Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Autosomal dominant polycystic kidney disease (ADPKD) (MIM 173900) is a systemic disorder characterized by age-dependent occurrence of bilateral, multiple renal cysts as well as a variety of extrarenal manifestations. The latter include cysts in the liver bile ducts, pancreatic ducts, seminal vesicles, and arachnoid membrane, as well as noncystic manifestations, such as intracranial aneurysms and dolichoectasias, aortic root dilatation and aneurysms, mitral valve prolapse, and abdominal wall hernias. Over the past several decades, the study of this disease has yielded remarkable progress and insights. The mutated genes and their respective protein products were identified by positional cloning, the occurrence of multiple somatic mutations were implicated in the molecular pathogenesis, a comprehensive array of orthologous gene animal models have been developed, and a much neglected organelle, the primary cilium, has become the focus of investigation not just in this disease but in a whole panoply of structural kidney diseases as well as more diverse biological processes. There has been improved understanding of the clinical disease and the variation it exhibits, and several directed therapeutic clinical trials based on preclinical and bench investigations are beginning to yield results in patients. Still, the goals of understanding the basic disease mechanisms and finding effective treatments remain a work in progress.
Keywords
polycystic kidney, cilia, polycystic liver, calcium signaling, polycystin, TRPP2
Autosomal dominant polycystic kidney disease (ADPKD) (MIM 173900) is a systemic disorder characterized by age-dependent occurrence of bilateral, multiple renal cysts as well as a variety of extrarenal manifestations. The latter include cysts in the liver bile ducts, pancreatic ducts, seminal vesicles, and arachnoid membrane, as well as noncystic manifestations, such as intracranial aneurysms and dolichoectasias, aortic root dilatation and aneurysms, mitral valve prolapse, and abdominal wall hernias. Over the past several decades, the study of this disease has yielded remarkable progress and insights. The mutated genes and their respective protein products were identified by positional cloning, the occurrence of multiple somatic mutations were implicated in the molecular pathogenesis, a comprehensive array of orthologous gene animal models have been developed, and a much neglected organelle, the primary cilium, has become the focus of investigation not just in this disease but in a whole panoply of structural kidney diseases as well as more diverse biological processes. There has been improved understanding of the clinical disease and the variation it exhibits, and several directed therapeutic clinical trials based on preclinical and bench investigations are beginning to yield results in patients. Still, the goals of understanding the basic disease mechanisms and finding effective treatments remain a work in progress.
ADPKD affects between 1 in 400 and 1 in 1000 live births in all ethnic populations worldwide. Mutations in either of two genes, PKD1 or PKD2 , respectively encoding to protein products called polycystin-1 (PC1) than polycystin-2 (PC2), result in ADPKD. It is the most common single gene disorder that can lead to premature death in man. Approximately 2100 ADPKD patients start renal replacement therapy yearly in the United States. Worldwide yearly incidence rates for end stage renal disease (ESRD) caused by ADPKD in men and women, respectively, are 8.7 and 6.9 per million (1998–2001, United States), 7.8 and 6.0 per million (1998–1999, Europe), and 5.6 and 4.0 per million (1999–2000, Japan). Age-adjusted sex ratios greater than unity (1.2–1.3) suggest a more progressive disease in men than in women. In the United States the incidence of ESRD due to ADPKD has increased by 10.8 percent from 1996–1998 to 2007–2008. Similarly, in Denmark ESRD incidence secondary to ADPKD increased from 6.45 per million in 1990–1995 to 7.59 per million in 2002–2007. These trends may be due to improved patient survival before the onset of ESRD. At the same time the age at ESRD in ADPKD patients has increased in both countries. In Denmark, age-adjusted male-to-female ratio for onset of ESRD has changed from 1.6 to 1.1, indicating a trend toward similar progression in both genders in recent years.
The diagnosis of ADPKD usually relies on imaging testing ( Figure 80.1 ). Renal ultrasound is commonly used because of cost and safety. Counseling should be done before testing an individual with a family history for the presence of ADPKD. Benefits of testing include certainty regarding diagnosis that may influence family planning, early detection and treatment of disease complications, and identification of genetically unaffected family members for living related-donor renal transplantation. The potential for discrimination in terms of health insurability and employment associated with a positive diagnosis has been reduced by the Genetic Information Nondiscrimination Act (GINA) but it does not apply to life, disability or long-term care insurance. Additionally, the psychological burden of knowing affliction by a chronic disease should be considered in the decision to test.
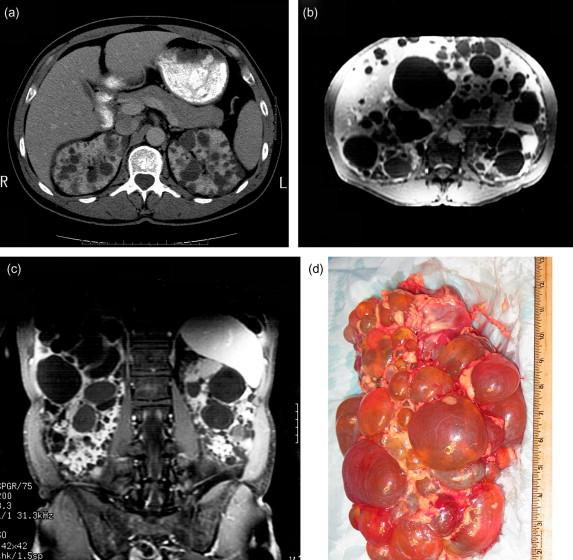
The occurrence and severity of the cystic lesions are highly variable. Cysts have been observed in utero and have been detected as incidental findings in 80-year-old patients with otherwise normal blood pressure and kidney function. In genetic terms, the expressivity, or severity, of the phenotype, in this disease is highly variable. By contrast the penetrance is virtually complete if it is expressed as a function of age. Individuals over the age of 30 carrying a causative gene mutation will invariably manifest cysts sufficient for diagnosis when assessed with an appropriately sensitive imaging study. For this reason, the gold standard for diagnosis of ADPKD remains imaging studies of the kidney. The ultrasound criteria, referred to as the modified Ravine criteria, take into account the age-dependent penetrance of the disease by requiring increasing numbers of cysts with increasing age to make the diagnosis. For individuals at 50% risk for the disease (i.e., those with a family history), these criteria include at least two unilateral or bilateral cysts in individuals younger than 30 years; two cysts in each kidney in individuals 30 to 59 years old; and four cysts in each kidney in individuals 60 years or older. In the absence of a family history of ADPKD, bilateral renal enlargement and cysts or the presence of multiple bilateral cysts with hepatic cysts together with the absence of other manifestations suggesting a different renal cystic disease provide presumptive evidence for the diagnosis of ADPKD.
