Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The autonomic nervous system extends to every organ in the human body, creating a dizzying array of central and peripheral nerves, nuclei, ganglia, and neurotransmitters that often defy conventional attempts at learning through memorization. Although colloquial understanding of the autonomic nervous system is discovered in the medical students’ mantra of “fight or flight,” true comprehension of the system often occurs only after disease-specific disturbances of autonomic function are encountered in clinical practice. The autonomic nervous system modulates blood pressure, heart rate, thermoregulation, motility of the gastrointestinal system, micturition, pupillary function, and salivary gland secretion, among other things ( ). Dysfunction of one, or all, of these processes may occur in neuromuscular disorders that affect the autonomic nervous system. This chapter briefly reviews the anatomic structure and clinical implications of autonomic dysfunction, the evaluation of the autonomic nervous system, neuromuscular diseases that result in autonomic disturbances, and treatment options in patients with dysautonomia. A graphic overview of the autonomic nervous system is shown in Fig. 5.1 .
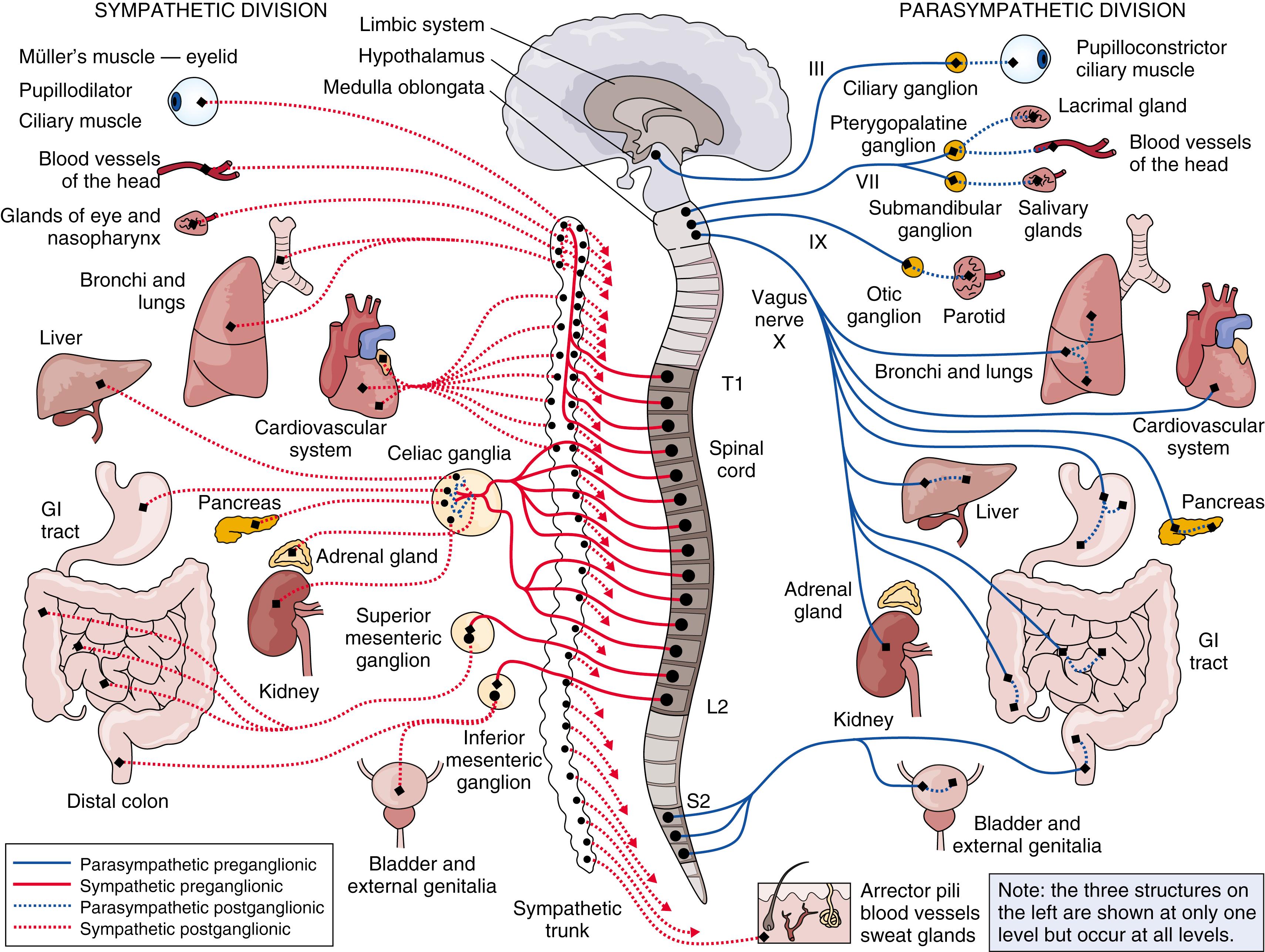
The sympathetic nervous system is composed of cells located in the lateral horn of the spinal cord (thoracic to lumbar levels), and for this reason it has been referred to as the thoracolumbar system . The cell bodies of sympathetic preganglionic neurons are located from T1 to L3 in the intermediolateral columns ( ). The preganglionic neurons project ipsilaterally out the white rami to the paravertebral chain, where they synapse on postganglionic neurons in adjacent ganglia. There is a rough anatomic distribution to the ganglia, with the upper thoracic ganglia projecting to the head and the lumbar ganglia projecting to the lower extremities and lower trunk. The axons of the preganglionic sympathetic neurons are relatively short because of the close proximity of the paravertebral ganglia, and they use acetylcholine as their neurotransmitter. In contrast, the postganglionic neurons have longer axons that stimulate directly on their target end-organ through the neurotransmitter norepinephrine. One notable exception to this rule is the innervation to the postganglionic sudomotor system (i.e., the sweat glands), which also uses acetylcholine as the postganglionic neurotransmitter ( ).
