Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Sexually transmitted infections (STIs) include a wide variety of organisms that are transmitted through intimate contact involving skin or mucosal surfaces of the oropharynx, vagina, penis, and rectum. STIs can generally be divided into five broad categories (syndromes): urethritis, genital ulcers, epithelial cell disorders, vaginal discharge, and ectoparasites ( Table 264-1 ). Gender inclusive models ( Chapter 215 ) and programs of care are recommended for all persons who would benefit from such services.
| SYNDROME | ORGANISM |
|---|---|
| URETHRITIS | |
| Gonococcal | Neisseria gonorrhoeae ( Chapter 275 ) |
| Nongonococcal | Chlamydia trachomatis ( Chapter 294 ) Trichomonas vaginalis ( Chapter 324 ) Mycoplasma genitalium ( Chapter 293 ) Ureaplasma urealyticum ( Chapter 293 ) Neisseria meningitidis ( Chapter 274 ) Herpes simplex (primary infection, Chapter 345 ) |
| GENITAL ULCERS | |
| Genital herpes | Herpes simplex ( Chapter 345 ) |
| Syphilis | Treponema pallidum ( Chapter 295 ) |
| Chancroid | Haemophilus ducreyi ( Chapter 276 ) |
| Granuloma inguinale (donovanosis) | Klebsiella granulomatis ( Chapter 292 ) |
| Lymphogranuloma venereum | Chlamydia trachomatis (serovars L1, L2, or L3) |
| Monkeypox | Monkeypox ( Chapter 343 ) |
| EPITHELIAL CELL INFECTIONS | |
| Genital warts | Human papillomavirus ( Chapter 344 ) |
| Cervical neoplasia | Human papillomavirus types 16 and 18 ( Chapter 344 ) |
| Molluscum | Molluscum contagiosum ( Chapter 343 ) |
| FEMALE GENITAL DISCHARGE | |
| Bacterial vaginosis | Gardnerella vaginalis , anaerobes ( Chapter 273 ) |
| Vaginitis | Trichomonas vaginalis ( Chapter 324 ) Candida albicans ( Chapter 310 ) |
| Cervicitis | Neisseria gonorrhoeae ( Chapter 275 ) Chlamydia trachomatis ( Chapter 294 ) Trichomonas vaginalis ( Chapter 324 ) Herpes simplex ( Chapter 345 ) |
| Pelvic inflammatory disease | Neisseria gonorrhoeae ( Chapter 275 ) Chlamydia trachomatis ( Chapter 294 ) |
| ECTOPARASITES | |
| Pubic lice | Pthirus pubis |
| Scabies | Sarcoptes scabiei |
The interaction between the host and the sexually transmitted pathogen plays a critical role, and characteristic tissue changes offer clues about etiology. Several sexually transmitted pathogens cause local inflammation only ( Neisseria gonorrhoeae , Chlamydia trachomatis , Trichomonas vaginalis ), with the potential for local tissue invasion ( N. gonorrhoeae , C. trachomatis ) or systemic dissemination ( N. gonorrhoeae ). Some sexually transmitted pathogens cause tissue ulceration ( Treponema pallidum , Haemophilus ducreyi , herpes simplex viruses 1 and 2, Klebsiella granulomatis ). Human papillomaviruses cause epithelial cell changes and predispose to neoplasm. Several sexually transmitted pathogens (human immunodeficiency virus [HIV], hepatitis B and C viruses, cytomegalovirus) routinely enter through the genital tract without causing any local changes.
Other important systemic diseases that can be acquired through sexual transmission include Zika virus, Ebola virus, Lassa fever, and monkeypox ( Chapters 343 , 351 , and 352 ). Although these infections can present with genital cutaneous lesions that can be confused with herpes simplex virus, they can cause systemic signs and symptoms that can be fatal.
Sexually transmitted infections are among the most common infections worldwide, but most are never reported. The World Health Organization (WHO) estimates that nearly 1 million people worldwide become infected every day with any of four curable sexually transmitted infections: chlamydia, gonorrhea, syphilis, and trichomoniasis. Each year, an estimated 2.5 million incident cases of gonorrhea, chlamydia, and syphilis are reported in the United States alone. However, the total estimated number of cases of all sexually transmitted infections is likely much greater because of under-reporting and because many infections are subclinical and may escape detection. Sexually transmitted infections are generally transmissible whether they are symptomatic or asymptomatic.