Revised ultrasound criteria have been proposed to improve the diagnostic performance of ultrasonography in ADPKD ( Table 80.1 ). The presence of at least three (unilateral or bilateral) renal cysts and of two cysts in each kidney have a positive predictive value of 100% in 15 to 39 and 40 to 59 year-old at-risk individuals, respectively. For at-risk individuals ages 60 years and older, four or more cysts in each kidney are required. Although the positive predictive values of these criteria are very high, their sensitivity and negative predictive value are low, particularly when applied to 15- to 59-year-old PKD2 patients. This is a problem in the evaluation of potential kidney donors where exclusion of the diagnosis is important. Information on the age of ESRD in other affected family members may be helpful in this setting. A history of at least one affected family member with ESRD secondary to ADPKD by age 55 years has 100% positive predictive value for PKD1 . Conversely, a history of at least one affected family member without ESRD by age≥70 years has 100% positive predictive value for PKD2 . More strict criteria have therefore been proposed to exclude a diagnosis of ADPKD in an individual at risk from a family with an unknown genotype ( Table 80.1 ). An ultrasound scan finding of normal kidneys or one renal cyst in an individual age 40 years or older has a negative predictive value of 100%. The absence of any renal cyst provides near certainty that ADPKD is absent in at-risk individuals ages 30 to 39 years with a negative predictive value of 98.3%. A negative or indeterminate ultrasound scan result does not exclude ADPKD with certainty in an at-risk individual younger than 30 years of age. In this setting, negative results on magnetic resonance imaging (MRI) or contrast-enhanced computed tomography (CT) provide further assurance, but there are insufficient data to quantify its predictive accuracy. Jointly considering the proposed sonographic criteria, family history information, and findings on high resolution imaging studies routinely obtained during donor evaluations usually allows a determination of disease status in the majority of potential living related kidney donors with high level of certainty.
| Family Genotype | Unknown | PKD1 * | PKD2 † | ||||
|---|---|---|---|---|---|---|---|
| Age | Revised criteria for positive diagnosis | PPV | SEN | PPV | SEN | PPV | SEN |
| 15–29 | ≥3 cysts, unilateral or bilateral | 100 | 81.7 | 100 | 94.3 | 100 | 69.5 |
| 30–39 | ≥3 cysts, unilateral or bilateral | 100 | 95.5 | 100 | 96.6 | 100 | 94.9 |
| 40–59 | ≥2 cysts in each kidney | 100 | 90.0 | 100 | 92.6 | 100 | 88.8 |
| ≥60 | ≥4 cysts in each kidney | 100 | 100 | 100 | 100 | 100 | 100 |
| Revised criteria for diagnosis exclusion | NPV | SPEC | NPV | SPEC | NPV | SPEC | |
| 15–29 | ≥1 cyst | 90.8 | 97.1 | 99.1 | 97.6 | 83.5 | 96.6 |
| 30–39 | ≥1 cyst | 98.3 | 94.8 | 100 | 96.0 | 96.8 | 93.8 |
| 40–59 | ≥2 cysts | 100 | 98.2 | 100 | 98.4 | 100 | 97.8 |
* 100% (PPV) if one affected family member has ESRD by age 55 yrs.
† 100% PPV if one affected family member does not have ESRD by age ≥ 70 yrs
Genetic testing can be used when the imaging results are equivocal and/or when a definite diagnosis is required in a younger individual, such as a potential living related kidney donor. Prenatal and preimplantation genetic testing are rarely considered for ADPKD. Genetic testing is performed by direct sequence analysis. Direct sequencing yields mutation detection rates of approximately 85%. However, as most mutations are unique and up to one third of PKD1 changes are predicted to be single amino acid substitution changes, the causative nature of some sequence changes is difficult to prove. Genetic testing can be helpful with de novo mutations in the absence of a family history. A unique variant in either PKD1 or PKD2 present in the affected child but absent in both parents in the setting of documented maternity and paternity can identify the new mutation.
The clinical sine qua non for ADPKD is the presence of multiple cysts in both kidneys and the consequent increase in total kidney size. The development of cysts in ADPKD probably starts in the embryo. They continue to form and grow during the remaining life time. Cyst expansion along with associated inflammation, fibrosis, and tubular dropout results in a loss of filtering nephrons, but glomerular filtration rate remains stable for decades thanks to the compensatory capacity of the kidney. During this often-silent phase, mild polyuria due to impaired urinary concentrating capacity, elevated blood pressure, microalbuminuria, and, occasionally, low-grade proteinuria can develop. When enough filtering nephrons have been lost, the glomerular filtration rate (GFR) begins to fall precipitously. Extrarenal manifestations of the disease are rare in childhood. Cysts in the liver usually start later than in the kidney. Patients may seek medical attention at any point in the course of the disease for renal or extrarenal, cyst-related or non–cyst-related manifestations.
The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) has provided the best clinical information on how cysts develop and grow. Two hundred and forty-one nonazotemic patients have been followed prospectively with yearly magnetic resonance imaging (MRI) examinations to assess kidney and cyst growth. Total kidney volume and cyst volumes increased exponentially. At baseline, total kidney volume was 1060±642 ml, and the mean increase over three years was 204 ml, or 5.3% per year. The rates of change of total kidney and total cyst volumes, and of right and left kidney volumes, were strongly correlated, suggesting that kidney enlargement was due to cyst enlargement. Baseline total kidney volume predicted the subsequent rate of increase in renal volume and was associated with declining GFR in patients with baseline total kidney volume above 1500 ml after the first three years of follow-up. The association between baseline total kidney volume has become increasingly strong with an extended follow-up of eight years, qualifying total kidney volume as a prognostic biomarker in ADPKD.
Hypertension (BP>140/90 mmHg) is present in approximately 50% of 20-34 year old ADPKD patients with normal renal function and increases to nearly 100% of patients with ESRD. Its development is accompanied by a reduction in renal blood flow, increased filtration fraction, abnormal renal handling of sodium, and extensive remodeling of the renal vasculature.
The association between renal size and prevalence of hypertension has supported the hypothesis that stretching and compression of the vascular tree by cyst expansion causes ischemia and activation of the renin–angiotensin system. Whether the classic, circulating renin-angiotensin system is inappropriately activated is controversial. There is stronger evidence for the activation of a local intrarenal renin-angiotensin system. Evidence for the latter includes (1) partial amelioration of the reduced renal blood flow, increased renal vascular resistance and increased filtration fraction in the setting of acute or chronic administration of an ACEI; (2) shift of immunoreactive renin from the juxtaglomerular apparatus to the walls of the arterioles and small arteries; (3) ectopic synthesis of renin in the epithelium of dilated tubules and cysts; and (4) ACE-independent generation of angiotensin II by a chymase-like enzyme.
It has been suggested that a primary disruption of polycystin protein function in the vasculature may also play a role in the early development of hypertension and renal vascular remodeling. Supportive evidence includes expression of the respective PKD1 and PKD2 gene products in vascular smooth muscle and endothelium, and enhanced vascular smooth muscle contractility and impaired endothelial dependent vasorelaxation in heterozygous blood vessels. Recent studies have shown that nitric oxide endothelium-dependent vasorelaxation is impaired in small subcutaneous resistance vessels from patients with normal renal function before development of hypertension. Other factors proposed to contribute to hypertension in ADPKD include increased sympathetic nerve activity and plasma endothelin-1 levels and insulin resistance.