Activation of the sympathetic nervous system facilitates energy expenditure in urgent situations. Bronchial dilation occurs to improve respiratory capacity. There is constriction of the arrector pili muscles, resulting in hairs standing on end. The piloerection response is seen prominently in households cohabitated by feline and canine species as the domesticated cat attempts to frighten the dog by appearing larger. Sphincters of the anus and bladder tighten (to prevent fluid release), with simultaneous relaxation of the detrusor muscle (to prevent fluid expulsion). Activation of the cardiovascular system results in increased heart rate and contractility, and vasodilation occurs in blood vessels to the lungs, heart, and striated muscle. Conversely, vasoconstriction occurs in the skin and gastrointestinal tract to improve blood flow to the other organ systems. During sympathetic activation, the pupil widens as a result of constriction of the dilator muscle, the tarsal muscle of the eyelid contracts, elevating the eyelid, and tightening of the orbital muscle results in protrusion of the eyeball. These ocular adjustments result in an increase in visual field size but are well suited to stereotyping for cartoonists who create a caricature of a face with retracted eyelids and bulging eyes.
The parasympathetic nervous system is composed of cells located in the brain stem and the sacral region of the spinal cord, and for this reason it has been referred to as the craniosacral system. The cranial preganglionic neurons project to the cranial nerves (CNs) with autonomic activity: III, VII, IX, and X. Unlike the sympathetic nervous system, the parasympathetic postganglionic neurons are located near end-organ systems, resulting in long preganglionic axons and relatively short postganglionic axons ( ).
The brain stem nuclei involved in CN parasympathetic innervation include the following. (1) CN III: Preganglionic neurons from the Edinger-Westphal nucleus extend down the oculomotor nerve and synapse at the orbital ciliary ganglion, where postganglionic neurons extend to the ciliary muscles and iris, resulting in accommodation and pupillary constriction. (2) CN VII: Pontine preganglionic fibers from the superior salivatory nucleus extend down the facial nerve to the pterygopalatine ganglion, with postganglionic fibers extending to the lacrimal gland (tear production) and the cranial vasculature (resulting in vasodilation). Pontine preganglionic fibers also extend to the submandibular ganglion, with postganglionic fibers continuing on to the salivary glands (resulting in salivation). (3) CN IX: Medullary preganglionic fibers from the inferior salivatory nucleus extend down the glossopharyngeal nerve to the otic ganglion, where postganglionic fibers continue on to the parotid gland (resulting in salivation). (4) CN X: By far the largest parasympathetic output, the preganglionic fibers from the dorsal motor nucleus of the vagus and the ventrolateral portion of the nucleus ambiguus extend down the vagus nerve to various ganglia. The fibers extending from the dorsal motor nucleus of the vagus provide input to the gastrointestinal tract (enteric system, described in more detail later), the respiratory tract, and some cardiac input, whereas fibers extending from the nucleus ambiguus extend primarily to the heart. The primary response to vagal activation is cardiac inhibition, visceromotor activation, and salivation ( ).
Sacral parasympathetic output begins in the lateral gray matter of segments S2–S3, with preganglionic fibers extending down the ventral roots to the splanchnic nerves. The parasympathetic fibers extend to the colon, bladder, and sexual organs. The Onuf nucleus innervates the rectal and urethral sphincters and the pelvic floor. Selective denervation of the Onuf nucleus in Parkinson disease enables differentiation (in some cases) from multiple system atrophy, although this has not been found to be widely reproduced ( ).
The overall effect achieved with activation of the sacral parasympathetic system results is urination (relaxation of the bladder sphincter with simultaneous contraction of the detrusor muscle to facilitate micturition), defecation (relaxation of the rectal sphincter with increased peristalsis), and penile erection (ejaculation is mediated via sympathetic innervation). Unlike the sympathetic nervous system, the parasympathetic nervous system uses acetylcholine for both preganglionic and postganglionic neural transmission.
Although it was historically subsumed under the auspices of the parasympathetic nervous system, recent evidence suggests that the enteric nervous system is a discrete component of the autonomic nervous system. The enteric system is made up of two interconnected ganglia influenced by both sympathetic and parasympathetic pathways. The first ganglia are located between the longitudinal and circular muscle layers of the gastrointestinal tract, known as the myenteric or Auerbach plexus, and project to both external and internal muscle layers. The second ganglia are located in the submucosal layer, known as the submucosal or Meissner plexus, and project to the mucosal layer. Neurons from both the myenteric plexus and the submucosal plexus innervate nearby tissue as well as anteriorly and posteriorly along adjacent regions of the gastrointestinal tract. The enteric nervous system controls peristalsis, secretion, and absorption along the gastrointestinal tract ( ).
A number of testing techniques are available for clinical evaluation of the autonomic nervous system; far more are used for research investigation. This chapter describes the most commonly used clinical tests but does not provide a comprehensive review. For additional details on evaluation of the autonomic nervous system, more comprehensive references are suggested ( ). The overall utility of autonomic testing has been reviewed and given a level B recommendation (probably effective) by an expert panel for the evaluation of autonomic neuropathy and a level C recommendation (possibly effective) in the evaluation of distal small fiber neuropathy ( ).
Autonomic testing provides functional information on the parasympathetic, sympathetic adrenergic, and sympathetic cholinergic systems. Any patient presenting with suspected dysfunction of the autonomic nervous system resulting in orthostatic hypotension, syncope, postural tachycardia syndrome, peripheral neuropathy, or thermoregulatory abnormalities is a candidate for tests of autonomic function. Other common symptoms that suggest autonomic dysfunction include postural dizziness, visual graying in the upright position, impaired cognition, coat hanger headache (due to ischemia of the strap muscles of the neck and shoulders), lightheadedness, platypnea (shortness of breath in the upright position), weakness, and lethargy ( ).
Adequate preparation for autonomic testing is critical to obtain reliable and reproducible results. All medications that affect autonomic function should be discontinued for five half-lives before testing, if clinically appropriate, with guidance from the patient’s treating physician as necessary. All patients referred for autonomic evaluation should avoid caffeine and nicotine on the day of testing. Food intake should be kept to a minimum, with no food 3 hours before testing.
Medications that commonly interfere with testing include anticholinergics (antihistamines, antidepressants, decongestants), antihypertensives, volume expanders (e.g., fludrocortisone), and volume contractors (e.g., diuretics) and should be discontinued for five half-lives, if possible. Analgesics (opioids or over-the-counter) and items that cause structural changes to blood flow (e.g., compression stockings or corsets) should be avoided the day of testing ( ).