Despite efforts to control the epidemic of sexually transmitted infections, the numbers of new infections continue to grow. This epidemic disproportionately impacts specific populations and geographies, sometimes leading to long-term health consequences such as infertility, poor birth outcomes, cancer, and HIV infection. In the United States, social determinants of health contribute to an unequal burden of sexually transmitted infections in Black, American Indian/Alaska Native, and Latinx communities ( Chapter 4 ).
Chlamydia ( Chapter 294 ), gonorrhea ( Chapter 275 ), and syphilis ( Chapter 295 ) are reportable to the U.S. Centers for Disease Control and Prevention (CDC). Overall, primary and secondary syphilis rates have increased every year since 2000 and increased by 6.8% from 2019 to 2020. Although rates of syphilis are lower among women compared with men, rates in women have increased nearly 150% from 2016 to 2020, and rates of congenital syphilis increased by 250% during that time period. Men who have sex with men account for almost 60% of syphilis cases among men. For gonorrhea, the overall rate is about 150 cases per 100,000 population among women and 225 cases per 100,000 in men, but rates in men and women ages 20 to 24 years are closer to 750 cases per 100,000.
Emerging pathogens also must be considered in certain groups of patients. Some of these pathogens are asymptomatic but are of public health significance (e.g., Ebola [ Chapter 351 ], Zika [ Chapter 352 ] viruses in genital secretions), whereas others represent relatively newly recognized pathogens (e.g., Neisseria meningitidis urethritis [ Chapter 274 ] and monkeypox [ Chapter 343 ]).
Seroprevalence data and national surveys suggest a high burden of viral sexually transmitted infections. Antibodies to herpes simplex virus 2 (HSV-2) are found in the serum of about 12% of the population (ages 14 to 49 years), with seropositivity more frequent in non-Hispanic Black Americans (about 35%) compared with non-Hispanic Whites (about 8%), and in women (about 16%) compared with men (about 8%).
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic significantly impacted the availability of services for patients who have sexually transmitted infections, especially in urban settings. The longer-term ramifications of missed opportunities for screening and intervention remain to be seen.
The spread of sexually transmitted infections depends on the organism, the host, the length of time an infected person remains contagious, and the number of people exposed. These parameters can be summarized as:
where R o is the basic reproductive rate of an infection, or the mean number of secondary cases a typical single infected person will cause in a population; B is the efficiency of transmission; D is the duration of infectiousness; and C is the number of sexual partners.
Sexually transmitted pathogens depend entirely on human-human transmission, although T. vaginalis may have some inanimate sources. The efficiency of transmission reflects the infectiousness of the index case (which depends on the concentration and phenotype of the organism in the genital tract) and the susceptibility of the sexual partner (which reflects the resistance of the host, whether it is hereditary, acquired, or innate). Because immunity to STIs is rare, reinfections are common, and vaccines have been developed only for hepatitis A, hepatitis B, and human papillomavirus.
Each sexually transmitted organism produces a characteristic syndrome because each pathogen has a proclivity for one or more tissues and, when symptomatic, can evoke a predictable inflammatory response. For example, gonococci ( Chapter 275 ) that infect the male urethra generally produce an intense neutrophilic response that leads to a purulent discharge and pain with urination, whereas C. trachomatis ( Chapter 294 ) is less likely to produce such a response in the same tissue and is more likely to produce a mild, watery discharge or no symptoms at all.
Because sexually transmitted infections are more prevalent in certain geographies and populations, coinfections are common. Detection of one sexually transmitted organism should lead to other tests. Sexually transmitted pathogens also can be synergistic: for example, sexually transmitted infections also contribute to the acquisition and transmission of HIV and hamper efforts to prevent its transmission. Genital ulcers disrupt the genital mucosal epithelium and allow the entry of HIV. Inflammation caused by ulceration recruits macrophages and lymphocytes, thereby increasing the number of target cells for HIV and the number of receptors per cell. Sexually transmitted infections also can increase the HIV viral load in genital secretions, including semen and vaginal secretions, thereby contributing to a greater risk of transmitting HIV.
