Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Soft tissue sarcomas are a complex family of rare malignant neoplasms that show mesenchymal differentiation. Benign soft tissue tumors are more common than their malignant counterparts. Both benign and malignant mesenchymal tumors can cause diagnostic confusion. The previous chapters of this book have discussed the characteristics of these tumors as groups based on their morphologic similarities and stressed the features that allow their proper classification primarily based on histologic, immunophenotypic, and clinical features. Classification schemes for sarcomas, on which specific diagnoses are based, have gone through many iterations, with histochemistry, electron microscopy, and immunohistochemistry all making important contributions. Currently, molecular genetic approaches to members of this tumor family play an important role in their classification. More pangenomic approaches—such as comparative genomic hybridization, gene expression arrays, and next-generation sequencing (NGS)—are poised to make important contributions not only to our biologic understanding but also to classification, prognostication, and treatment approaches for these tumors. The basis and role of current clinical molecular testing is presented to demonstrate the practical applications of these methods to the daily practice of a surgical pathologist. Although diagnostic cytogenetic features of benign soft tissue neoplasms are discussed here, this chapter focuses chiefly on malignant tumors, because molecular testing is most commonly used in the differential diagnosis of sarcomas.
Soft tissue sarcomas can be divided broadly into two groups based on their cytogenetic features. One group is made up of sarcomas with simple cytogenetics consisting of relatively normal chromosomal complements and featuring chromosomal translocations (e.g., Ewing sarcoma and synovial sarcoma) or single-gene mutations (e.g., gastrointestinal stromal tumor [GIST] and desmoid fibromatosis), which can be used to support a specific diagnosis. The other group is made up of those with aneuploidy and complex cytogenetic features that lack specificity. The latter sarcomas are listed in Table 18.1 . A complex karyotype derived from an undifferentiated pleomorphic sarcoma is depicted in Fig. 18.1 . These are commonly associated with TP53 and/or RB1 (retinoblastoma) disruption and often show telomere dysfunction. Although they are not specific, the existence of such complex karyotypes can imply malignancy. It is in this tumor family that high-throughput genetic techniques allowing examination of the entire cancer genome may ultimately have great impact. One example of the utility of this approach is the recent discovery that malignant peripheral nerve sheath tumors have loss of histone H3 K27 trimethylation (H3K27me3). Loss of H3K27me3 can be detected by immunohistochemistry, is diagnostically useful, and portends a worse prognosis. Other examples in this class of tumors include IDH 1/2 and COL2A1 mutations in chondrosarcomas and MYC amplification in radiation-associated angiosarcomas.
| Sarcoma Type | Cytogenetic Alterations | Molecular Alterations |
|---|---|---|
| Angiosarcoma | Complex with various recurrent mutations including MYC in postradiation cases | The molecular alterations are complex, usually with extensive copy number alterations (deletions > gains), but often involving the TP53 and/or RB1 genes and pathways, sometimes combined with telomere dysfunction (± ATRX loss-of-function mutations) |
| Chondrosarcoma | Complex with various recurrent mutations such as IDH1/2 in conventional chondrosarcomas | |
| Leiomyosarcoma | Complex with frequent deletion of 1p | |
| Malignant peripheral nerve sheath tumor | Complex; loss-of-function mutations in NF1 often with EED or SUZ12 , resulting in loss of histone H3 K27 trimethylation | |
| Pleomorphic liposarcoma | Complex | |
| Pleomorphic rhabdomyosarcoma | Complex | |
| Undifferentiated pleomorphic sarcoma | Complex |
Within the group of sarcomas with relatively simple cytogenetic changes are some that lack recurrent molecular diagnostic features (e.g., embryonal rhabdomyosarcoma). These are listed in Table 18.2 , along with other, mostly benign soft tissue neoplasms for which testing is not generally indicated or utilized. Finally, Table 18.3 includes the group of soft tissue sarcomas (approximately one-third of all soft tissue sarcoma types) associated with genetic changes that are diagnostic when encountered and that provide the molecular basis for commonly used diagnostic tests. With this classification scheme in mind, the chapter presents the types of molecular testing available and then briefly discusses the entities listed in Table 18.3 , with a focus on situations where molecular diagnostics are particularly relevant and helpful.