A number of terms are frequently used when describing symptoms related to autonomic dysfunction, such as orthostatic hypotension , orthostatic intolerance , and postural tachycardia . A brief overview of these terms is provided in Table 5.1 .
| Term | Definition |
|---|---|
| Orthostatic intolerance | Symptoms that develop in the upright position that are relieved by recumbancy. No physiologic measurements required. |
| Orthostatic hypotension | A sustained drop in systolic blood pressure of ≥20 mm Hg, or diastolic blood pressure of ≥10 mm Hg within 3 minutes of moving from the supine to standing position or head up tilt to ≥60 degrees. |
| Postural tachycardia | A sustained increase in pulse of ≥30 beats per minute within the first 10 minutes of moving from the supine to standing position or head up tilt to ≥60 degrees. |
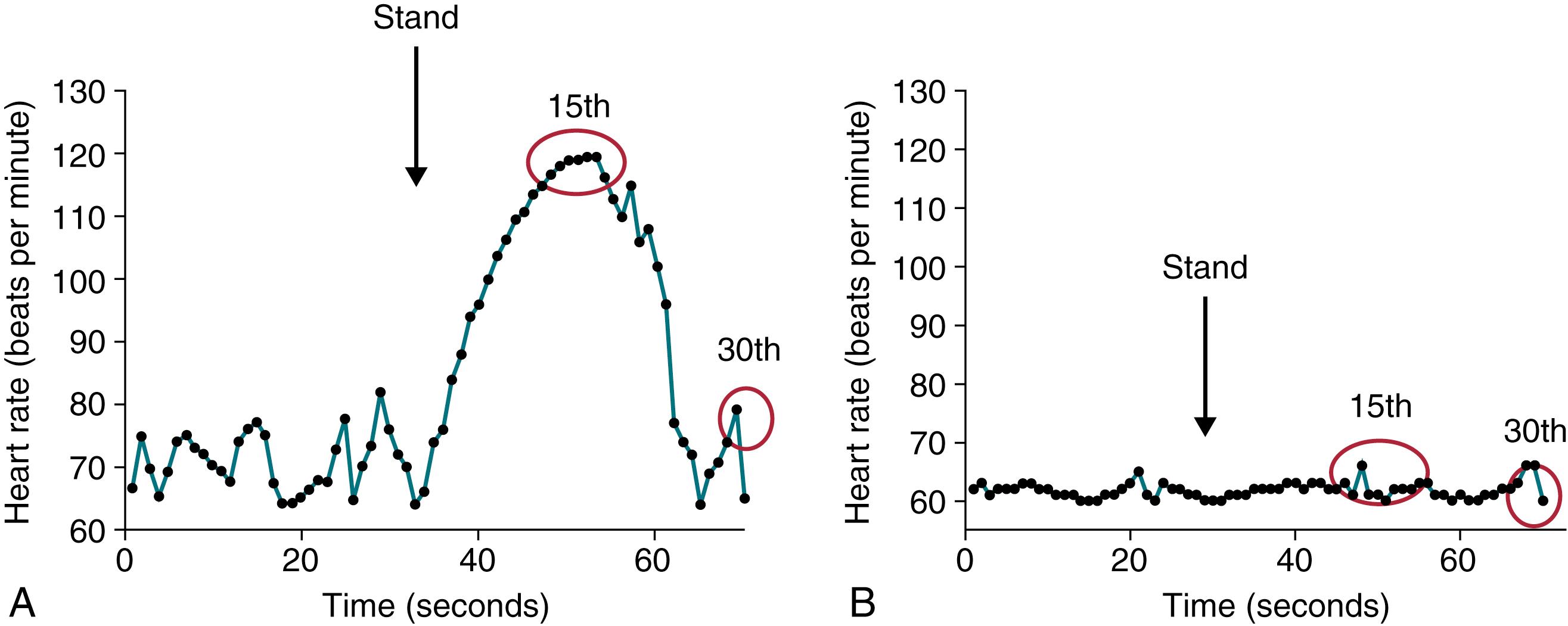
The transition from supine to standing causes hemodynamic stress on the cardiovascular system as approximately 500 to 1000 mL of blood moves from the central to the peripheral vasculature ( ). The immediate response to orthostatic stress occurs in the first 30 seconds, beginning with a rapid decrease in blood pressure and systemic resistance, followed by a rapid increase in peripheral vascular resistance, cardioacceleration, and blood pressure overshoot ( ). These dynamic changes allow two tests of autonomic function to be determined: (1) orthostatic vital signs and (2) the 30:15 ratio.
To measure orthostatic vital signs, blood pressure and heart rate should be measured in the supine position after an adequate period of recumbency (typically, at least 5 minutes). Patients then move to the standing position, where blood pressure and heart rate are monitored again after 3 minutes. A diagnosis of orthostatic hypotension is made when a decrease in systolic blood pressure of 20 mm Hg or a decrease in diastolic blood pressure of 10 mm Hg occurs from the supine to the standing position ( ). There is a frequent misunderstanding among practitioners that an increase in pulse of 25 points or greater leads to a diagnosis of “orthostatic by pulse.” In the appropriate clinical setting, this finding suggests that the patient may be hypovolemic, but there is appropriate tachycardia preventing orthostatic hypotension from occurring. However, in a normovolemic patient, a sustained increase in pulse of 30 points or greater (to a maximum of 120 beats per minute) from the supine to the standing position within 10 minutes results in a diagnosis of postural tachycardia syndrome (if the appropriate symptoms occur with standing and have been present for a sufficient duration of time) ( ; ).
The 30:15 ratio is a measure of parasympathetic function that occurs when subjects move from the supine to the standing position. The immediate response to orthostatic stress is tachycardia, typically maximal at the 15th heartbeat after standing, followed by bradycardia, most pronounced at the 30th heartbeat after standing. The ratio of the RR interval at beat 30 to the RR interval at beat 15, called the 30:15 ratio, is an index of cardiovagal function ( Fig. 5.2 ) ( ).
The tilt-table is an important tool in the evaluation of the autonomic nervous system. When a subject is tilted to an angle of 60 to 70 degrees, the orthostatic stress that occurs is similar to that of standing, but the muscles of the legs are relaxed. The “muscle pump” effect that occurs with the legs during standing is a powerful countermaneuver to prevent orthostatic hypotension ( ).