The diagnosis of hypertension in ADPKD is often made late. Twenty-four hours ambulatory blood pressure monitoring of children or young adults without hypertension may reveal elevated blood pressures and attenuated nocturnal blood pressure dipping and exaggerated blood pressure response during exercise, which may be accompanied by left ventricular hypertrophy and diastolic dysfunction. Early detection and treatment of hypertension is important because cardiovascular disease is the main cause of death and uncontrolled blood pressure increases the risk for proteinuria, hematuria, faster decline of renal function and morbidity and mortality from valvular heart disease and aneurysms.
Pain is the most frequent symptom (~60%) reported by adult patients. Acute pain may be associated with renal hemorrhage, passage of stones and urinary tract infections. Some patients develop chronic flank pain without identifiable etiology other than the cysts.
VEGF produced by the cystic epithelium may promote angiogenesis, hemorrhage into cysts and gross hematuria. Rarely, a hemorrhagic cyst can rupture into the subcasular or retroperitoneal space. Symptomatic episodes likely underestimate the frequency of cyst hemorrhage as over 90% of ADPKD patients have hyperdense (CT) or high signal (MRI) cysts reflecting blood or high protein content. Most hemorrhages resolve within two to seven days. If symptoms last longer than one week or if the initial episode occurs after the age of 50 years, investigation to exclude neoplasm should be undertaken.
Approximately 20% of ADPKD patients have kidney stones. Their composition is usually uric acid and/or calcium oxalate. Metabolic factors include decreased ammonia excretion, low urinary pH and low urinary citrate concentration. Urinary stasis secondary to the distorted renal anatomy may also play a role. Stones may be difficult to differentiate from cyst wall and parenchymal calcification, which also occur with increased frequency.
As in the general population urinary tract infections affect females more frequently than males. Most are caused by enterobacteriaciae. CT and MRI are sensitive to detect complicated cysts and provide anatomic definition, but the findings are not specific for infection. Nuclear imaging ( 67 Ga or 111 In-labeled leukocyte scans) is useful but false negative and positive results are possible. 18 fluorodeoxyglucose ( 18 FDG) positron-emission computed tomography (PET/CT) is currently the most helpful imaging modality to detect a cyst infection, but it is not widely available, is expensive, and currently not approved by the Centers for Medicare and Medicaid Services (CMS) for the diagnosis of infection. Cyst aspiration should be considered when the clinical setting and imaging are suggestive and blood and urine cultures are negative.
Renal cell carcinoma is a rare cause of pain in ADPKD. It does not occur more frequently than in the general population, but it may present at an earlier age with frequent constitutional symptoms and a higher proportion of sarcomatoid, bilateral, multicentric, and metastatic tumors. A solid mass on ultrasound, speckled calcifications on CT, and contrast enhancement, tumor thrombus and regional lymphadenopathies on CT or MRI should raise the suspicion for a carcinoma.
The development of renal failure is highly variable. In most patients renal function is maintained within the normal range, despite relentless growth of cysts, until the 4 th to 6 th decade of life. By the time renal function starts declining, the kidneys usually are markedly enlarged and distorted with little recognizable parenchyma on imaging studies. At this stage, the average rate of GFR decline is approximately 4.4–5.9 mL/min/year. The mutated gene ( PKD1 or PKD2 ) is a significant determinant of the severity of disease and position of the mutation within PKD1 and possible modifier genes also contribute to the clinical course of ADPKD (see following sections). Other risk factors for a worse prognosis include male gender (particularly in PKD2 ), black race, first episode of hematuria before the age of 30, onset of hypertension before the age of 35, hyperlipidemia, low HDL, and sickle cell trait.
Several mechanisms account for renal function decline. The CRISP study has confirmed previous studies suggesting a strong relationship between renal enlargement and functional decline. CRISP has shown that kidney and cyst volumes are the strongest predictors of renal functional decline. CRISP also found that renal blood flow (or vascular resistance) is an independent predictor of functional decline. This points to the importance of vascular remodeling in the progression of the disease and may account for cases where the decline of renal function seems to be out of proportion to the severity of the cystic disease. Angiotensin II, transforming growth factor-β, and reactive oxygen species may contribute to the vascular lesions and interstitial fibrosis by stimulating the synthesis of chemokines, extracellular matrix, and metalloproteinase inhibitors. Other factors such as heavy use of analgesics may contribute to chronic kidney disease (CKD) progression in some patients.
PLD is the most common extrarenal manifestation. It is associated with both PKD1 and PKD2 genotypes. The cysts arise by excessive proliferation and dilatation of biliary ductules and peribiliary glands. Estrogen receptors are expressed in the epithelium lining the hepatic cysts and estrogens stimulate hepatic cyst derived cell proliferation. Bile duct cyst growth is also promoted by growth factors and cytokines secreted into the cyst fluid.
Hepatic cysts are rare in children. Their frequency increases with age and may have been underestimated by ultrasound and CT studies. Their prevalence by MRI in the CRISP study is 58%, 85 and 94% in 15 to 24, 25 to 34 and 35 to 46 year old participants. Hepatic cysts are more prevalent and hepatic cyst volume is larger in women than in men. Women who have multiple pregnancies or who have used oral contraceptive agents or estrogen replacement therapy have worse disease suggesting an estrogen effect on hepatic cyst growth.
Typically, PLD is asymptomatic, but symptoms have become more frequent as the lifespan of ADPKD patients has lengthened with dialysis and transplantation. Symptoms may result from mass effect or from complications related to the cysts. Symptoms typically caused by massive enlargement of the liver or by mass effect from a single or a limited number of dominant cysts include dyspnea, early satiety, gastroesophageal reflux, and mechanical low back pain. Other complications caused by mass effect include hepatic venous outflow obstruction, inferior vena cava compression, portal vein compression or bile duct compression presenting as obstructive jaundice.
Symptomatic liver cyst complications include cyst hemorrhage, infection and rarely torsion or rupture. The typical presentation of cyst infection is with localized pain, fever, leukocytosis, elevated sedimentation rate and often elevated alkaline phosphatase. It is usually monomicrobial and caused by enterobacteriaceae. MRI is very sensitive for identifying complicated hepatic cysts. On CT scanning, fluid-debris levels within cysts, cyst wall thickening, intracystic gas bubbles, and heterogeneous or increased density have been associated with infection. Radionuclide imaging and more recently 18 F-fluorodoxyglucose PET/CT scanning have been used for diagnosis.
Mild dilatation of the common bile duct has been observed in 40% of patients studied by CT and may rarely be associated with episodes of cholangitis. Rare associations of PLD include congenital hepatic fibrosis, adenomas of the ampulla of Vater, and cholangiocarcinoma.
