | Allopurinol, febuxostat |
| Hypoxanthine-guanine phosphoribosyltransferase (HGPRT) | Active purine analogs—especially 6-TG | Lesch–Nyhan syndrome (rare) |
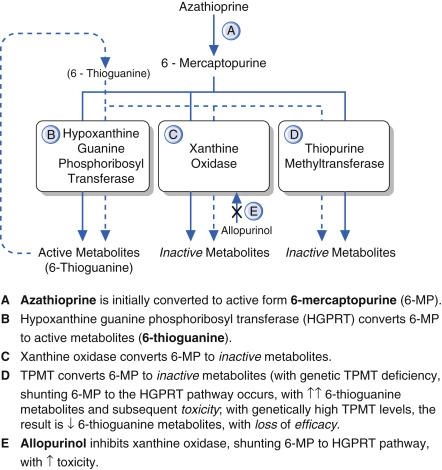
Azathioprine is rapidly converted to 6-MP upon absorption; conceptually, azathioprine is a ‘prodrug’. This conversion occurs mainly in erythrocytes. Q15.1 The fate of 6-MP is determined by one of the following three competing pathways:
Anabolized to its active form, a purine analog 6-TG, by the enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRT);
Degraded by TPMT to inactive metabolites; or
Degraded by xanthine oxidase (XO) to inactive metabolites.
The anabolic pathway leads to the active metabolites, the purine analogs thioguanine monophosphate and other 6-TG metabolites (including diphosphate and triphosphate metabolites). Both degradative pathways lead to inactive, nontoxic metabolites. Reduced activity of either of the degradative pathways will shift more of the 6-MP into the HGPRT active (or anabolic) pathway, leading to excessive clinical immunosuppression, with an increased risk of myelosuppression.
Q15.1 TPMT activity is reduced or absent in certain patients with a genetic polymorphism. TPMT enzyme function testing and enzyme allele sequencing are readily available. The enzyme function assay is available for clinical use and measures the activity of TPMT in red blood cells (RBC) and correlates well with systemic TPMT activity. Through this functional assay system, three groups of patients have been identified: those with high activity, intermediate activity, or low activity. Patients with low TPMT activity have markedly increased accumulation of 6-TG metabolites, which increases the risk of catastrophic myelosuppression; these patients should not be treated with azathioprine. Patients with high levels of this enzyme are commonly therapeutically underdosed, unless the clinician appropriately adjusts the traditional dose upward. Research into ethnic variation at the TPMT locus revealed that certain mutations appear to be unique to a given ethnic group. This diversity among ethnicities makes it difficult to create a genetic test to identify all possible mutations in TPMT leading to a low-activity phenotype. The functional enzyme assay is a relatively simple solution to identify patients with varying levels of TPMT activity phenotype. In addition, it appears that TPMT is inducible and that the enzyme activity is not a constant but has an activity range. Induction of TPMT may contribute to nonresponse. Despite the imperfections of these tests, the literature supports genotypic and, more importantly, phenotypic testing (enzyme activity) of TPMT activity to minimize the risk of myelosuppression and optimally dose azathioprine.
Q15.2 Should a laboratory provide a genotypic result for TPMT without quantitative enzymatic levels, the genotype can be easily interpreted by the number of wild type alleles. Because the TPMT alleles exhibit codominance, the number of wild type alleles are directly proportional to enzymatic activity. Effectively, homozygous wild type alleles equate to high TPMT activity, heterozygous wild type to medium activity, and homozygous mutant alleles to low activity. Therefore, dosing is easily translatable (see Table 15.1 ). Of note, a small proportion of heterozygous wild type individuals may tolerate homozygous dosing.
Q15.3 Decreased activity of XO occurs as the result of a drug interaction with allopurinol. Allopurinol inhibits the XO pathway and coadministration of allopurinol or febuxostat with azathioprine results in the production of more active metabolite 6-TG analogs. This in turn leads to excessive immunosuppression and increased risk for myelosuppression. In patients receiving allopurinol or the similar drug febuxostat, who also require azathioprine therapy, the dose of azathioprine should be reduced by 75%.