Testing requires a period of recumbency, typically 20 minutes, followed by a gradual rise to a head-up tilt angle of 60 to 70 degrees. Testing for many laboratories extends to 45 minutes for adequate assessment of autonomic function ( ). Testing combines the use of oscillometric blood pressure recording at regular intervals and noninvasive beat-to-beat blood pressure recordings ( ). A diagnosis of orthostatic hypotension is made when a sustained decrease in systolic blood pressure of 20 mm Hg or a decrease in diastolic blood pressure of 10 mm Hg occurs from the supine to the upright position within the first 3 minutes of head-up tilt. Decreases in blood pressure after 3 minutes have been described as delayed orthostatic hypotension ( ; ). A sustained increase in pulse of 30 points or greater (to a maximum of 120 beats per minute) from the supine to the standing position within 10 minutes, in a normovolemic patient, results in a diagnosis of postural tachycardia syndrome ( ).
The heart rate varies during inspiration and expiration and has been described as the sinus arrhythmia. The maximal heart rate variation with respiration occurs at a breathing rate of 5 to 10 breaths per minute. Patients are provided with a visual or auditory cue to regulate their breathing in combination with continuous electrocardiographic monitoring. Approximately 1 minute of respiration is recorded, and the average variation between the maximal and minimal heart rates is described ( Fig. 5.3 ). This test provides a measure of parasympathetic function and can be compared with age- and sex-matched normative values ( ).

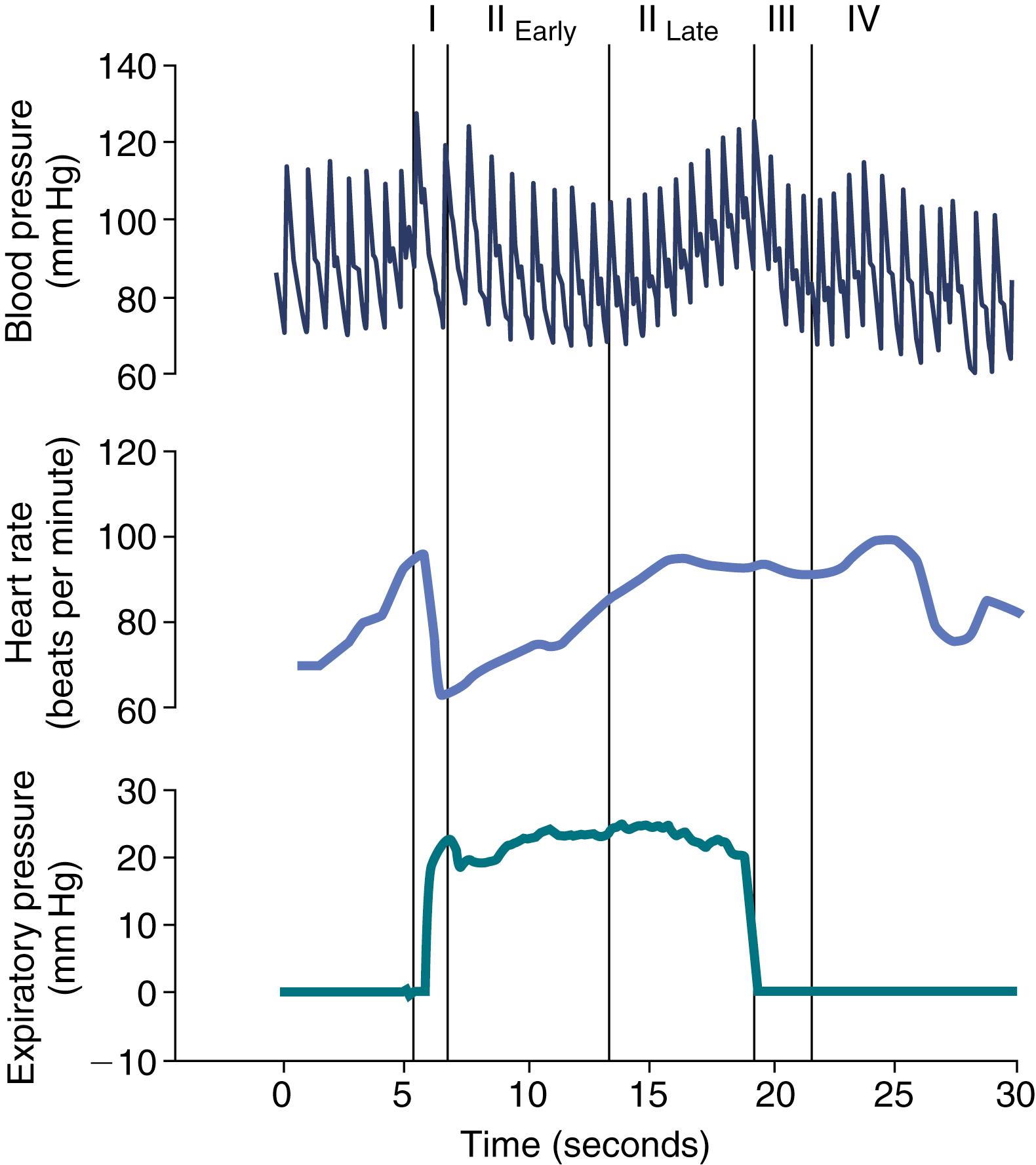
The Valsalva maneuver is a relatively simple test to perform, but it results in rapid and complex hemodynamic shifts that require continuous electrocardiographic and beat-to-beat blood pressure recordings to measure. The subject, in a supine position, blows into a tube with a pressure of approximately 40 mm Hg for 15 seconds ( ). The procedure is similar to blowing up a stiff balloon. The breathing tube should have an air leak to prevent glottic closure.
The Valsalva maneuver has four parts, as seen in Fig. 5.4 . Phase 1 occurs during the onset of exhalation with straining against resistance. The increase in intrathoracic pressure causes compression of the great vessels and an increase in blood pressure. Phase 2 of the Valsalva maneuver begins with decreased venous return (because of increased intrathoracic pressure) and decreased stroke volume, cardiac output, and blood pressure (phase 2 early), followed by sympathetically mediated peripheral vasoconstriction and an increase in blood pressure and heart rate (phase 2 late). Phase 3 occurs with cessation of forced exhalation, resulting in decreased intrathoracic pressure and a transient decrease in blood pressure. Phase 4 may extend for several minutes from the end of phase 3 and leads to an increase in blood pressure over baseline levels secondary to increases in stroke volume and cardiac output with peripheral vasoconstriction ( ).