| Tumor Type | Cytogenetic Alterations | Molecular Alterations |
|---|---|---|
| Embryonal rhabdomyosarcoma | Trisomies 2q, 8, and 20 | LOH at 11p15 |
| Hibernoma | 11q13 rearrangements | MEN1 deletions |
| Leiomyoma | 12q15 rearrangements | HMGA2 overexpression MED12 mutation (uterine) ALK rearrangement (GI) |
| Lipoblastoma | 8q11–13 rearrangements | HAS2-PLAG1 fusion |
| COL1A2-PLAG1 fusion | ||
| Spindle cell/sclerosing rhabdomyosarcoma | 11p15 mutation | MYOD1 mutation |
| Spindle cell/pleomorphic lipoma | 13q or 16q deletions | RB1 locus and unknown |
| Chondroid lipoma | t(11;16)(q13;p12–13) | C11orf95-MKL2 fusion |
| Neurofibroma | Monosomy 17 | NF1 mutation and LOH |
| Pericytoma | t(7;12)(p21–22;q13–15) | ACTB-GLI fusion |
| Schwannoma | Monosomy 22 | NF2 mutation and LOH |
| Tenosynovial giant cell tumor | 1p13 rearrangements | CSF1 overexpression |
| Sarcoma Type | Cytogenetic Alterations | Molecular Alterations |
|---|---|---|
| Alveolar rhabdomyosarcoma | t(2;13)(q35;q14) | PAX3-FOXO1A fusion |
| t(1;13)(p36;q14), double minutes | PAX7-FOXO1A fusion | |
| t(2;2)(q35;p23) | PAX3-NCOA1 fusion | |
| t(X;2)(q35;q13) | PAX3-AFX fusion | |
| Alveolar soft part sarcoma | t(X;17)(p11;q25) | TFE3-ASPSCR1 fusion a |
| Aneurysmal bone cyst | t(16;17)(q22;p13) | CDH11-USP6 fusion |
| Angiomatoid fibrous histiocytoma | t(12;16)(q13;p11) | FUS-ATF1 fusion |
| t(12;22)(q13;q12) | EWSR1-ATF1 fusion a | |
| t(2;22)(q33;q12) | EWSR1-CREB1 fusion | |
| Clear cell sarcoma | t(12;22)(q13;q12) | EWSR1-ATF1 fusion a |
| t(2;22)(q33;q12) | EWSR1-CREB1 fusion | |
| Cellular fibroma of tendon sheath | 17p13 | USP6 with unknown fusion partners |
| Desmoid fibromatosis | Trisomies 8 and 20 and loss of 5q21 | CTNNB1 or APC mutation a |
| Desmoplastic small round cell tumor | t(11;22)(p13;q12) | EWSR1-WT1 fusion |
| Dermatofibrosarcoma protuberans | Ring form of chromosomes 17 and 22 | COL1A1-PDGFB fusion |
| t(17;22)(q21;q13) | COL1A1-PDGFB fusion | |
| Dermatofibroma/fibrous histiocytoma | t(3;11)(p21;q13) | LAMTOR1-PRKCD fusion NUMA1-SFMBT1 fusion |
| Epithelioid fibrous histiocytoma | 2p23 | VCL-ALK fusion SQSTM1-ALK fusion |
| Endometrial stromal sarcoma | t(7;17)(p15;q21) | JAZF1-SUZ12 fusion |
| t(6;7)(p21;7p15) | JAZF1-PHF1 fusion | |
| t(6;10)(p21;p11) | EPC1-PHF1 fusion | |
| Epithelioid hemangioendothelioma | t(1;3)(p36;q25) | WWTR1-CAMTA1 fusion |
| t(X;11)(p11;q22) | YAP1-TFE3 fusion | |
| Epithelioid hemangioma of bone with atypical features | t(7;19)(q22;q13) | ZFP36-FOSB fusion |
| Ewing sarcoma | t(11;22)(q24;q12) | EWSR1-FLI1 fusion |
| t(21;22)(q12;q12) | EWSR1-ERG fusion | |
| t(2;22)(q33;q12) | EWSR1-FEV fusion | |
| t(7;22)(p22;q12) | EWSR1-ETV1 fusion | |
| t(17;22)(q12;q12) | EWSR1-E1AF fusion | |
