Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intracerebral vascular malformations constitute an important cause of intracranial hemorrhage in younger patients. The spectrum of vascular malformations ranges from sporadic congenital lesions such as brain arteriovenous malformations (AVMs) to genetically determined familial disorders that may progress over time.
Arterial aneurysms, moyamoya disease, and arteriovenous dural fistulas are considered acquired lesions.
Developmental venous anomalies represent a variant of physiologic venous drainage and pose minimal, if any, risk of becoming symptomatic.
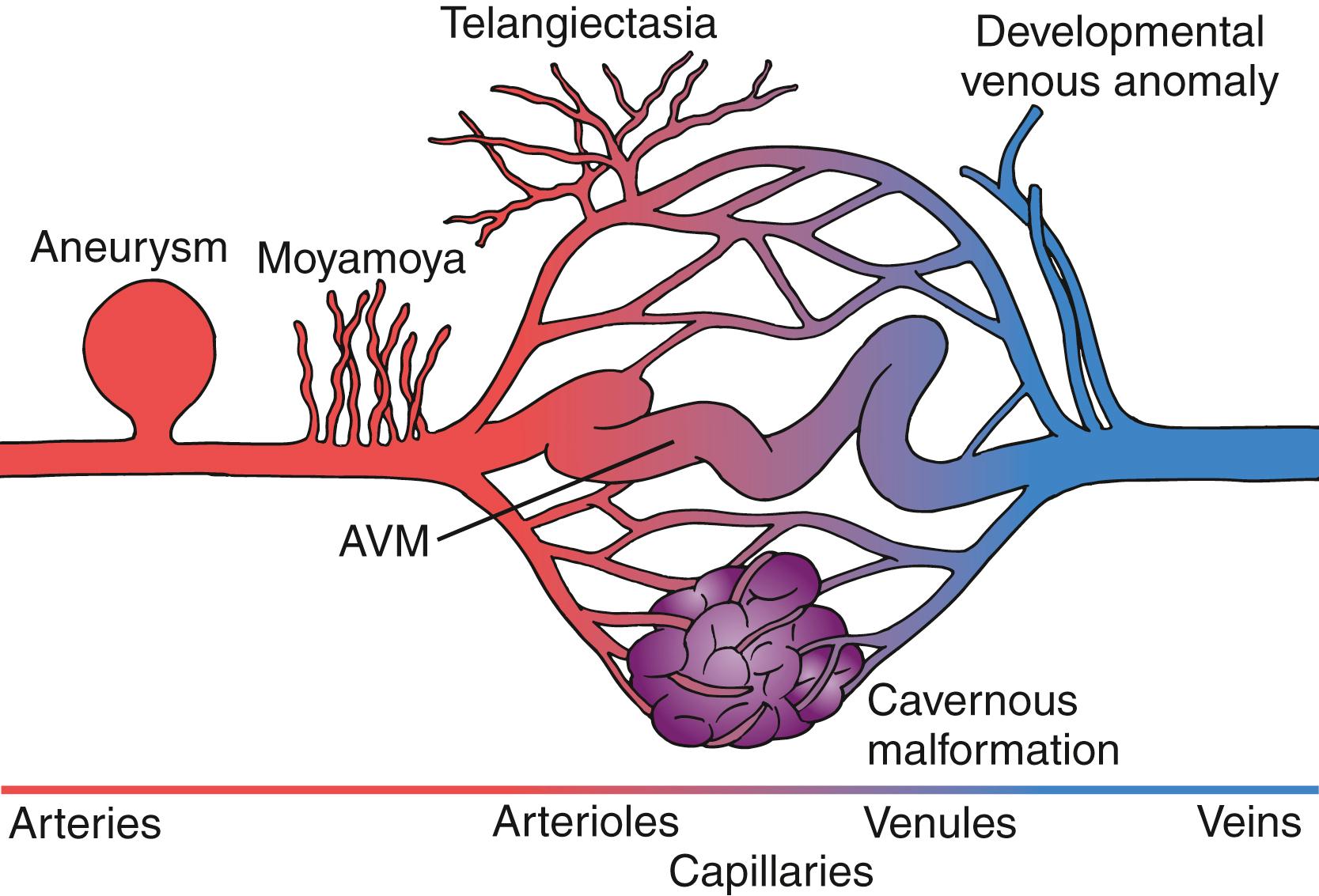
Among the “true” vascular malformations, only cerebral cavernous malformations and AVMs are of clinical relevance, while capillary telangiectasias constitute asymptomatic, incidental imaging findings.