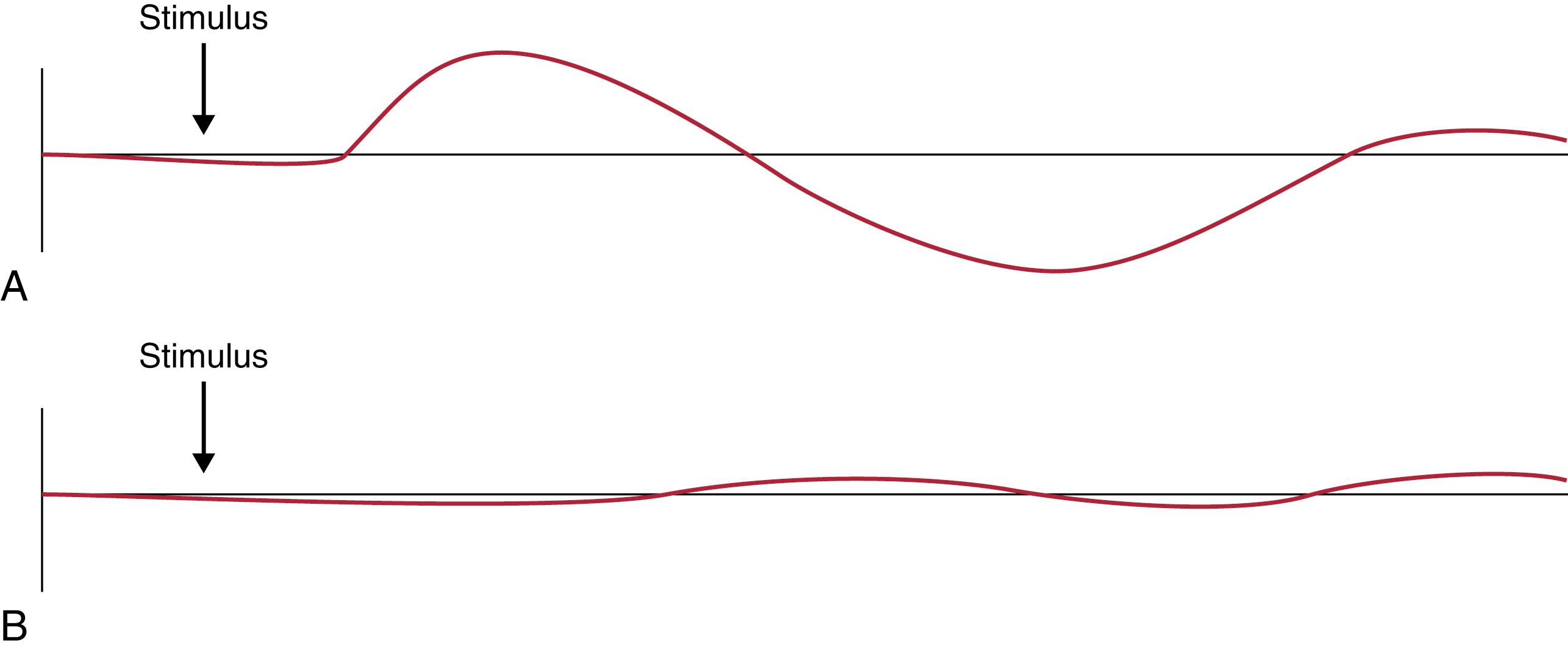
The sympathetic skin response detects electrical potential changes between the dorsal and ventral surfaces of the hands and feet. A stimulus, such as an inspiratory gasp or electric shock, results in electrical potential changes to the palms of the hands and soles of the feet. This test can be easily performed with any electromyogram or evoked potential device. A typical example in a healthy individual and a patient with neuropathy is shown in Fig. 5.5 . The sympathetic skin response is a surrogate measure of sudomotor function but does not actually measure sweat production ( ). Attempts to correlate response amplitude and latency with disease severity have met with mixed results. The absence of a sympathetic skin response is usually considered abnormal, although age-related changes occur in the lower extremities of individuals older than 50 years ( ). In addition, some central disorders, such as stroke, may result in loss of the sympathetic skin response ( ). More recent efforts to improve upon measurements of sweat function through use of an electrochemical skin conductance device (Sudoscan) have provided very mixed results ( ). At the present time, electrochemical skin conductance is not recommended for clinical use.
The thermoregulatory sweat test detects the host’s ability to generate sweat in response to an increase in core temperature. The subject is placed in a chamber where the external temperature is increased to a degree sufficient to increase the core temperature by 1°C to 1.5°C ( ). An indicator dye, typically alizarin red or iodinated corn starch, covers the individual and changes color in the presence of sweat. Photographic mapping of sweat patterns can determine patterns of abnormalities. A normal response is seen with symmetrical sweat production over the entire body (there is individual variation in maximal sweat production), as shown in Fig. 5.6 . Specific abnormalities can be seen in diseases of both the central nervous system and the peripheral nervous system because the thermoregulatory sweat test measures both the preganglionic and postganglionic response to an increase in core temperature. An abnormal response may suggest a specific diagnosis through a distribution of sweat loss but cannot differentiate a preganglionic lesion from a postganglionic lesion ( ).
Quantitative sudomotor axon reflex testing provides a measure of postganglionic sweat production. Activation of local sudomotor fibers through iontophoresis of a cholinergic agonist (typically, acetylcholine) results in the direct stimulation of local sweat glands. However, a local axon reflex occurs when an antidromic response is generated, travels to a more proximal nerve branch point, and then travels orthodromically to neighboring sweat glands that were not directly activated by the cholinergic agonist ( ). A small capsule over the skin is used to detect changes in humidity and provide a quantifiable measure of postganglionic sweat production. Well-established normative values have been published, and abnormalities can be seen early in distal small fiber neuropathies of many causes ( ).