| inv(22)(q12;q12) | EWSR1-ZSG fusion | |
| t(16;21)(p11;q12) | FUS-ERG fusion a | |
| t(19;der)ins.inv(21;22) | EWSR1-ERG fusion | |
| t(17;22)(q12;q12) | EWSR1-ETV4 fusion | |
| t(6;22)(p21;q12) | EWSR1-POU5F1 fusion | |
| t(1;22)(q36.1;q12) | EWSR1-PATZ1 fusion | |
| t(2;22)(q31;q12) | EWSR1-SP3 fusion | |
| t(20;22)(q13;q12) | EWSR1-NFATc2 fusion | |
| t(2;16)(q35;p11) | FUS-FEV fusion | |
| t(15;19)(q14;p13.1) | BRD4-NUT fusion a | |
| Ewing-like tumor | t(4;19)(q35;q13) or t(10;19)(q26;q13) |
CIC-DUX4 fusion |
| t(X;19)(q13;q13) | CIC - FOXO4 fusion | |
| Inv(X)(p11.4; p11.22) | BCOR-CCNB3 fusion | |
| t(X;4)(p1.4;q31.1) | BCOR - MAML3 fusion | |
| t(X;22)(p1.4;q13) | ZC3H7B - BCOR fusion | |
| Extraskeletal myxoid chondrosarcoma | t(9;22)(q22;q12) | EWSR1-NR4A3 fusion |
| t(9;17)(q22;q11) | TAF2N-NR4A3 fusion | |
| t(9;15)(q22;q21) | TCF12-NR4A3 fusion | |
| t(3;9)(q11;q22) | TFG-NR4A3 fusion | |
| Fibrosarcoma, infantile/cellular mesoblastic nephroma | t(12;15)(p13;q26) | ETV6-NTRK3 fusion a |
| Trisomies 8, 11, 17, and 20 | ||
| Gastrointestinal stromal tumor | Monosomies 14 and 22; deletion on 1p | KIT or PDGFRA mutation; rarely BRAF , SDHA , SDHB , SDHC , or SDHD mutations |
| Inflammatory myofibroblastic tumor | t(1;2)(q22;p23) | TPM3-ALK fusion a |
| t(2;19)(p23;p13) | TPM4-ALK fusion | |
| t(2;17)(p23;q23) | CLTC-ALK fusion a | |
| t(2;2)(p23;q13) | RANBP2-ALK fusion | |
| t(2;2)(p23;q35) | ATIC-ALK fusion a | |
| t(2;11)(p23;p15) | CARS-ALK fusion | |
| t(2;4)(p23;q21) | SEC31L1-ALK fusion | |
| t(2;12)(p23;p12) | PPFIBP1-ALK fusion | |
| Intimal sarcoma | Chromosome 12 | Amplification of MDM2 |
| Low-grade fibromyxoid sarcoma | t(7;16)(q33;p11) | FUS-CREB3L2 fusion |
| t(11;16)(p11;p11) | FUS-CREB3L1 fusion | |
| Lipofibromatosis-like neural tumor | 1q23 | NTRK1 with various partners including TFR and TPM3 |
| Mesenchymal chondrosarcoma | t(8;8)(q13;q21) | HEY1-NCOA2 fusion |
| Myoepithelial tumors of soft tissue | t(6;22)(p21;q12) | EWSR1-POU5F1 fusion |
| t(19;22)(q13;q12) | EWSR1-ZNF444 fusion | |
| t(1;22)(q23;q12) | EWSR1-PBX1 fusion | |
| Myxoid/round cell liposarcoma | t(12;16)(q13;p11) | FUS-DDIT3 fusion |
| t(12;22)(q13;q12) | EWSR1-DDIT3 fusion | |
| Myxoinflammatory fibroblastic sarcoma/hemosiderotic fibrolipomatous tumor | t(1;10)(p22;q24) | TGFBR3-MGEA5 fusion |
| 3p11–12 (ring chromosomes) | Amplification of VGLL3, CHMP2B | |
| Nodular fasciitis | t(17;22)(p13;q13) | MYH9-USP6 fusion |
| Ossifying fibromyxoid tumor | 6p21 | PHF1 with various partners; one major partner is EP400 |
| Primary pulmonary myxoid sarcoma of the lung | t(2;22)(q33;q12) | EWSR1-CREB1 fusion |
| Pseudomyogenic hemangioendothelioma | t(7;19)(q22;q13) | SERPINE1-FOSB fusion |