Seminal vesicles, pancreas, and arachnoid membrane cysts are present in 40% (males), 5%, and 8% of patients, respectively. Seminal vesicle cysts rarely result in infertility. Defective sperm motility is another cause of male infertility in ADPKD. Pancreatic cysts are almost always asymptomatic, with very rare occurrences of recurrent pancreatitis and possibly chance associations of intraductal papillary mucinous tumor or carcinoma reported in ADPKD. Arachnoid membrane cysts are asymptomatic, but may increase the risk for subdural hematomas. Spinal meningeal diverticula may occur with increased frequency and rarely present with intracranial hypotension due to cerebrospinal fluid leak. Ovarian cysts are not associated with ADPKD.
These include intracranial aneurysms and dolichoectasias, thoracic aortic and cervicocephalic artery dissections, and coronary artery aneurysms. They are caused by alterations in the vasculature directly linked to mutations in PKD1 or PKD2 . The respective protein products, PC1 and PC2, are expressed in vascular smooth muscle cells (VSMC). Pkd2 +/− VSMCs from mice exhibit increased rates of proliferation and apoptosis and Pkd2 +/− mice have an increased susceptibility to vascular injury and premature death when induced to develop hypertension. Defective structural integrity of blood vessels occur in mice lacking PC1.
Intracranial aneurysms (ICA) occur in approximately 6% of patients with a negative, and 16% of those with a positive family history of aneurysms. They are most often asymptomatic. Focal findings such as cranial nerve palsy or seizure result from compression of local structures. The risk of rupture depends on many factors including the size of the aneurysm. Rupture carries a 35–55% risk of combined severe morbidity and mortality. The mean age at rupture is lower than in the general population (39 years versus 51 years). Most patients with ICA have normal renal function and up to 29% will have normal blood pressure at the time of rupture.
Mitral valve prolapse occurs in up to 25% of ADPKD patients on echocardiography. Aortic insufficiency may occur in association with dilatation of the aortic root. Although these lesions may progress with time, they rarely require valve replacement. Screening echocardiography is not indicated unless a murmur is detected on examination. Clinically inconsequential pericardial effusions are a common incidental finding in ADPKD.
Colonic diverticulosis and diverticulitis are more common in ESRD patients with ADPKD than in those with other renal diseases. Whether this increased risk extends to patients prior to ESRD is uncertain. There have been reports of extracolonic diverticular disease. It may become clinically significant in a minority of patients. Subtle alterations in polycystin function that may enhance the smooth muscle dysfunction during aging may underlie the development of diverticula.
Isolated autosomal dominant PLD (ADPLD; MIM 174050) also occurs as a genetically distinct disease in the absence of renal cysts. Like ADPKD, ADPLD is genetically heterogeneous, with two genes identified ( PRKCSH and SEC63 ) accounting for approximately one-third of isolated ADPLD cases. ADPLD often goes undetected even in first-degree relatives of patients with highly symptomatic polycystic liver disease. As in the case of polycystic liver disease associated with ADPKD, isolated ADPLD is more severe in women than in men. Liver function tests remain normal and when symptoms develop, these are related to mass effects or complications such as cyst hemorrhage or infection. Patients with isolated ADPLD may also be at increased risk for intracranial aneurysms and valvular heart disease.
Understanding of the clinical spectrum of polycystic kidney diseases as well as clues to the underlying molecular pathogenesis have been significantly advanced by the discovery over the past decade of the central role of cilia in cyst formation in the kidney. The ‘cilia hypothesis’ for polycystic disorders has been reviewed extensively (e.g., ). It is now appreciated that defects in cilia-basal body-centriole-related proteins—i.e., those found in the cilial membrane, cilial axoneme, the basal body or pericentriolar region—are varyingly associated with clinical spectrum of disease that can include cystic kidneys, bile duct and pancreatic duct cysts, retinal degeneration and retinitis pigmentosa, situs inversus (incorrect left-right body axis), anosmia, infertility, and hydrocephalus.
There are two general classes of cilia: the motile “9+2” structures and the non-motile ‘9+0’ structures that are also referred to as “primary cilia” ( Figure 80.2 ). In the kidney, the apical surface of every tubular epithelial cell, with the exception of mature intercalated cells, is decorated by a single primary cilium, a hair-like structure enclosed by a membrane continuous with the cell membrane and a containing the central axoneme composed of nine peripheral microtubule doublets without a central pair (hence, 9+0). The non-motile primary cilium is rooted in the centrosome, the microtubule organizing center of the cell. The centrosome is composed of a mother and a daughter centriole and a cloud of pericentriolar material around the mother centriole. During interphase, the distal end of the mother centriole known as the basal body gives rise to the primary cilium. Cilia are assembled and maintained by a process called intraflagellar transport (IFT) in which the components of the ciliary axoneme are assembled at the basal bodies into large transport particles called rafts. A region at the base of the cilia composed of transition fibers provides a compartmental demarcation between the cilium and the rest of the cell. Kinesin-2 and cytoplasmic dynein motor proteins mediate the anterograde and retrograde traffic of rafts along the axoneme, respectively. Kinesin-2 forms a heterotrimeric complex composed of two motor subunits KIF3A and KIF3B or KIF3C and a tail-associated non-motor accessory subunit, KAP3. When the cell prepares to divide in the S-phase, the primary cilium is reabsorbed and each centriole divides into new mother and daughter centrioles that migrate to the poles of the mitotic spindle.
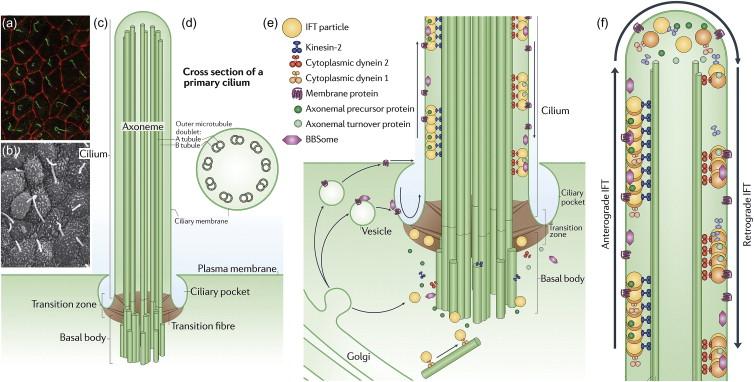
Several lines of complementary evidence converged on cilia as the central organelle in the pathogenesis of ADPKD. Among these were the findings that the PC1 ortholog in C. elegans is expressed in cilia and that several mouse models of recessive polycystic kidney disease targeting genes not orthologous to either PKD1 or PKD2 exhibited the phenotypic combination of cystic kidney defects and left-right axis abnormalities. This is significant because left-right axis determination has been established as a cilia dependent phenotype. The connection was further strengthened when one of these recessive polycystic kidney disease genes, Tg737 (also called polaris or IFT88), was identified as a component of the intraflagellar transport machinery necessary for ciliary biogenesis. Further direct evidence of the cilia link with ADPKD came with the demonstration of the localization of PC2 and PC1 in the cilia of kidney epithelia and the discovery that Pkd2 −/− mice have defects in left-right axis formation. Previous studies in ciliated MDCK cells had shown the mechanical deflection of cilia resulted in cellular calcium transients that could be detected by calcium sensitive fluorescent dyes. The discovery that PC1 and PC2 were expressed in cilia coupled with the knowledge that PC2 function as a cation channel (see below) led to the hypothesis that the polycystins were responsible for the cilia-dependent calcium transients observed in cultured cells. This hypothesis was supported by the observation that cells lacking PC1 failed to respond with cellular calcium transients upon deflection of cilia by laminar shear stress under flow. This finding was combined with the discovery that PC2 is required for left-right axis formation led to studies that showed that lateralized PC2-dependent calcium signals played a role in downstream signaling and vertebrate body axis specification. PC2 may be part of a mechanosensory complex that sensed the leftward flow generated by cilial movement in left-right axis formation, although a more complex mechanism for the lateralized nodal calcium signal may be operational.