Tests of sympathetic adrenergic, sympathetic cholinergic, and parasympathetic function are shown in Table 5.2 . Many tests fall under both sympathetic adrenergic and parasympathetic categories because of their effects on both heart rate and blood pressure. A large number of additional tests can be performed by other subspecialty groups in ophthalmology, gastroenterology, urology, cardiology, and others. For a complete review of available tests, the reader is referred to a number of excellent texts devoted entirely to these topics ( ; ).
| Sympathetic Adrenergic | Parasympathetic | Sympathetic Cholinergic |
|---|---|---|
| Blood pressure response standing | Heart rate response to standing (30:15 ratio) | Sympathetic skin response |
| Blood pressure response to tilt table testing | Heart rate response to tilt table testing | Thermoregulatory sweat testing |
| Valsalva maneuver: phase 2 blood pressure recovery and phase 4 overshoot | Valsalva heart rate ratio | Quantitative sudomotor axon reflex testing |
| Isometric exercise: blood pressure response a | Isometric exercise: heart rate response a | Silicone impression testing a |
| Cold pressor test: blood pressure response a | Cold pressor test: heart rate response a | Quantitative direct and indirect axon reflex testing a |
| Plasma catecholamine levels a | Deep breathing: heart rate response | Acetylcholine sweat-spot a |
One of the challenges inherent in autonomic testing is the differentiation of peripheral and central disorders of autonomic regulation, a problem compounded by the frequently overlapping test results. Most autonomic tests cannot differentiate between peripheral and central causes of autonomic disturbance in isolation; the results are usually combined with a detailed history and examination by a specialist to narrow the differential diagnosis. A few tests are more likely to differentiate central from peripheral autonomic disorders and are described in Table 5.3 .
| Test | Central Autonomic Disorder | Peripheral Autonomic Disorder |
|---|---|---|
| Plasma catecholamines a | Normal or slightly elevated (150–300 pg/mL plasma norepinephrine) | Low (≤100 pg/mL of plasma norepinephrine) |
| Thermoregulatory sweat test b | Abnormal | Abnormal |
| Quantitative sudomotor axon reflex test b , c | Normal | Abnormal |
a A minority (up to 20%) of patients will have plasma catecholamine levels that fall between the two ranges, resulting in diagnostic ambiguity.
b Thermoregulatory sweat testing and postganglionic tests of sudomotor function can be combined to identify central or peripheral disorders. If both tests are abnormal, it is a peripheral disorder; if only the thermoregulatory sweat test is abnormal, it is central.
c Any postganglionic test of sudomotor function can be used (Quantitative sudomotor axon reflex testing (QSART), silicone impressions, acetylcholine sweat-spot testing or quantitative direct and indirect reflex testing (QDIRT)).
Diabetic autonomic neuropathy is the most common form of autonomic neuropathy in the developed world ( ). It is a system-wide disorder, affecting all parts of the autonomic nervous system ( ). The incidence of this form of neuropathy increases with age, with duration of disease, and with chronic hyperglycemia, and it is almost universally accompanied by features of concomitant distal sensorimotor polyneuropathy ( ). The symptoms of autonomic neuropathy generally occur well after the onset of the endocrinologic manifestations of diabetes mellitus. However, evidence of subclinical autonomic dysfunction may be seen as early as 1 year after initial diagnosis ( ). The prevalence of diabetic autonomic neuropathy is dependent on the criteria used for diagnosis and the population studied. A population-based study in the United States found that symptomatic diabetic autonomic neuropathy affected 5.5% of patients with diabetes mellitus ( ). In a multicenter study in Europe involving nearly 1200 patients, there was evidence of abnormalities in the results of tests of autonomic function in 25.3% of patients with type 1 diabetes mellitus and in 34.3% of patients with type 2 diabetes mellitus ( ).
Autonomic neuropathy is associated with an increase in overall mortality and with a higher likelihood of sudden death, especially when cardiovascular autonomic neuropathy is present ( ). In a prospective study ( ), 56% of patients with diabetic cardiovascular autonomic neuropathy were dead at 5 years, and half of them died unexpectedly and possibly of causes related to underlying dysautonomia.
There are multiple hypotheses as to the pathogenesis of diabetic autonomic neuropathy, and the etiology is likely multifactorial. A metabolic injury to nerve fibers secondary to hyperglycemia, neurovascular insufficiency, autoimmune damage, and a neurohormonal growth factor deficiency are among the processes implicated ( ). As with most neuropathies, diabetic autonomic neuropathy is a length-dependent process and clinical manifestations are first seen in processes affecting longer nerves. Accordingly, the vagus nerve (the longest of the autonomic nerves) is affected early in the disease course. Because this nerve is responsible for approximately 75% of parasympathetic function, symptoms may occur from the beginning and may be widespread ( ). The clinical features of diabetic autonomic neuropathy are reflective of the multiple organ systems that it affects. Accordingly, these are discussed on a system-by-system basis.
Cardiovascular autonomic neuropathy results from damage to autonomic nerves that innervate the heart and blood vessels, thereby leading to abnormalities in heart rate control and peripheral vascular dynamics. The prevalence is estimated to be approximately 17% of patients with type 1 diabetes mellitus and 22% with type 2 diabetes mellitus ( ). The 5-year mortality rate among diabetic patients with symptomatic cardiovascular autonomic neuropathy is estimated to be five times higher than that of those without cardiovascular autonomic neuropathy ( ). The manifestations of cardiovascular autonomic neuropathy are multiple and reflect both parasympathetic and sympathetic dysfunction.
Exercise intolerance is frequently seen in patients with cardiovascular autonomic neuropathy. Early in the course of the disease, an increase in resting heart rate is often observed as a result of vagal neuropathy and unopposed sympathetic activity and may be the first sign of autonomic neuropathy. As the disease progresses, a fixed heart rate is observed ( ). Eventually, reduced response in heart rate and blood pressure during physical activity leads to decreased cardiac output, reflecting impairment of both parasympathetic and sympathetic responses that typically augment cardiac output during exercise.