The syndromic approach focuses on diagnosis based on signs and symptoms, followed by empiric treatment of the index case and concomitant treatment of sexual partners. The syndromic approach is particularly critical in resource-constrained countries or wherever laboratory tests are not available or their cost is prohibitive. In the United States, microbiologic diagnosis is preferred because it confirms the choice of empiric therapy or redirects subsequent care; permits the detection and monitoring of resistance to treatment; and enables specific diagnoses to be reported to public health authorities, as is required by state law for many sexually transmitted infections.
Newer point of care nucleic acid amplification tests (NAATs) for sexually transmitted infections allow diagnosis and treatment for some syndromes (e.g., vaginitis) and specific infections (e.g., gonorrhea and chlamydia) at the same outpatient visit. However, when test results are not available at the same visit, the most appropriate syndromic treatment should be provided empirically at the point of care to resolve the infection and to reduce subsequent transmission.
The diagnosis of a sexually transmitted infection suggests condomless intercourse and serves as a marker for potential HIV infection. Any person undergoing evaluation or treatment for an STI should be tested for HIV infection, because early diagnosis ( Chapter 355 ) and treatment ( Chapter 357 ) of HIV infection has major personal and public health benefits, including reducing secondary HIV transmission.
Although most sexually transmitted infections are self-limited and readily treated, the comprehensive and proper management of the patient with a sexually transmitted infection requires considerable skill. First, the correct syndrome must be recognized, and a decision about specific diagnostic tests must be made. Second, empiric therapy must be provided and must be sufficiently broad to promise cure or reduced duration of illness. Third, the clinician is obligated to search for other sexually transmitted infections of public health or personal significance. Fourth, the clinician must deal with the patient’s sexual partners, through either referral or expedited partner therapy. Fifth, the patient needs counseling and adjunctive preventive measures, where appropriate. Such measures might include: use of condoms; vaccination for hepatitis A, hepatitis B, and human papillomavirus; use of post-exposure doxycycline (200 mg within 72 hours after condomless sex) to reduce bacterial sexually transmitted infections in men who have sex with men by about two-thirds ; or antimicrobials to prevent or treat another sexually transmitted infection, such as incubating syphilis or HIV infection.
Urethritis is characterized by some combination of urethral discharge and dysuria, but prostatitis ( Chapter 114 ) can cause similar complaints. Women with urethritis typically have some combination of dysuria and pyuria, which must be differentiated from bacterial cystitis ( Chapter 263 ).
Urethritis is diagnosed when one or more of the following are demonstrated: (1) mucopurulent or purulent urethral discharge, (2) Gram stain of urethral secretions demonstrating two or more leukocytes per oil immersion microscopic field, (3) positive leukocyte esterase test result on first-void urine, or (4) microscopic examination of first-void urine demonstrating 10 or more leukocytes per high-power field. If no urethral discharge can be expressed from the urethral meatus, a calcium alginate swab can be inserted 5 mm into the urethra; the material collected is transferred to a slide by rolling the swab along the glass.
Urethritis is caused by a limited group of pathogens (see Table 264-1 ) that may be difficult to visualize microscopically or to grow in culture. Nevertheless, specific diagnosis may enhance the management of sexual partners, and the results from such tests should be reported to the health department.
A Gram stain of urethral discharge is a simple and rapid diagnostic test to document both urethritis and possible gonococcal infection ( Chapter 275 ), which is characterized by the detection of leukocytes containing intracellular gram-negative diplococci ( Fig. 275-1 ). Because confirmation of gonococcal urethritis does not exclude concomitant infection with Chlamydia or Mycoplasma , NAATs, which are highly sensitive and specific for the detection of these and other organisms, are routinely performed on the urethral discharge owing to the high probability of coinfection. However, clinicians should use caution in relying solely on NAATs for diagnosing gonorrhea because it does not always identify other sexually transmitted Neisseria species, such as Neisseria meningitidis.