Subsequently, an ever growing number of genes for human diseases that include cystic changes in the kidney have been identified in their function linked to cilia. These “reverse genetic” discoveries have been complemented by a forward genetic screen using random insertional mutagenesis in zebrafish that resulted in a cystic pronephric kidney phenotype in fish which identified a number of intraflagellar transport related proteins, as well as Pkd2 , among the target genes. Similarly, in the metanephric mouse kidney, conditional inactivation of the Kif3a component of the heterotrimeric kinesin-2 anterograde motor resulted in loss of cilia and consequent cyst formation during kidney development. Taken together the data support a strong functional connection between defects in cilia structure or function and cyst formation in lumen forming epithelia including kidney tubules, bile ducts and pancreatic ducts.
Primary cilia are increasingly implicated in a wide variety of important morphogenic signaling pathways (e.g., ) which in turn account for the wide spectrum of clinical features associated with syndromes when cilia function is disturbed. Association with cilia, basal body or pericentriolar region has also been reported for the ever expanding groups of genes mutated in recessive “ciliopathy” syndromic disorders with pleiotropic manifestations that include varying degrees and penetrance of kidney cyst formation. These diseases include nephronophthisis (NPHP), Joubert syndrome (JBTS), Meckel-Gruber syndrome (MKS), Bardet-Biedl syndrome (BBS) and oro-facial-digital syndrome (OFD). In addition, fibrocystin, the ARPKD gene product is expressed in cilia as well as the apical membrane of distal nephron tubular cells.
Subsets of the recessive ciliopathies have coalesced into phenotypic and genetic continuums. NPHP, JBTS, and MKS comprise a phenotypic spectrum roughly proceeding from less severe to more severe, respectively. Manifestations at the NPHP end of the spectrum include kidney and liver fibrosis, kidney cysts and retinal defects. JBTS patients also have cerebellar vermal hypoplasia and cognitive impairment while at the most severe end of the spectrum, MKS patients manifest with occipital encephalocele and are commonly nonviable. BBS shares kidney and retinal defects and in addition is characterized by digital defects, obesity, anosmia and cognitive impairment. Recessive mutations in over thirty genes have been identified among the ciliopathy syndromes. Mutations in same genes (e.g., CEP290 , NPHP1 , BBS4 , MKS1 ) give rise to more than one of clinical syndromes indicating that the phenotypic continuum is mimicked by genotypic overlap. In the most extreme example, mutations in CEP290 has been associated with NPHP, JBTS MKS and BBS. This interrelationship of a spectrum of clinical syndromes with recessive mutations in the same gene likely results from a complex interplay of locus heterogeneity, multiple allelism, modifier gene effects and possibly more complex multigenic inheritance.
Recent work has established that the functional protein complexes composed of the recessive ciliopathy gene products are part of the transition zone and trafficking complexes that determine the molecular composition cilia ( Figure 80.2 ). Mutations in at least nine genes have been associated each with NPHP and JBTS, six genes with MKS, and an additional 14 genes associated with BBS. Extensive cell biological and proteomic analysis has shown that the NPHP, JBTS and MKS associated gene products comprise the transition zone fibers that are critical gatekeepers determining the entrance and exit of components into and out of cilia. The BBS gene products assemble into the BBSome which functions in the trafficking of integral membrane proteins to and from cilia. The other key elements of cilia structure and function are the IFT proteins that, in conjunction with the kinesin and dynein motor proteins, are required to form structurally intact cilia. While mice and fish exhibit polycystic phenotypes with mutations in IFT proteins that result in abnormal or absent cilia, no human diseases with recessive loss of function in IFT genes and absent cilia have been identified possibly because these are embryonically lethal. Taken together, these recent discoveries suggest that recessive ciliopathies result in complex perturbations in the molecular composition of cilia. These findings also define the molecular relationship between the syndromic kidney cystic diseases resulting from ciliopathies with the non-syndromic polycystic kidney diseases, ADPKD and ARPKD. The latter both result from mutations in individual integral membrane proteins that are among the much larger group of client proteins of the BBSome and transition zone complex as they function to determine molecular composition of cilia. Ciliopathy syndromes therefore result from complex perturbations of cilia composition whereas ADPKD and ARPKD result from the discrete absence of individual molecular components of cilia that are not know to otherwise affect delivery or retention of additional ciliary proteins.
Mutations in at least two genes cause the clinical presentation of ADPKD—a property referred to as genetic heterogeneity. The two genes, PKD1 located on chromosome 16p13.3 and PKD2 located on chromosome 4q21, have been isolated by positional cloning. The genomic segments occupied by the PKD1 and PKD2 genes are of similar size (~45–50 kb), but PKD1 contains 46 exons that encode ~12 kb of open reading frame, compared to 15 exons and ~3 kb of open reading frame for PKD2 . Two thirds of the 5’ end of human PKD1 , both exons and introns, are duplicated multiple times with very high sequence fidelity (>95%) in more proximal regions of chromosome 16. PKD1 accounts for approximately 85% of affected families in European populations, with the remaining 15% resulting from PKD2 mutations. Analysis of the gene locus-dependent clinical phenotypes have highlighted the increased severity of PKD1 . The mean age of the composite endpoint of death or ESRD is 53 years for PKD1 and 69.1 years for PKD2 ; both differed significantly from the control population (78 years). Given this difference, it is would be expected that the relative prevalence of PKD2 will increase in patient subgroups stratified based on age of onset of ESRD. In one study, 39.1% of patients reaching ESRD after age 63 had PKD2 mutations. Using only age of presentation with ESRD as an endpoint, PKD1 patients have a median age of onset of 54.3 compared with 74.0 for PKD2 patients. The prevalence of hypertension is four-fold lower in PKD2 compared to PKD1 and the occurrence of urinary tract infections and hematuria is also reduced in the former compared to PKD1 . The value of these gene locus-based clinical differences in providing specific prognostic information to patients is limited due to the marked intrafamilial and interfamilial variation in clinical manifestations of ADPKD.