Orthostatic hypotension is also a typical feature of cardiovascular autonomic neuropathy and is defined as a decrease in blood pressure of greater than 20 mm Hg systolic or greater than 10 mm Hg diastolic in response to postural change ( ). Orthostatic hypotension occurs secondary to damage to efferent sympathetic vasomotor fibers, predominantly in the splanchnic vasculature, reduced cardiac output, and a reduction in the normal increase in plasma norepinephrine ( ). Patients typically present with symptoms of lightheadedness or presyncope on change of position. Other symptoms may include nonspecific dizziness, weakness, fatigue, visual blurring, and neck pain ( ). Many patients may remain asymptomatic despite significant decreases in blood pressure on change of position ( ).
Other symptoms seen in cardiovascular autonomic neuropathy are intraoperative cardiovascular lability and sudden death, possibly as a result of malignant arrhythmogenesis ( ). In addition, silent myocardial infarction, likely as a result of cardiac denervation, is estimated to occur in approximately one third of patients with diabetes mellitus ( ).
Gastrointestinal symptoms are relatively common in patients with diabetes mellitus and are often caused by underlying autonomic neuropathy. The prevalence of gastrointestinal symptoms was reported to be as high as 76% in patients with type 2 diabetes mellitus in one series ( ). Symptoms are reflective of widespread disease and include esophageal dysfunction secondary to vagal neuropathy. This may manifest as heartburn or dysphagia for solids. Gastroparesis diabeticorum is seen in up to 50% of patients with diabetes mellitus. This can produce such symptoms as early satiety, anorexia, postprandial nausea and vomiting, and epigastric discomfort. In addition, delayed gastric emptying interferes with nutrient delivery to the small bowel and can have far-reaching implications ( ). Diarrhea, which is typically nocturnal, watery, and profuse, is frequently seen in those with diabetes. This may alternate with constipation as a result of gut dysmotility and is estimated to occur in approximately 60% of patients with diabetes ( ). Fecal incontinence may also occur as a result of anal sphincter incompetence or reduced rectal sensation.
Neurogenic bladder is seen in approximately one third to one half of patients with diabetes mellitus. Early on, deafferentation leads to impaired bladder sensation and an increased threshold for initiating the micturition reflex. This leads to an increase in bladder capacity with urinary retention. Later, parasympathetic dysfunction results in reduced detrusor activity with urinary hesitancy and incomplete emptying. This causes further retention and overflow incontinence, as well as predisposing patients to urinary tract infections ( ).
Erectile dysfunction may actually be the earliest sign of diabetic autonomic neuropathy and is seen in 35% to 75% of men with diabetes mellitus ( ). The pathogenesis is likely multifactorial and due to neuropathy (leading to reduced cholinergic activation), vascular, metabolic, endocrinologic, pharmacologic, and psychological factors ( ). In addition to erectile dysfunction, ejaculatory failure and retrograde ejaculation may be seen ( ).
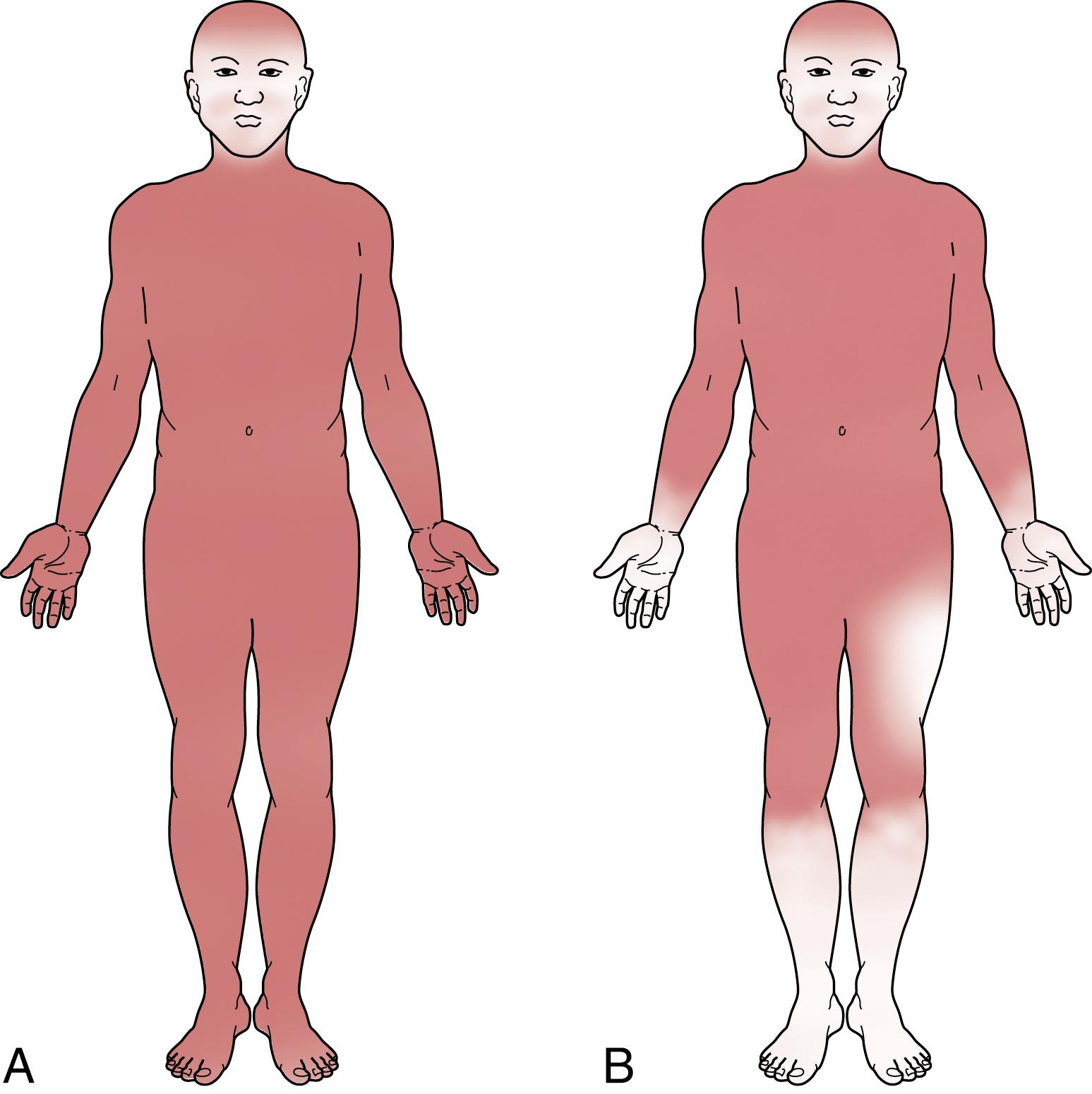
Sudomotor dysfunction is a common feature of diabetic autonomic neuropathy. It typically manifests first as anhidrosis of the extremities in a stocking-glove distribution, conforming to the length dependency of the neuropathy. This progresses to involve the upper aspects of the limbs, the anterior abdomen, and the top of the head and may ultimately result in global anhidrosis ( ). Hyperhidrosis of the trunk may be seen early in the disease as a compensatory phenomenon. Gustatory sweating (abnormal production of sweat over the face, head, neck, shoulders, and chest after eating) is also occasionally observed ( ). The gustatory pattern of sweating is believed to occur secondary to cervical sympathetic denervation and aberrant reinnervation ( ).