Pharmacological inhibition or genetically reduced activity of either catabolic pathway respectively (XO or TPMT) will result in excessive HGPRT conversion of azathioprine (via 6-MP), with clinically enhanced immunosuppression and risk of myelosuppression.
Azathioprine is supplied by various manufacturers as scored tablets, including 50-, 75-, and 100-mg tablets; an injectable formulation with 100 mg per vial is also available. Whereas historically, empiric dosing was titrated up gingerly to a goal of 2.5 mg/kg per day, current dosing is based on pretreatment TPMT activity testing. Q15.4 There is some variability between laboratories; however, in the Mayo Laboratories in Rochester, Minnesota, low levels of TPMT activity are less than 6.3 U/mL, intermediate levels 6.3 to 15 U/mL, and normal levels 15.1 to 26.4 U/mL. These values correlate with suggested genotypic dosing (see Table 15.1 ). Note, these are only suggested dosing schemes and clinical response may be variable. If clinical response is inadequate, 6-TG nucleotide and 6-methylmercaptopurine can be measured to determine the etiology of nonresponse (i.e., recalcitrant disease, underdosing, nonadherence). Similarly, the utility of measuring 6-TG nucleotides in children with inflammatory bowel disease to ensure azathioprine therapeutic dosing and toxicity mitigation has also been illustrated.
Q15.5 Azathioprine’s active metabolites are 6-TG monophosphate and other 6-TG metabolites. Azathioprine is structurally similar to the endogenous purines adenine and guanine, but substitutes a thiol group for the amino or the hydroxyl group, respectively. The exact mechanism by which this purine analog leads to immunosuppressive and anti-inflammatory effects is not known. However, the structural similarity of 6-TG to endogenous purines allows it to be incorporated into deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), thus inhibiting purine metabolism and cell division. Azathioprine also affects T-cell, B-cell, and antigen-presenting cell function. T-cell-mediated function is depressed, and antibody production is diminished in B cells. The altered production of antibodies is integral in the treatment of immunobullous dermatoses such as pemphigus vulgaris and bullous pemphigoid.
Box 15.1 contains indications for azathioprine.
Organ transplantation
Severe rheumatoid arthritis
Bullous pemphigoid
Pemphigus vulgaris
Cicatricial pemphigoid
Leukocytoclastic vasculitis
Wegener granulomatosis
Polyarteritis nodosa
Eosinophilic granulomatosis with polyangitis
Behçet syndrome
Pyoderma gangrenosum
Systemic lupus erythematosus
Discoid lupus erythematosus
Dermatomyositis/polymyositis
Relapsing polychondritis
Eosinophilic fasciitis
Contact dermatitis
Atopic dermatitis
Hand dermatitis
Lichen planus (oral and cutaneous)
Psoriasis and psoriatic arthritis
Chronic actinic dermatitis
Persistent light reaction
Polymorphous light eruption
Sarcoidosis (especially pulmonary features)
Erythema multiforme (persistent)
Chronic graft-versus-host disease
Leprosy
Vitiligo
Azathioprine is only formally approved by the US Food and Drug Administration (FDA) for use in organ transplantation and in patients with severe rheumatoid arthritis (RA). However, licensed indications in Europe include atopic dermatitis, pyoderma gangrenosum, angioedema, urticaria, urticarial vasculitis, systemic lupus erythematosus (SLE), dermatomyositis, bullous pemphigoid, and pemphigus vulgaris. The moderately potent immunosuppressive and anti-inflammatory effects, a reasonable risk–benefit profile, and the low cost have resulted in a variety of ‘off-label’ uses. Dermatologists should follow the guidelines in Box 15.2 to determine the appropriateness of azathioprine use.
Disease should be life threatening.
Disease should be reversible or controllable.
Disease is unresponsive to less potentially risky therapies.
Disease should have measurable clinical or laboratory measures of improvement.
Risks and adverse effects should be carefully discussed with the patient.
Alternative therapies should be discussed with patient.
Patient should be compliant with various laboratory monitoring tests and regular follow-up.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here