Surgical treatment indications for cerebral cavernous malformations are limited. Neurosurgical excision is generally offered for cavernous malformations associated with therapy-resistant epilepsy, progressive neurologic deficit, enlargement of the lesion, or symptomatic intracerebral hemorrhage.
Brain AVMs are usually treated after they become symptomatic with hemorrhage. Intervention of unruptured brain AVMs has been associated with a higher risk of stroke and neurologic deficits than medical management. The discussion of treatment options for patients with asymptomatic AVMs should include the risks of the intervention versus the risk of hemorrhage based on the angio-architecture of the lesion and life expectancy of the patient.
Intracerebral vascular malformations constitute an important cause of intracranial hemorrhage, especially in younger patients. However, this group of pathologies is quite heterogeneous and comprises a wide spectrum ranging from sporadic congenital lesions to genetically determined familial disorders that may progress over time.
Although guidelines for the diagnostic workup after acute intracerebral hemorrhage (ICH) have been published, no diagnostic criteria have been established or prospectively validated. Therefore, the timing and preference of a diagnostic tool (i.e., computed tomography angiography [CTA], magnetic resonance imaging [MRI], magnetic resonance angiography [MRA], magnetic resonance venography, and cerebral angiography) vary depending on specific practices, public health policies, and hospital preferences. Current recommendations from the AHA/ASA and the European Stroke Organization (ESO) favor angiography for all cases without a clear cause of hemorrhage, particularly for young, normotensive patients (Class IIa; Level of Evidence B) . , Nonetheless, there is no consensus for timing of cerebral angiography after acute ICH, as it usually depends on the patient’s clinical status and the neurosurgeon’s judgment about the need for surgical intervention. Moreover, an underlying arteriovenous malformation (AVM) may be compressed by mass effect of the initial hematoma and only be visible on follow-up angiography. Similarly, cavernous malformations presenting with hemorrhage beyond the limits of the actual malformation may only be identified on MRI after hematoma resorption, even several months after the index bleed. Some “primary intracerebral hemorrhages” may be the result of an underlying intracranial vascular malformation that is undiagnosed.
Vascular malformations may arise from any segment of the different functional units of the brain vasculature, including arteries, arterioles, capillaries, venules, and veins. This may be due to either developmental derangement during the time of the vessel formation or may occur later in time based on external risk factors and genetic predisposition ( Fig. 30.1 ). Some of these anomalies are associated with an increased risk of hemorrhage, as structural changes of the vascular wall may lead to progressive hemodynamic changes and lower resistance to intraluminal volume and pressure. As a general rule, the risk of spontaneous hemorrhage appears to be low if the vascular malformation is diagnosed unruptured. Most types have been associated with higher bleeding rates if hemorrhage occurred at initial presentation.
Aneurysms do not constitute vascular malformations sensu stricto, but their formation represents the most frequently observed structural anomaly of intracranial arteries after endoluminal changes due to atherosclerosis. The pathogenesis of aneurysm formation is multifactorial. Increased hemodynamic stress secondary to turbulent flow forces causes excessive wear, tear, and eventual breakdown of the internal elastic lamina of the blood vessels, resulting in structural fatigue. Consequently, patients with hyperdynamic flow patterns as a result of anomalous collateral pathways or other high-flow states are predisposed to accelerated degenerative changes in the vessel wall and subsequent aneurysm development. Hypertension, cigarette smoking, and connective tissue disease probably play a contributory rather than causal role in this process. There is growing evidence that inflammation plays an important role in the pathogenesis and growth of intracranial aneurysms. ,
Aneurysm rupture constitutes the principal cause of non-traumatic subarachnoid hemorrhage (SAH) with an overall crude annual incidence between 10 and 15 in 100,000 in the Western world and up to 30 in 100,000 in high-risk populations such as Asia or Finland. Both aneurysm development and growth can be affected by familial predisposition and additional risk factors such as increasing age, female sex, smoking, and alcohol consumption. Looking at age alone, most prospective and retrospective studies have independently demonstrated a steady increase of annual incidence rates with age, with values ranging from below 5 per 100,000 for those younger than 35 years and rates between 30 and 40 per 100,000 for individuals aged 75 or older. , Two large prospective studies have reported on the natural history of unruptured intracranial aneurysms. The International Study of Unruptured Intracranial Aneurysms (ISUIA) prospectively assessed 1692 patients with 2686 unruptured aneurysms (6544 patient-years) in the United States, Canada, and Europe, and the Unruptured Cerebral Aneurysms Study (UCAS), a Japanese cohort, followed 6697 aneurysms in 5720 patients (11,660 aneurysm-years). Both studies demonstrated that larger aneurysm size and location in the posterior (i.e., vertebrobasilar) circulation increased the risk of hemorrhage.