Other manifestations of diabetic autonomic neuropathy include reduced or absent pupillary response to light and lacrimal gland dysfunction (resulting in dry eyes). Although impaired awareness of hypoglycemia as a result of blunting of the normal catecholamine surge has long been believed to be secondary to autonomic neuropathy in patients with diabetes, the literature on this topic is controversial. Some studies found that peripheral neuropathy was a risk factor for episodes of severe hypoglycemia ( ; ), although others looking specifically at the presence of autonomic neuropathy found that neuropathy produced at best a modest increase in the risk of severe hypoglycemia because of a reduction in counter-regulatory catecholamine responses ( ; ).
Overall, amyloidosis is a rare disease ( ). There are multiple forms of amyloidosis, including primary (AL) amyloidosis (typically caused by a plasma cell dyscrasia), secondary (AA) amyloidosis (typically caused by an underlying chronic inflammatory condition), and hereditary amyloidosis ( ). Of these, dysautonomia is seen in the primary and hereditary forms ( ). Neuropathy is present in 20% to 50% of patients with amyloidosis. The typical presentation is that of a distal painful small-fiber sensory neuropathy ( ; ). Autonomic neuropathy is also frequently observed and involves both sympathetic and parasympathetic nerve fibers ( ). Symptoms of autonomic dysfunction typically manifest early in the disease process and may be the presenting symptom in approximately 40% of cases ( ). These include orthostatic hypotension, heart block, anhidrosis, erectile dysfunction, gastroparesis, pupillary abnormalities, constipation, and diarrhea.
The pathogenesis of the neuropathy in amyloidosis involves the deposition of insoluble beta-fibrillar proteins in the epineurium, perineurium, and endoneurium, as well as in the vasa nervorum, with resultant infiltrative, inflammatory, toxic, or ischemic damage to axons ( ).
Primary amyloidosis is most commonly of the amyloid light chain (AL) type and is a plasma cell dyscrasia whereby monoclonal immunoglobulin light chains are deposited as amyloid ( ). Patients typically present in the sixth or seventh decade. The survival is poor (median range, 13–35 months), despite treatment with prednisone and melphalan ( ).
There are many forms of hereditary amyloidosis, and the most common cause is mutant production of transthyretin protein ( ). Inheritance is autosomal dominant, and the disease usually presents in the third to fifth decade ( ). Autonomic symptoms are usually prominent. Survival is better than in the primary form of amyloidosis, but death generally occurs 5 to 15 years after diagnosis. There are reports of increased survival with orthotopic liver transplantation, although the autonomic symptoms typically do not improve ( ). The exciting development of novel therapies that target transthyretin (TTR) formation or TTR deposition for hereditary amyloidosis (hATTR) is one of the first major breakthroughs in treating a progressive neuropathy. The TTR therapies target different stages of protein formation or misfolding, with several now available for clinical use. The treatments for TTR amyloidosis generally fall into one of two categories: (1) they stabilize TTR amyloid or (2) they knock down TTR through a reduction in RNA degradation or gene silencing (although several novel therapies with additional mechanisms of action are in development) ( ).
Tafamidis is a drug that binds to TTR at the thyroxine binding sites and stabilizes the tetramer of the TTR transport protein, slowing the breakdown of the TTR into monomers and slowing the development of amyloid deposition in tissues. Tafamidis has proven efficacy in the treatment of TTR cardiomyopathy and has received Food and Drug Administration (FDA) approval for a reduction in cardiovascular mortality and hospitalizations related to cardiomyopathy ( ). Tafamidis also has shown benefits in the treatment of early polyneuropathy through slowing of disease progression; however, it has not demonstrated efficacy in moderate- to late-stage polyneuropathy and does not have FDA approval for this treatment. The results for tafamidis do vary across TTR mutation subtypes, and therefore it is difficult to make general recommendations regarding therapy.
Diflunisal is a nonsteroidal anti-inflammatory drug (NSAID) that stabilizes TTR tetramers and reduces the rate of disease progression. Diflunisal has shown a reduction in the rate of neuropathy progression over 2 years compared to placebo, although the adverse events related to chronic NSAID use have raised concerns about chronic treatment ( ). Diflunisal does not currently have FDA approval for treatment of hATTR.
Inotersen is an antisense oligonucleotide therapy that reduces the TTR amyloid RNA and protein levels. It has been FDA approved for the treatment of early-stage polyneuropathy in individuals with hATTR ( ). In a longitudinal study of 172 patients treated with either inotersen or placebo, there was a significant an improvement in symptoms and signs of neuropathy progression in the inotersen-treated group ( ).
Patisiran is a double-stranded small interfering RNA (ribonuclein acid) that targets TTR. Patisiran has been FDA approved for the treatment of polyneuropathy in hATTR. In a study of 225 patients with hATTR polyneuropathy, there was a significant improvement in symptoms and signs of neuropathy development with patisiran compared to placebo after 18 months of treatment ( ).
Direct comparisons between treatment for hATTR polyneuropathy have not been conducted; therefore, specific treatment recommendations are limited ( ). Treatment recommendations will largely vary by availability, cost, formulation (oral vs. intravenous), side effect profiles, and comorbidities. However, despite the gaps in knowledge, the development of therapies for hATTR polyneuropathy is an enormous scientific and clinical advance.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here