| Sclerosing epithelioid fibrosarcoma | t(11;22)(p11;q12) | EWSR1-CREB3L1 fusion |
| t(11;16)(p11;p11) | FUS-CREB3L1 fusion | |
| t(7;16)(p22;p11) | FUS-CREB3L2 fusion | |
| Solitary fibrous tumor | Inv(12)(q13q13) | NAB2-STAT6 fusion |
| Synovial sarcoma | ||
| Biphasic | t(X;18)(p11;q11) | Predominantly SS18-SSX1 fusion |
| Monophasic | t(X;18)(p11;q11) | SS18-SSX1 , SSX2, or SSX4 fusion |
| Tenosynovial giant cell tumor | t(1;2)(p13;q35) | COL6A3-CSF1 fusion |
| Well-differentiated liposarcoma/dedifferentiated liposarcoma | Ring form of chromosome 12 | Amplification of MDM2, CDK4, and others |
a These alterations are also present in other tumor types (including carcinomas, leukemias, or lymphomas).
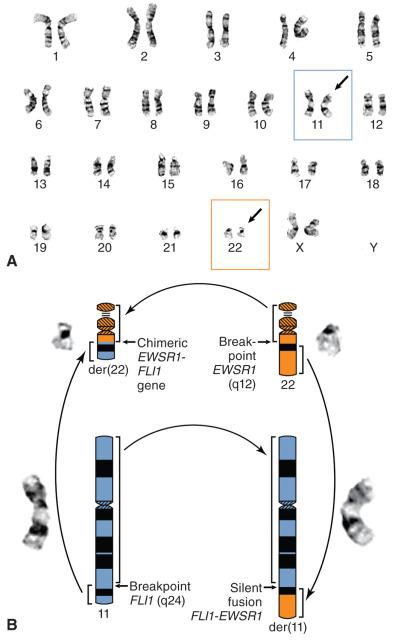
Over the past several decades, there has been a considerable increase in our understanding of the genetic basis of cancer, including sarcomas. Sarcomas have been amenable to a classic cytogenetic approach because, like many hematopoietic malignancies, a considerable subset has chromosomal translocations that can be detected by standard karyotyping of fresh tumor samples grown in culture. Ewing sarcoma was the first sarcoma and solid tumor noted to harbor such a translocation ( Fig. 18.2 ). For this reason and because the features of this tumor make it an ideal archetype, it is used in the following text to demonstrate the methods and principles of molecular diagnostic testing. Subsequently, an alphabetically ordered discussion of each soft tissue tumor with relevant molecular diagnostic features is briefly presented, including reference to other chapters in this book where the lesions are discussed in more detail.
It is interesting to note that the majority of sarcomas with unique chromosomal translocations tend to occur in younger patients, whereas those with more complex cytogenetic features tend to present in older ones. Furthermore, many soft tissue sarcomas with differentiation patterns that do not clearly recapitulate features of a known mature mesenchymal tissue (e.g., Ewing sarcoma, synovial sarcoma, and alveolar soft part sarcoma) harbor translocations ( Box 18.1 ). The significance of these intriguing associations is unclear. Each of these entities is discussed in detail in the preceding chapters; thus, here the discussion is strictly limited to the relevant molecular diagnostic considerations. The reader is encouraged to refer to the prior chapters and sections covering the individual entities for additional information. The role of these genetic events in the molecular pathogenesis of these neoplasms is a fascinating and rapidly evolving field, but a detailed discussion of each sarcoma presented subsequently is beyond the scope of this chapter.