The molecular genetic mechanisms of cyst formation in ADPKD are required to explain two salient clinical features of the disease. The first is to explain why the disease occurs in such a focal manner given that the ADPKD gene mutations are autosomally inherited and all cells in the body carry a mutated allele. The second is to determine the basis of the marked intra-and interfamilial variability in disease severity.
The apparent paradox of the focal nature of ADPKD is highlighted by the observation in microdissection studies of early ADPKD kidneys that localized cystic dilatations occur in kidney tubules that appear otherwise normal despite the presence of the same germline mutation in all the cells. A molecular explanation for this observation has come with the discovery that cyst lining cells from human ADPKD cysts have loss of heterozygosity (LOH) in the chromosomal regions of the respective PKD genes in both kidney tubular and bile ductular cysts. The LOH indicates loss of the functional gene from the wild type allele through focal somatic second hit mutations that define cyst formation as a recessive phenotype at the cellular level. The absence of a remnant wild type allele in a subset of cells from a cyst with LOH suggests that the cysts are clonal in origin—i.e., arising from a single cell.
The conclusions from these studies were challenged on the basis of: (1) Immunolocalization studies using anti-PC1 antibodies showing evidence of residual expressed protein in human cysts, (2) the relatively low rate of detection of LOH in cysts, (3) the relatively high rate of somatic mutations required to account for the thousands of independent cysts, and (4) the fact that the finding of LOH in cysts established an association, but not a causal link. In retrospect, immunolocalization studies were focused on cellular staining patterns that did not examine cilia expression of the protein; the latter is now thought to be the primary site of PC1 function in ADPKD. Additionally, approximately 30% of germline mutations in PKD1 are predicted to be non-synonymous amino acid substitution mutations which are predicted to yield immunoreactive protein products. If a similar proportion of missense variants occurs among second hit mutations, resulting cysts be positive for PC1 by immunostaining. The relatively low detection rate of LOH in cysts may reflect limitations in comprehensive screening of PKD1 for pathogenic mutations. Even a decade later, the success rate of PKD1 mutation detection is still ~85%. When more effective sequence-based mutation detection was applied to cysts from patients with germline PKD2 mutations, the rate of detection of second hits was the same as the sensitivity of mutation detection suggesting that effectively all cysts had second hits. The rate of somatic mutations required to account for the thousands of cyst that develop in ADPKD are in keeping with the rate of measured HPRT mutation frequencies in kidney epithelial cells ranging from 5×10 −5 in the first decade to 2.5×10 −4 after the eighth decade of life.
A variant of the two hit hypothesis was suggested by studies examining kidney cysts from patients with defined PKD2 mutations. Cyst cells were initially screened for second hit mutations in PKD2 , followed by screening for PKD1 mutations in the subset of cysts in which second hit PKD2 mutations could not be found. Evidence of loss of a PKD1 allele in these latter cysts led to the proposal the trans-heterozygous somatic mutations involving an allele of the PKD gene other than the one affected by the germline mutation can give rise to cysts. However, trans-heterozygous mutations are unlikely to be sufficient for cyst formation. Compound heterozygous individuals with bilineal inheritance of a PKD1 mutation and a PKD2 mutation show more severe polycystic kidney disease as do trans-heterozygous mice, but the overall severity of phenotype in both man and mouse is within the range observed for individuals with mutations at just one of the gene loci.
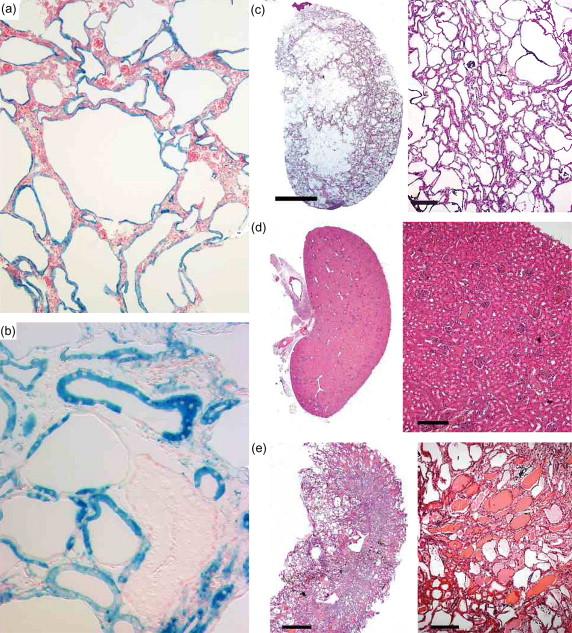
Mouse models have established the causal link between second hits in a single Pkd gene and cyst formation ( Figure 80.3 ). The occurrence of kidney cysts after embryonic day 14.5 in conventional germline knockout animal models of Pkd1 and Pkd2 anticipated a link between homozygous loss of polycystin genes and cyst formation. A novel mouse model of Pkd2 was the first to established a causal relationship between somatic inactivation of the normal allele and cyst formation in adult mice. In this model, a serendipitous modified allele, Pkd2 WS25 , resulted from insertion of a disrupted exon 1 in tandem with the wild type exon1. Pkd2 WS25 expresses functional PC2 but is prone to genomic rearrangement converting it to either a null or true wild type allele. The dosage of this allele correlates with propensity toward cyst formation and the cyst lining cells in adult mouse kidneys did not express PC2. Subsequently, the conditional Cre-lox system has been used to bypass the embryonic lethality of null mice and achieve homozygous inactivation of Pkd genes in a tissue in a selective and temporally controlled manner. These studies confirmed that somatic second hit mutations in either Pkd gene are sufficient for cyst formation. Similar conditional inactivation approaches have been applied to cilia ablation models targeting genes whose products are required for intraflagellar transport ( Kif3a , Ift88 ). The latter showed that somatic loss of intact cilia also results in a polycystic kidney phenotype. The Cre-lox system offers spatial and temporal control of Pkd gene inactivation but has the limitation that inactivation events occur simultaneously in large tracts of cells in either selected nephron segments or along the entire nephron. This is not believed to be the prevalent pattern of inactivation in human disease; in this regard the Pkd2 WS25 model remains unique in that Pkd2 inactivation is thought to occur by stochastic mechanism involving individual cells. Interestingly, the molecular events underlying Pkd2 inactivation in this model are not believed to have a nephron segment specific bias, yet the majority of cysts arise from distal nephron segments. More recent mouse studies suggest that collecting duct cyst growth is particularly sensitive to PC1 dosage. These findings have been part of the rationale for the potential benefit of vassopress-2 receptor antagonists in the treatment of ADPKD.