Recent US population estimates suggest an overall mortality rate of 2.77 per 100,000 person-years attributable to aneurysm rupture. Most of the larger outcome studies indicate that roughly one-third of patients who suffer a first-ever SAH die in the acute state, another one-third will survive with a disabling deficit, and only one-third will not be disabled from the event. The probability of survival seems to be lower in women and has been shown to depend on the degree of initial neurologic impairment, commonly graded according to the Glasgow Coma or Hunt and Hess scales.
Middle cerebral artery (MCA) aneurysms or dissecting or mycotic aneurysms may lead to intraparenchymal hemorrhage ( Fig. 30.2 ). Therefore, whenever an intracerebral hematoma reaches down to the level of the carotid tip or circle of Willis arteries, or if ICH is associated with subarachnoid bleeding into the basal cisterns, an underlying aneurysm should be excluded by MRA, CTA, or conventional angiography in the acute phase, as re-rupture rates seem to be similarly high as in cases with isolated SAH. Some arterial aneurysms develop in the context of an AVM and may add to the potential hazard of intracranial hemorrhage associated with such lesions (see below).
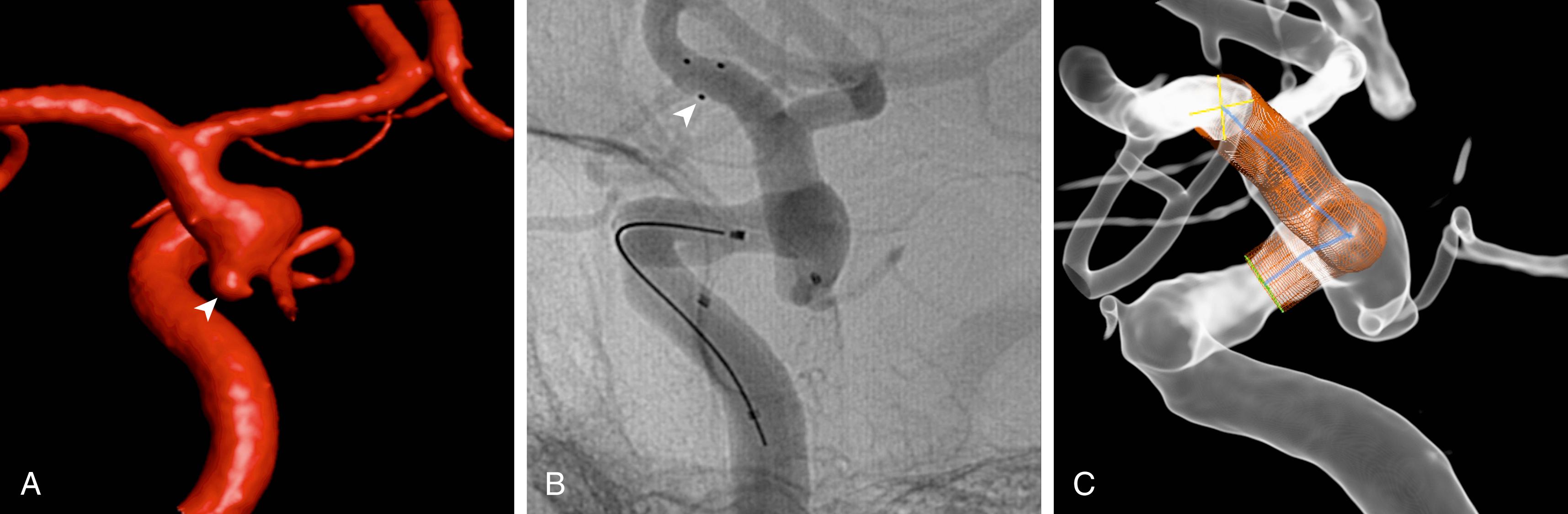
High-resolution vessel wall imaging (HR-VWI) using 3T and 7T MRI scans has emerged as a valuable tool in assessing unstable unruptured intracranial aneurysms. There are promising observations in the characterization of aneurysm wall enhancement as a biomarker of aneurysm wall inflammation, aneurysm growth, and rupture. , HR-VWI may also be used for non-invasive identification of the source of hemorrhage in patients with SAH and multiple aneurysms, which is essential for targeted treatment. However, current evidence is based mostly on retrospective studies with different criteria to define aneurysm instability and enhancement, as well as heterogeneous protocols on imaging, thus limiting its clinical use.