Nucleic acid amplification tests for gonorrhea, Chlamydia , and Trichomonas also can be applied to first-void urine samples (the meatus is intentionally not cleaned so that the urine is contaminated with these organisms), urethral swab material, or vaginal swab material. Extra-genital (e.g., rectal and throat swabs) NAATs are recommended for men who have sex with men or women with reported exposures, since urine-based and cervicovaginal testing alone can miss a large proportion of infected individuals.
In practice, patients and (in most cases) sexual partners must be treated with empiric therapy to cover the spectrum of potentially causative organisms before the results of definitive tests are available. This empiric treatment should be initiated as soon as possible after the clinical diagnosis and should be directly observed if feasible ( Table 264-2 ). , Doxycycline appears to be more effective than azithromycin for nongonococcal urethritis and is now the preferred treatment for chlamydia because of the increasing resistance of other concomitant infections (e.g., gonorrhea and M. genitalium ) to macrolides.
| GONOCOCCAL ∗ |
| Recommended |
| Ceftriaxone 500 mg injected intramuscularly once, and (if chlamydia has not been excluded) doxycycline 100 mg orally twice daily for 7 days (for persons weighing >150 kg, the ceftriaxone dose should be 1 g) |
| NONGONOCOCCAL |
| Recommended |
| Doxycycline 100 mg orally twice daily for 7 days |
| Alternative |
| Azithromycin 1 g orally as a single dose; or 500 mg orally as a single dose followed by 250 mg orally daily for 4 days |
| Recurrent or Persistent |
| If azithromycin was used for initial episode: moxifloxacin 400 mg orally once daily for 7 days If doxycycline was used for initial episode: azithromycin 1 g orally (single dose) PLUS For men who have sex with women who live in areas where T. vaginalis is highly prevalent:
|
If NAAT results suggest that empiric therapy is unlikely to be successful or if the patient fails to respond to empiric therapy, the antibiotic regimen must be adjusted. For M. genitalium , persistent or recurrent urethritis should be treated with moxifloxacin for 7 days. T. vaginalis , which also should be considered in the setting of treatment failure, is susceptible to metronidazole or tinidazole. Because treatment for urinary tract pathogens ( Table 263-6 ) may also resolve sexually transmitted urethritis, the clinician who is treating a presumed bladder infection should consider a possible sexually transmitted infection as well.
For presumed sexually transmitted proctitis, the recommended empiric treatment is ceftriaxone (500 mg IM once) plus doxycycline (100 mg orally twice daily for 7 days). For presumed sexually transmitted epididymitis, ceftriaxone (500 mg IM once) plus doxycycline (100 mg orally twice daily for 10 days) is recommended in heterosexual men, but ceftriaxone (500 mg IM once) plus levofloxacin (500 mg orally daily for 10 days) is preferred in men with suspected epididymitis due to enteric pathogens.
In the United States, HSV-1 and HSV-2 ( Chapter 345 ) and T. pallidum are responsible for virtually all genital ulcers, and HSV-1 and HSV-2 are by far the most common cause.
Genital herpes ( Chapter 345 ), which usually develops after an incubation period of less than 21 days, arises as clustered vesicles on an erythematous base. The vesicles become pustular and then rupture to form shallow, painful ulcers that may coalesce. The ulcers heal by crusting over, and the process is usually completed 2 to 3 weeks after the initial lesions appear. In the recent monkeypox outbreak, the lesions were initially confused with the vesicles of herpes simplex virus. Recurrences proceed through the same stages but generally persist only about 5 to 7 days. The first (incident) episode of HSV-2 infection may be accompanied by systemic signs and symptoms including fever and headache, the latter reflecting the spread of HSV to the central nervous system. HSV-2 can cause recurrent meningitis that may occur coincident with reactivation of the genital lesions.