Alveolar soft part sarcoma
Angiomatoid fibrous histiocytoma
Clear cell sarcoma of soft tissue
Desmoplastic small round cell tumor
Ewing sarcoma
Extraskeletal myxoid chondrosarcoma
Hemosiderotic fibrolipomatous tumor
Myoepithelioma/myoepithelial carcinoma/mixed tumor
Ossifying fibromyxoid tumor
Phosphaturic mesenchymal tumor
Synovial sarcoma
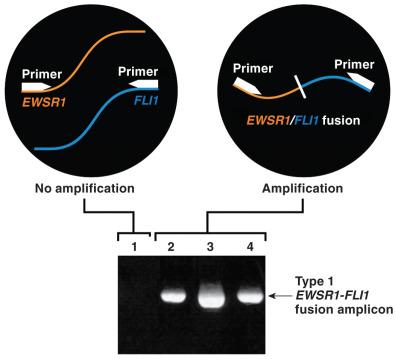
Ewing sarcoma was the first sarcoma recognized to have a recurrent cytogenetic abnormality, namely a balanced translocation between chromosomes 11 and 22 (see Fig. 18.2 ), which was initially discovered by chromosomal karyotypic analysis of cultured fresh human tumor. Further analysis refined the genetic intervals to 11q24 and 22q12, which were ultimately shown to contain the FLI1 and EWSR1 genes, respectively. Because this chromosomal rearrangement involves the transposition of material between two chromosomes without loss of genetic material, it is termed a balanced translocation. The two break points on chromosomes 11 and 22 occur in noncoding DNA introns; thus, when the chromosomes join, the exons from the two genes are in frame and ultimately produce a novel fusion or chimeric gene encoding a protein with an aberrant function ( Fig. 18.3 ). In Ewing sarcoma, this is a novel transcription factor wherein FLI1 provides the DNA-binding domain and the EWSR1 portion acts as a transactivator. This transcriptional activation may involve additional protein intermediaries, which is a topic of active research. This translocation is likely an early and necessary pathogenetic event, because the vast majority of well-characterized cases of Ewing sarcoma contain this or an analogous rearrangement. Additional genetic events are probably necessary, with this fusion gene likely relying on a specific cellular compartment to exert its effect (so-called lineage addiction or specificity).
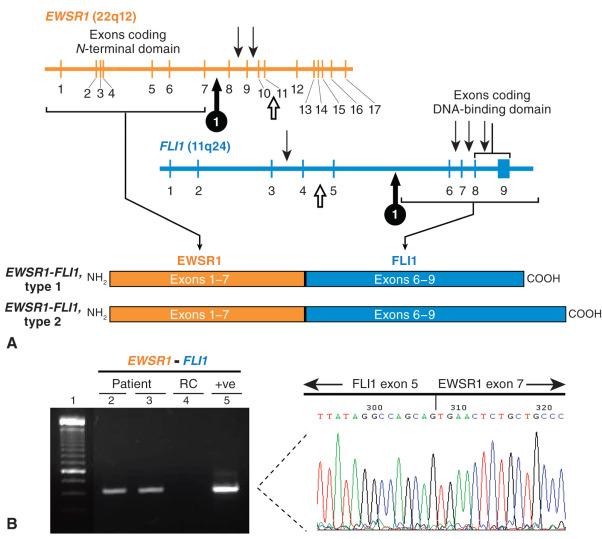
The break points within both FLI1 and EWSR1 can involve multiple different introns in each gene; thus, numerous different fusion transcripts and corresponding proteins have been described ( Fig. 18.4 ). The EWSR1 gene occupies approximately 40 kb and is composed of 17 exons that encode a protein with homology to a putative RNA-binding site of RNA polymerase II. The precise function of the native Ewsr1 protein is unclear. Approximately 80% of the break points in EWSR1 are located in introns 7 or 8 and result in a fusion transcript containing exons 1 to 7. Two particular fusion events are most common, constituting at least 80% of the fusions between these two genes, which are termed type 1 (60%) and type 2 (20%). The remaining EWSR1-FLI1 fusion types and other fusion variants are relatively rare as single entities (with the exception of EWSR1-ERG, about 5%) but as a whole may account for up to 10% of all cases. This may be an overestimate owing to the propensity of researchers to report rare events in the literature ( Table 18.4 ), as many of these variants exist only as single case reports. There also appear to be cases of Ewing sarcoma in which a fusion gene cannot be demonstrated. Some of these tumors are now known, with the help of NGS, to represent histologically similar tumors with alternative translocations, including CIC-DUX4 and BCOR-CCNB3 . Despite overlapping round cell morphology, these tumors appear to behave differently from Ewing sarcomas (see later discussion) and thus fall broadly under the rubric of unclassified round cell sarcoma in the classification scheme of the World Health Organization (WHO). Additional alternative fusions may be discovered, which might help classify these tumors when traditional morphologic features and immunohistochemical markers are insufficient.