The International Subarachnoid Aneurysm Trial (ISAT) demonstrated that in patients with aneurysmal SAH endovascular coiling is more likely to result in independent survival at 1 year than neurosurgical clipping. The survival benefit continues at least 7 years; this trend has been confirmed by other studies. , Treatment decisions between endovascular coiling versus clipping should be based on different factors such as patient’s age and clinical condition, angio-architecture of the aneurysm, and the indication for additional hematoma evacuation as in the cases of ACOM or MCA aneurysms with a large intraparenchymal component. New technologies such as flow diversion and intrasaccular devices allow the endovascular treatment of wide-necked aneurysms or technically challenging aneurysms such blister-like aneurysms. More detailed information on aneurysm rupture and management are available in Chapter 29, Chapter 68, Chapter 71 .
A telangiectasia is a small, dilated blood vessel (arteriole, venule, or capillary) that is apparent near the surface of skin or mucous membranes. At the level of the arterioles, telangiectasias may develop as clusters of pencil-like vessels located mainly in the brainstem or cerebellum. Even though considered true vascular malformations, they have long been considered innocuous lesions of only minor clinical significance, as they may not be a source of symptomatic hemorrhage. They may demonstrate small microhemorrhages on histologic examination, but the size of the hemorrhage does not appear to be large enough to become symptomatic. Telangiectasias may be detected on MRI with and without contrast ( Fig. 30.3 ).
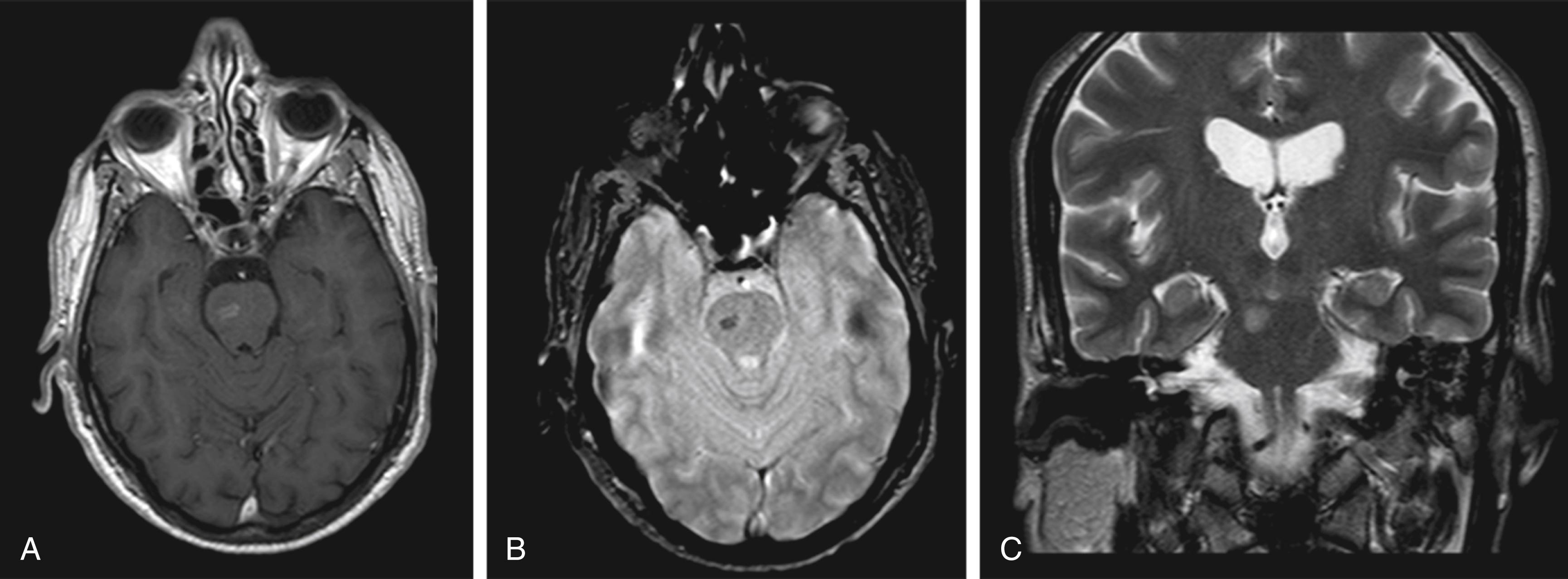
Familial hemorrhagic telangiectasia (i.e., Osler-Weber-Rendu syndrome) is an autosomal dominant disorder associated with multiple vascular malformations elsewhere in the body (characteristically mucocutaneous), which constitute tiny vascular nodules with arteriovenous shunting. Their number may increase during lifetime with the organs most affected being the mucosa, skin, lungs, brain, and liver. A variety of clinical manifestations may be present, including epistaxis, gastrointestinal bleeding, and iron deficiency anemia. If located in the brain, these lesions may behave similar to sporadic AVMs and may cause spontaneous rupture with intracranial hemorrhage (see below).