About 20% of infected individuals manifest the classic genital presentation, 60% have mild and atypical signs and symptoms, and the remaining 20% are completely asymptomatic. Viral shedding occurs more often in symptomatic individuals (20% of days) compared with asymptomatic individuals (10% of days), thereby posing an ongoing risk to sexual partners. Nucleic acid testing of swabs of lesions has a 97% sensitivity for detecting herpes virus. However, serologic screening for genital herpes is associated with a high rate of false-positive test results and potential psychosocial harms. Treatment with antiviral medications can shorten the duration of symptoms and may be helpful for preventing recurrence of HSV-2 ( Table 264-3 ). Counseling for patients should include a discussion of the risk of shedding, disclosure, and the avoidance of sex in the viral prodrome or during outbreaks.
| GENITAL HERPES |
| First Episode |
| Acyclovir 400 mg orally 3 times/day for 7-10 days or Famciclovir 250 mg orally 3 times/day for 7-10 days or Valacyclovir 1 g orally 2 times/day for 7-10 days (Treatment can be extended if healing is incomplete after 10 days of therapy.) |
| Recurrent Episodes of HSV-2 |
| Acyclovir 800 mg orally 2 times/day for 5 days or Acyclovir 800 mg orally 3 times/day for 2 days or Famciclovir 1 g orally 2 times/day for 1 day or Famciclovir 500 mg orally once, followed by 250 mg 2 times/day for 2 days or Famciclovir 125 mg 2 times/day for 5 days or Valacyclovir 500 mg orally 2 times/day for 3 days or Valacyclovir 1 g orally once daily for 5 days |
| Chronic Suppression of HSV-2 |
| Acyclovir 400 mg orally 2 times/day or Valacyclovir 500 mg orally once a day or Valacyclovir 1 g orally once a day or Famciclovir 250 mg orally 2 times/day |
| SYPHILIS |
| Primary or Secondary |
| Benzathine penicillin G 2.4 million units IM in a single dose |
| Latent |
| Early latent syphilis: Benzathine penicillin G 2.4 million units IM in a single dose Late latent syphilis: Benzathine penicillin G 7.2 million units total, administered as 3 doses of 2.4 million units IM each at 1-week intervals |
| LYMPHOGRANULOMA VENEREUM |
| Doxycycline 100 mg orally 2 times/day for 21 days |
| Alternative Regimens |
| Azithromycin 1 g orally once weekly for 3 weeks or Erythromycin base 500 mg orally 4 times/day for 21 days |
| CHANCROID |
| Azithromycin 1 g orally in a single dose or Ceftriaxone 250 mg IM in a single dose or Ciprofloxacin 500 mg orally 2 times/days for 3 days or Erythromycin base 500 mg orally 3 times/day for 7 days |
| GRANULOMA INGUINALE (DONOVANOSIS) |
| Azithromycin 1 g orally once weekly or 500 mg daily for >3 weeks and until all lesions have completely healed |
| Alternative Regimens |
| Doxycycline 100 mg orally 2 times/day for at least 3 weeks and until all lesions have completely healed or Erythromycin base 500 mg orally 4 times/day for >3 weeks and until all lesions have completely healed or Trimethoprim-sulfamethoxazole one double-strength (160 mg/800 mg) tablet orally 2 times/day for >3 weeks and until all lesions have completely healed |
The ulcerative lesion of syphilis ( Chapter 295 and Fig. 295-1 )—the chancre—is indurated and typically painless. Some patients may have multiple ulcers rather than a single chancre. Swabs from a genital ulcer should be collected and examined by dark-field microscopy; the finding of motile spirochetes is diagnostic for syphilis. When darkfield microscopy is not available, primary syphilis can be diagnosed with serologic testing, but the nontreponemal and treponemal antibody tests may be negative in early primary infection. Secondary syphilis results when the spirochetes spread systemically, thereby leading to a characteristic maculopapular rash involving the palms and soles, alopecia, oral mucous patches, or condyloma latum.
All patients who present with a sexually transmitted infection must have serologic screening ( E-Fig. 264-1 ) for syphilis. (e.g., rapid plasma reagin test, Venereal Disease Research Laboratory [VDRL] test, toluidine red unheated serum test [TRUST]). If reactive, the diagnosis must be confirmed with a more specific treponemal antibody test (microhemagglutination assay– T. pallidum , fluorescent treponema antibody test). However, some large laboratories have switched to the reverse syphilis screening algorithm using newer automated treponemal tests for initial testing followed by a nontreponemal test ( Chapter 295 ).