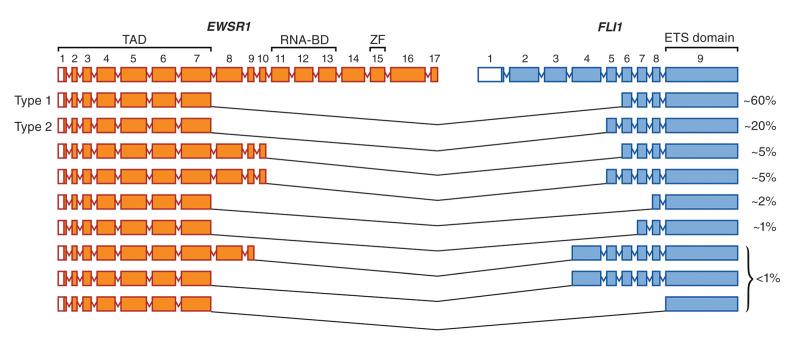
| Variant | Percentage (%) |
|---|---|
| EWSR1-FLI1 (all types) | 90 |
| EWSR1-ERG | 5 |
| EWSR1-FEV | <1 |
| EWSR1-ETV1 | <1 |
| EWSR1-ETV4 | <1 |
| EWSR1-ZSG | <1 |
| FUS-ERG | <1 |
| EWSR1-POU5F1 | <1 |
| EWSR1-PATZ1 | <1 |
| EWSR1-SP3 | <1 |
| EWSR1-NFATc2 | <1 |
| FUS-FEV | <1 |
| BRD4-NUT | <1 |
| None identified | Unknown |
The murine form of FLI1 contains a Friend murine leukemia retroviral integration site. This gene belongs to the DNA-binding protein superfamily and is rearranged in the erythroleukemias induced by this virus. Human Fli1 shows 70% amino acid sequence homology with Ets1, a gene expressed during cranial neural crest migration and apparently also during vasculogenesis, where it appears to act as a transcription factor. The alternative fusion partners that can substitute for FLI1 are primarily members of the ETS gene family. The DNA-binding domain of Fli1 substitutes for the RNA-binding portion of Ewsr1 in the fusion protein, creating a novel transcription factor. ERG (21q22) and four other described members of the ETS gene family can substitute for FLI1 in a small subset of cases. FUS (16p11) has been reported to substitute for EWSR1 in rare cases (see Chapter 8 for additional discussion of Ewing sarcoma). As illustrated in Fig. 18.5 , EWSR1 and FUS , a homologous gene, also contribute to multiple fusion genes present in a wide variety of soft tissue sarcomas.
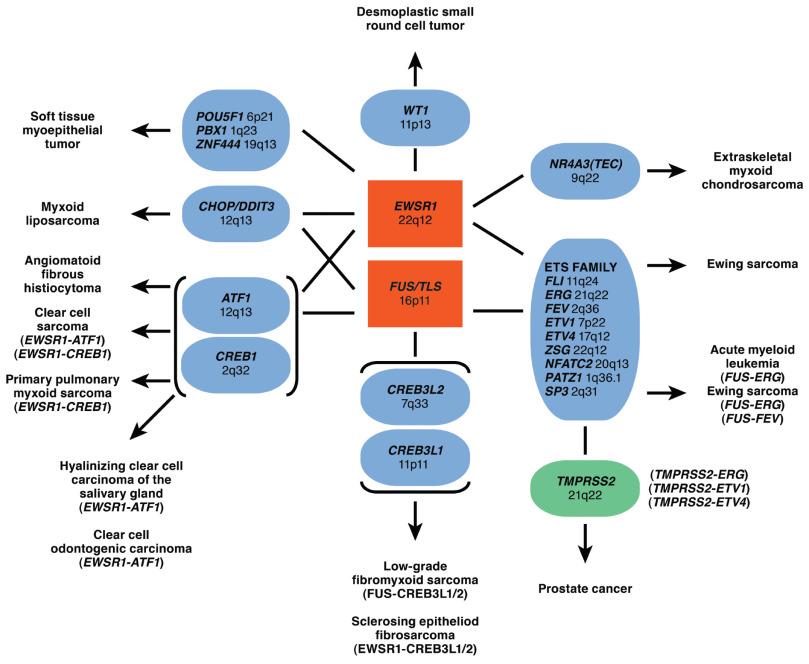
Previously, these fusion events had been considered to be unique to a specific tumor type; however, this assumption is no longer true. One example of this includes a class of tumors that have EWSR1 - CREB1 and EWSR1 - ATF1 translocations. These fusions are found in clear cell sarcoma, gastrointestinal clear cell sarcoma–like tumor, angiomatoid fibrous histiocytoma, and primary pulmonary myxoid sarcoma. Despite sharing the same translocation, these sarcomas have distinct histologic appearances, immunophenotypic findings, and behavior. EWSR1-ATF1 has also been described in nonmesenchymal tumors and is characteristic of hyalinizing clear cell carcinoma of the salivary gland and clear cell odontogenic carcinoma. Another example of overlap includes Ewing sarcoma and acute myeloid leukemia ( FUS - ERG ). Therefore not all fusion transcripts, nor the genes they involve, are necessarily exclusive to a single entity or to tumors of mesenchymal origin. This highlights the importance of the pathologist to correlate the molecular testing results with the histologic and immunohistochemical findings.