Moyamoya is an uncommon form of chronic cerebrovascular occlusive disease with angiographic findings of progressive stenosis or occlusion of the circle of Willis arteries together with a network of dilated perforating neo-vessels in the vicinity of the occlusion. The initial description arose from the angiographic appearance of this pathologic vascularization at the base of the brain: the tiny size and large number of vessels imaged made the combination look like a “cloud” or a “puff of smoke” instead of single arteries (which is called “moyamoya” in Japanese).
In the vascular network of perforating arteries (the so-called moyamoya vessels), various histologic changes can be observed: the distal portion of the carotid arteries as well as those constituting the circle of Willis often show fibrocellular intimal thickening, waving of the internal elastic lamina, and attenuation of the media. Dilated neo-vessels are found around the circle of Willis, and reticular conglomerates of small vessels may appear in the pia mater. With hemodynamic stress or aging, the dilated arteries with attenuated walls may predispose to the formation of microaneurysms, and their rupture is considered one of the mechanisms leading to parenchymatous hemorrhages in moyamoya patients.
Annual incidence estimates of symptomatic moyamoya range between 0.06 per 100,000 in Caucasians and 0.54 per 100,000 in Japan, but seem to be almost twice as high in women, and show two age distribution peaks around 10–20 and 40–50 years in Asian case series. In North American and European case series, the most common presentation phenotype is women in their 3rd, 4th, or 5th decade of life presenting with ischemic symptoms. , Clinically, ischemic and hemorrhagic symptoms are encountered. The ischemic type dominates in childhood, and clinically transient ischemic events occur more often than infarctions with persistent neurologic deficits. Of note, children with moyamoya disease often exhibit abnormalities on electroencephalography (EEG), the most characteristic of which is the “rebuild-up” phenomenon following hyperventilation. Normally, hyperventilation induces generalized high-voltage slow waves (the “build-up” phenomenon) that resolve after hyperventilation stops. The reappearance of generalized or localized high-voltage slow waves on EEG 20–60 seconds after the end of hyperventilation (the “rebuild-up” phenomenon) is considered pathognomonic of moyamoya disease, occurring in approximately two-thirds of affected children. Instead, hemorrhagic complications are more frequent in adult patients. Bleeding occurs often in repetitive intervals, and massive bleeding, although infrequent, is the principal cause of death.
Hereditary factors and ethnic origins may play a role in the occurrence or susceptibility to idiopathic moyamoya disease, as suggested by the occasional familial occurrence (12% in Japanese cases). Accumulating evidence suggests that the RNF213 gene on chromosome 17q25.3 is an important susceptibility factor for moyamoya disease. , A secondary moyamoya syndrome may be seen in association with other congenital diseases such as sickle cell anemia, β thalassemia, von Recklinghausen disease (neurofibromatosis type 1), tuberous sclerosis, and Down syndrome (trisomy 21). However, the clinical manifestation and disease progression are not congenital and may also be seen as a secondary complication of early-onset intracranial atherosclerosis, autoimmune disease, vasculitis, meningitis, post-radiation changes, vasospasm (after SAH), fibromuscular dysplasia, cranial trauma, and brain neoplasms. ,
Typical angiographic findings were considered indispensable for the diagnosis of moyamoya, but as the quality of MRI and MRA greatly improved, the diagnosis was also made if they clearly demonstrated all the findings indicative of moyamoya ( Fig. 30.4 ). Suzuki and colleagues followed patients with moyamoya disease and classified the angiographic progression into six stages: Stage 1: narrowing of carotid fork only; stage 2: initiation of basal moyamoya with dilatation of all main cerebral arteries; stage 3: intensification of moyamoya together with reduction of flow in the middle and anterior cerebral arteries; stage 4: minimization of moyamoya vessels; the proximal portions of the posterior cerebral arteries become involved; stage 5: reduction of moyamoya and absence of all main cerebral arteries; and stage 6: disappearance of moyamoya vessels; the cerebral circulation is supplied only by the external carotid system.