While somatic second hits are sufficient to initiate cyst formation, an increasing number of additional molecular factors affecting progression of polycystic kidney disease have been discovered using in vivo model systems. These factors include: (1) The critical role of the timing of second hit mutations with respect to developmental stage, (2) the discovery that reduced dosage of PC1, not just complete absence, is sufficient for graded cystic dilation, (3) the potential role of kidney injury in fostering PKD progression, and (4) the finding that loss of polycystins can exert effects on surrounding cells to promote disease progression. Evidence that timing of polycystin inactivation has a profound impact on the rate of cyst growth came from inducible conditional inactivation mouse models permissive for inactivation of Pkd genes either during kidney development or during adult life. Using such a model, Piontek et al. showed that inactivating Pkd1 in the early postnatal period when the mouse kidney is still undergoing active nephrogenesis resulted in rapid progression of polycystic kidney disease whereas inactivation of Pkd1 in the post-developmental adult kidney resulted in very slow progression polycystic kidney disease. A similar finding of rapid cyst growth following early inactivation and slow cyst growth following late inactivation was observed in a polycystic model based on cilia ablation following inactivation of Kif3a . The discovery of the timing of gene inactivation as a contributing factor to disease progression has led to consideration of the possibility that the majority of somatic second hit mutations in human ADPKD as well as the critical rapid growth phase of cysts occur during embryogenesis. Such a mechanism, if confirmed in patient studies, would potentially have significant implications for the timing of the most effective therapy in ADPKD.
The impact of reduced PC1 dosage, as opposed to complete loss, in polycystic kidney disease has emerged over the past several years. Two animal models expressing hypomorphic (i.e., reduced function) alleles of Pkd1 showed progressive cyst formation despite the presence of some residual Pkd1 activity. Similarly, loss of the protein bicaudal C results in reduced PC2 expression and cyst formation through de-repression of inhibitory microRNAs. The significant interplay of gene dosage and polycystic kidney disease has, however, best been defined by studies centered on the genes responsible for isolated polycystic liver disease without kidney cysts (ADPLD)—a disease with indistinguishable clinical features from the polycystic liver disease that occurs in ADPKD patients. Heterozygous loss of function mutations in two genes have been identified so far for this dominantly inherited disease: PRKCSH and SEC63 . PRKCSH encodes the non-catalytic β-subunit of glucosidase II (GIIβ), an N -linked glycan processing (glucose trimming) enzyme in the ER. The SEC63p protein is highly conserved from yeast to man and functions as part of the ER protein translocation machinery to deliver proteins into and through the ER membrane. The functional unit for this process is the multicomponent translocon ( Figure 80.4 ). The activities of the translocon are tightly coordinated with downstream events of protein folding, modification and assembly, thereby providing a direct link between membrane targeting (including the cilial membrane) and protein maturation, and between Sec63p and GIIβ functional pathways. While neither protein is expressed in cilia, the connection of these proteins to polycystic kidney diseases is underscored by the finding that Prkcsh and Sec63 conditional mouse models develop kidney cysts in addition to liver cysts by a two hit mechanism when either gene is inactivated in a tissue selective manner. The severity of the polycystic phenotype in the ADPLD gene knockouts can be worsened by reducing the Pkd1 gene dosage. Increasing Pkd1 dosage by either genetic or pharmacologic means on the other hand eliminated cyst formation. The ability to modulate severity of polycystic kidney disease in these models by manipulating Pkd1 dosage establishes PC1 is the rate limiting component of the cystic pathway in both ADPKD and ADPLD. Interestingly, the same study showed that PC1 dosage also affects the severity of the kidney disease Due to mutations in Pkhd1 , the ARPKD disease gene.
![Figure 80.4, The inter-relationship of isolated polycystic liver disease (ADPLD) with polycystic kidney disease (ADPKD). a : The two known polycystic liver disease gene products, the β subunit of glucosidase-II (GIIβ, indicated as Gluc II, red box) and SEC63 (red oval), are shown in relation to the multicomponent co-translational translocon. GII is an N-linked glycan processing (glucose trimming) enzyme in the ER that plays a major role in regulation of proper folding and maturation of glycoproteins. SEC63 acts as a docking site localizing the ER chaperone BiP to the luminal exit site of the translocon. BiP plays a direct role in gating of the translocation channel and in polypeptide transport via an ATP-dependent reaction that is activated by SEC63. There is a tight interconnection between protein translocation mediated by the multicomponent translocon and protein maturation involving the N-linked glycan processing enzymes, providing a direct link between the functions of the ADPLD gene products. Co-translational maturation events in this pathway include signal peptide cleavage, the transfer and trimming of N -linked glycans including the activity of GII, disulfide bond formation, transmembrane domain integration, chaperone binding and protein folding. SPC, signal peptidase complex; SS, signal sequence; PDI, protein disulfide isomerase; OST, oligosaccharyl transferase; Gluc I, glucosidase I; Gluc II, glucosidase II (α and β subunits); CNX, calnexin; CRT, calreticulin. (Modified and reprinted by permission from Elsevier: ref. [ 185 ], copyright 2003.) b: The critical effect of polycystin-1 (PC1) dosage on polycystic disease severity. Inactivation of the APDLD gene Prkcsh encoding GIIβ in collecting ducts result in kidney cyst formation due to impaired PC1 biogenesis. The severity of cyst formation is markedly increased by further reducing PC1 with Pkd1 +/− background and moderately increased by reducing polycystin-2 (PC2) on the Pkd2 +/− background. Over-expression of PC1 using the Pkd1 F/H -BAC transgene rescues the Prkcsh flox/flox ; Ksp-Cre;Pkd2 +/− cystic phenotype; Pkd2 -BAC has no effect. Ages, P42; scale bar, 2 mm. Figure 80.4, The inter-relationship of isolated polycystic liver disease (ADPLD) with polycystic kidney disease (ADPKD). a : The two known polycystic liver disease gene products, the β subunit of glucosidase-II (GIIβ, indicated as Gluc II, red box) and SEC63 (red oval), are shown in relation to the multicomponent co-translational translocon. GII is an N-linked glycan processing (glucose trimming) enzyme in the ER that plays a major role in regulation of proper folding and maturation of glycoproteins. SEC63 acts as a docking site localizing the ER chaperone BiP to the luminal exit site of the translocon. BiP plays a direct role in gating of the translocation channel and in polypeptide transport via an ATP-dependent reaction that is activated by SEC63. There is a tight interconnection between protein translocation mediated by the multicomponent translocon and protein maturation involving the N-linked glycan processing enzymes, providing a direct link between the functions of the ADPLD gene products. Co-translational maturation events in this pathway include signal peptide cleavage, the transfer and trimming of N -linked glycans including the activity of GII, disulfide bond formation, transmembrane domain integration, chaperone binding and protein folding. SPC, signal peptidase complex; SS, signal sequence; PDI, protein disulfide isomerase; OST, oligosaccharyl transferase; Gluc I, glucosidase I; Gluc II, glucosidase II (α and β subunits); CNX, calnexin; CRT, calreticulin. (Modified and reprinted by permission from Elsevier: ref. [ 185 ], copyright 2003.) b: The critical effect of polycystin-1 (PC1) dosage on polycystic disease severity. Inactivation of the APDLD gene Prkcsh encoding GIIβ in collecting ducts result in kidney cyst formation due to impaired PC1 biogenesis. The severity of cyst formation is markedly increased by further reducing PC1 with Pkd1 +/− background and moderately increased by reducing polycystin-2 (PC2) on the Pkd2 +/− background. Over-expression of PC1 using the Pkd1 F/H -BAC transgene rescues the Prkcsh flox/flox ; Ksp-Cre;Pkd2 +/− cystic phenotype; Pkd2 -BAC has no effect. Ages, P42; scale bar, 2 mm.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/AutosomalDominantPolycysticKidneyDisease/3_3s20B978012381462300080X.jpg)