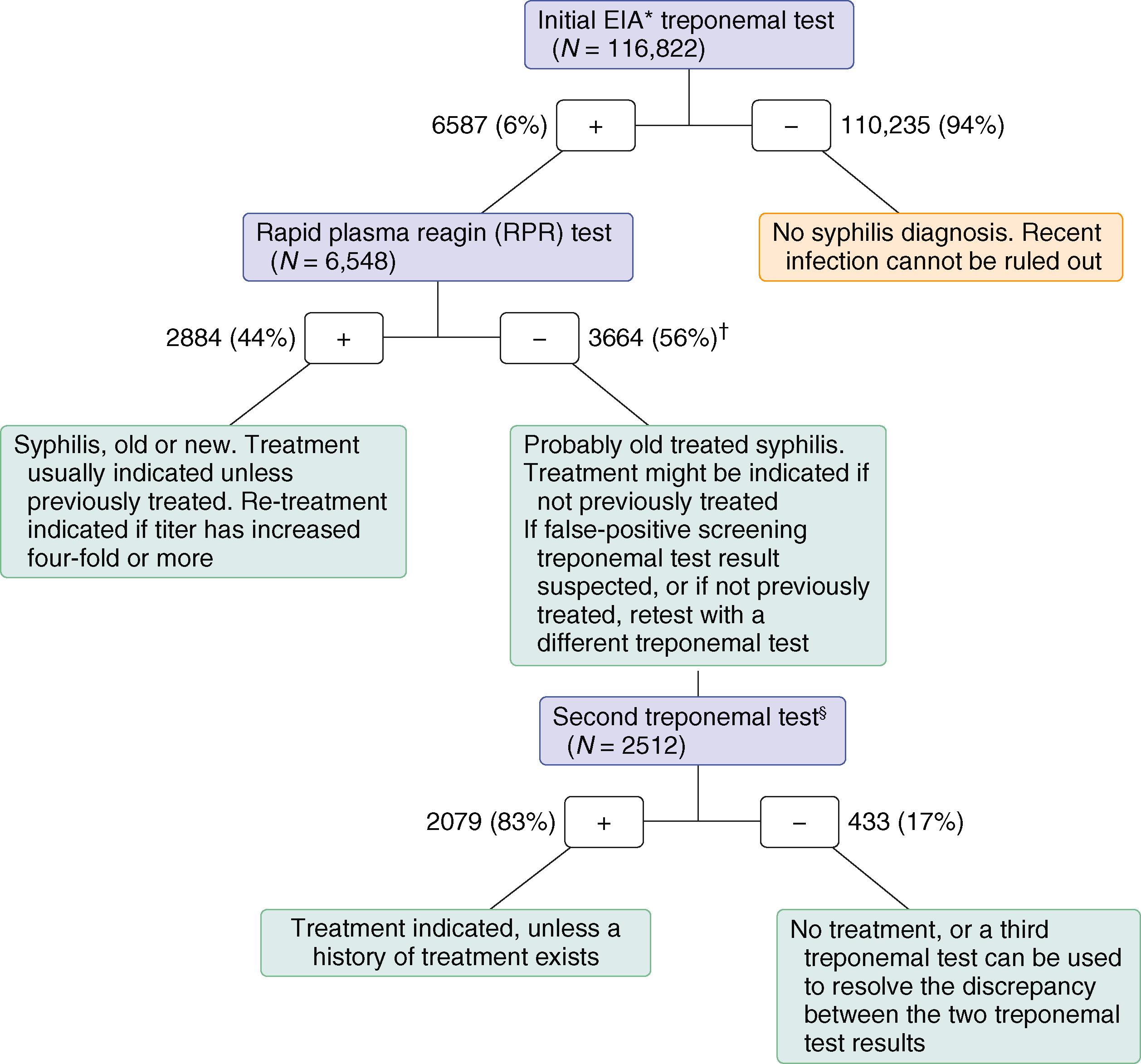
All patients diagnosed with syphilis should be questioned about ocular symptoms and symptoms suggestive of neurosyphilis; ocular and neurosyphilis require 14 days of intravenous aqueous penicillin G ( Table 295-4 ). Late latent syphilis and syphilis of unknown duration are managed with three weekly injections of intramuscular penicillin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here