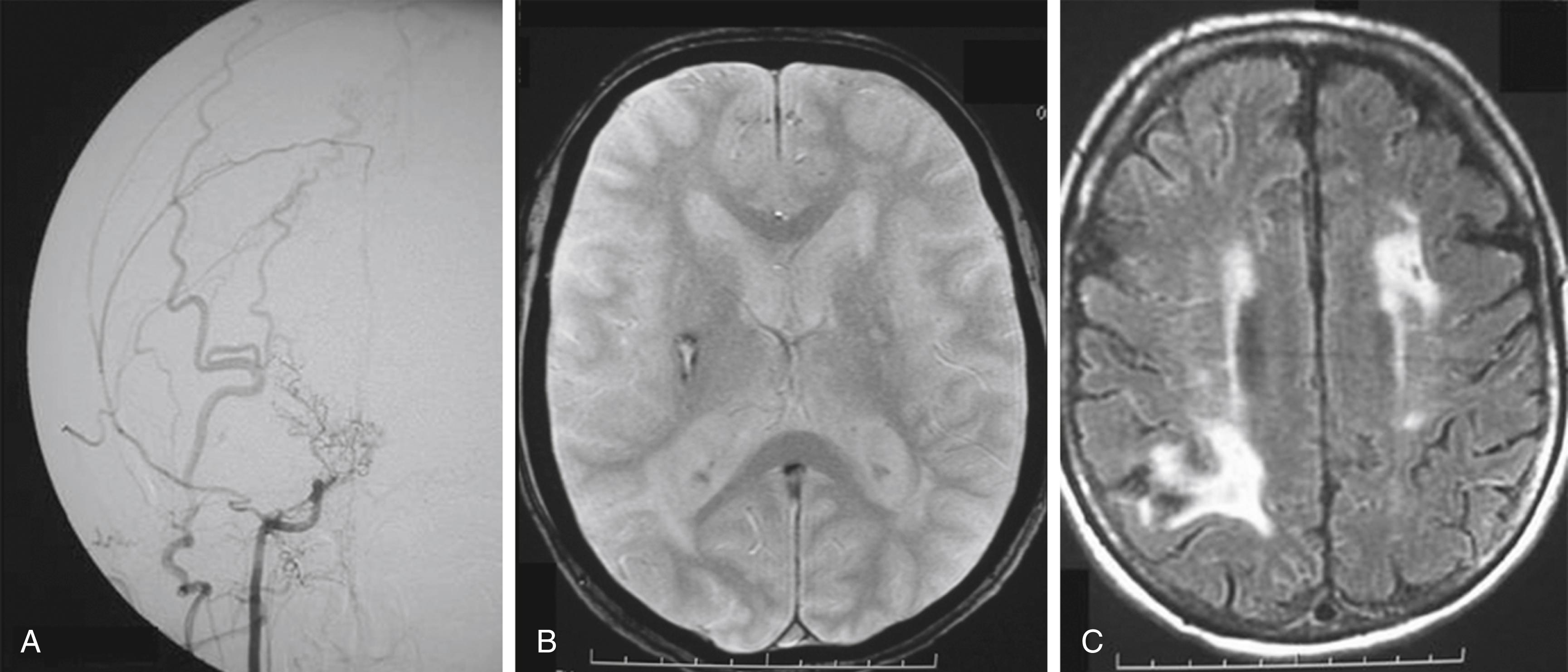
In advanced stages of moyamoya, some degree of early venous drainage may be seen on angiograms suggesting arteriovenous shunting at the level of the neo-vessel network. On the other hand, moyamoya-type vascular changes have been observed on high-flow feeding arteries in brain AVMs. Whether the two entities are linked biologically or whether the moyamoya type vascular pathology observed in AVM patients merely results from hemodynamic changes associated with the AVM remains yet unclear. More data on the management of moyamoya disease are available in Chapter 40 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here