Acute kidney injury following ischemia, nephrotoxin exposure or reduction of renal mass results in markedly augmented progression of cyst formation in animal models based on Pkd1 , Pkd2 , Kif3a or Ift88 . This is particularly true in adult onset models that otherwise show indolent progression in the absence of injury. These environmental “third hits” have been proposed as an essential part of disease progression in human ADPKD. While it is likely that kidney injury can exacerbate polycystic kidney disease, its contribution to disease progress in human ADPKD is uncertain given that cyst progression also occurs in animal models in the absence of injury. Finally, evidence is accumulating that there are effects promoting cyst formation that are active in the context of the whole kidney organ and may impact cells that have not lost polycystin expression. Perhaps the most intriguing of these was suggested by a unique chimeric animal model produced by aggregation of Pkd1 −/− embryonic stem cells with wild type morulae expressing LacZ as the wild type lineage marker ( Figure 80.3 ). These mice formed kidney cysts commensurate with the degree of Pkd1 −/− chimerism. The cyst lining epithelia were mosaic with both Pkd1 –/– and Pkd1 +/+ epithelial cells present in the early stages of cystogenesis. The Pkd1 –/– cyst cells appeared flat while the Pkd1 +/+ cells were cuboidal. Over time, Pkd1 −/− cells replaced the Pkd1 +/+ cells by inducing apoptosis via the c-Jun N-terminal kinase (JNK)-mediated pathway. These findings suggest the possibility that cells that lose PC1 expression induce cystic degeneration followed by programmed cell death in surrounding wild type tubular cells. Another study based on kidney specific inactivation of Pkd1 demonstrated the importance of the immune response mediated by macrophages in the progression of cyst formation in ADPKD. Orthologous gene models of ADPKD based on either Pkd1 or Pkd2 show infiltration of alternatively activated macrophages into the pericystic space early in the course of the disease. Pharmacologic depletion of the macrophages markedly slows the rate of growth of kidney cysts in these models. And aggregately, these animal models based on genes orthologous to human ADPKD are providing an increasingly complex and complete picture of the molecular genetic basis for disease progression. These studies also serve to emphasize the importance of using in vivo models to validate and refine data obtained from ex vivo tissue culture-based systems--ADPKD is after all a disease of three-dimensional organ structure and is most appropriately studied using in vivo models.
Studies have begun to provide clues as to the genetic determinants of disease progression. Interfamilial variation in ADPKD is strongly influenced by gene locus. PKD1 causes more severe disease than PKD2 , yet the gene products are thought to function in the same genetic and biophysical pathway. Either of two explanations can underlie this observed difference: mutation of PKD1 is inherently a more severe molecular lesion than mutation of PKD2 or the occurrence of the somatic second hit alterations that precipitate cyst formation more readily affect PKD1 than PKD2 . In general, the data support the latter mechanism. Homozygous inactivation of Pkd1 or Pkd2 in the mouse results in similar severity of kidney and pancreatic cystic disease, embryo edema, hemorrhage and embryonic lethality. While PC1 is rate limiting in models of dosage reduction, there is an absolute requirement for functional PC2 for PC1 to function normally. Indeed, the Pkd2 −/− mouse has the additional phenotypic features of randomization of left-right axis formation and defects in heart septum formation, neither of which are seen in Pkd1 −/− mice. Defects of the conotruncus, such as dual outlet right ventricle, have been a variable finding in Pkd1 −/− mice as have defects of bone ossification, and vascular integrity. These phenotypes have not been specifically investigated in Pkd2 −/− mice. In the genetic model organism Caenorhabditis elegans (nematode), mutation to either the Pkd1 ortholog, lov-1 or the Pkd2 ortholog, pkd-2 , results in identical defects in the stereotypical male mating behavior. Doubly mutant lov-1:pkd-2 worms do not show increased phenotypic severity.
The higher population frequency and increased clinical severity of PKD1 disease compared to PKD2 may be explained in large part by a higher frequency, respectively, of germline and somatic mutation affecting PKD1 . This in turn is certain to be influenced by the four-fold larger coding sequence of PKD1 compared to PKD2 . The recent recognition that PC1 is the rate limiting component of the polycystin complex coupled with the significantly higher rate of pathogenic non-synonymous amino acid substitution mutations in PC1 compared to PC2, further suggests that a broader range of mutations in PC1, than in PC2, will result in ADPKD. The strongest support for this theory that the frequency of mutation is primarily responsible for the locus specific differences in disease severity comes from the CRISP. At baseline, PKD1 kidneys had more cysts and were larger than PKD2 kidneys. However, while the absolute changes in kidney volume were greater for PKD1 (74.9 mL/year) than for PKD2 (32.2 mL/year), the relative rates of growth were not significantly different for the two genotypes (5.68% per year vs. 4.82% per year). This suggests that cyst initiation, but not cyst enlargement, is modulated by the disease gene; PKD1 is more prone to second hits than PKD2 , but there is no inherent difference in cyst growth potential once second hits occur in either gene.
Most of the several hundred PKD1 mutations described to date are unique to individual families. This suggests that each mutation essentially arose as a de novo event that was fixed in the population since it confers little or no significant reproductive disadvantage. The de novo mutation rate for PKD1 is estimated to range from 1.8×10 −5 to 6.9×10 −5 per generation. Studies in heterozygous mouse models support these theories. Pkd2 +/− mice uniformly show loss of PC2 immunoreactivity in cyst linings suggesting that homozygous somatic loss is the underlying cystogenic event; cyst linings in Pkd1 +/− mice uniformly showed presence of PC2 immunoreactivity allowing for the inference that it was PC1 that was lost in these cysts. In compound heterozygous Pkd1 +/− :Pkd2 +/− animals, 160 of 171 cysts (93%) showed uniformly positive staining for PC2, 8 (5%) were negative for PC2 and 3 (2%) could not be evaluated. This suggests that in compound heterozygous mice, Pkd1 was far more often the target of somatic loss in vivo .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here