A brief discussion of the various techniques available for the detection of fusion events, using the case of Ewing sarcoma as an example ( Box 18.2 ), follows.
Gene fusions occurring in the same neoplasm that involve a single gene such as EWSR1 with multiple partners such as FLI1 and ERG in Ewing sarcoma. EWSR1 - FLI1 and EWSR1 - ERG are fusion variants.
Within a single fusion variant, the different exon combinations between two genes such as EWSR1 and FLI1 are known as types. In this example, the type 1 fusion in Ewing sarcoma fuses exon 7 of EWSR1 to exon 6 of FLI1 , whereas type 2 combines exons 7 and 5 of these two genes, respectively, when transcribed.
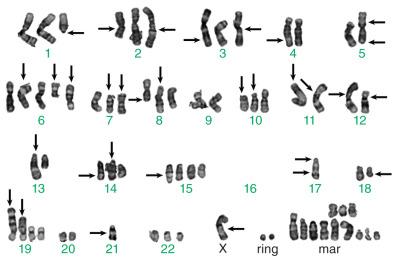
Chromosomal karyotyping is the traditional approach for cytogenetic evaluation; historically most chromosomal translocations were discovered in this fashion. In fact, data indicate that the number of translocations discovered in various families of tumors is directly proportional to the number of cases karyotyped. In effect, one must look for these alterations to find them. Increasingly, novel methodologies using NGS of RNA or DNA and innovative bioinformatic approaches have yielded exciting, novel discoveries in this area. Nonetheless, karyotype analysis remains an effective diagnostic and discovery approach in many situations. Fresh tumor cells are required for growth in culture and are treated to capture the cells in metaphase, where the chromosomes are condensed and amenable to analysis. Traditionally, the chromosomal spreads are treated with trypsin and stained with Giemsa to produce a G-banding pattern that allows identification of the 22 autosomal pairs and sex chromosomes. These are then formatted into a karyotype, as depicted in Fig. 18.2 for a case of Ewing sarcoma. A complex karyotype is depicted in Fig. 18.1 for comparison. Gross chromosomal abnormalities can be identified in this fashion, although small cryptic deletions, inversions, and translocations can be overlooked. Techniques such as spectral karyotyping and fluorescence in situ hybridization (FISH), discussed further on, can augment this methodology.
The main advantage of karyotyping is that it is open-ended; it evaluates all the chromosomes in a cell and is not targeted to a particular chromosomal event. Disadvantages include the fact that tumor cells, even malignant ones, are surprisingly difficult to grow in culture, and this technique requires highly skilled personnel both for growing cells and for the subsequent interpretation. In addition, as previously mentioned, small chromosomal alterations can be difficult to detect by karyotyping and can be missed.
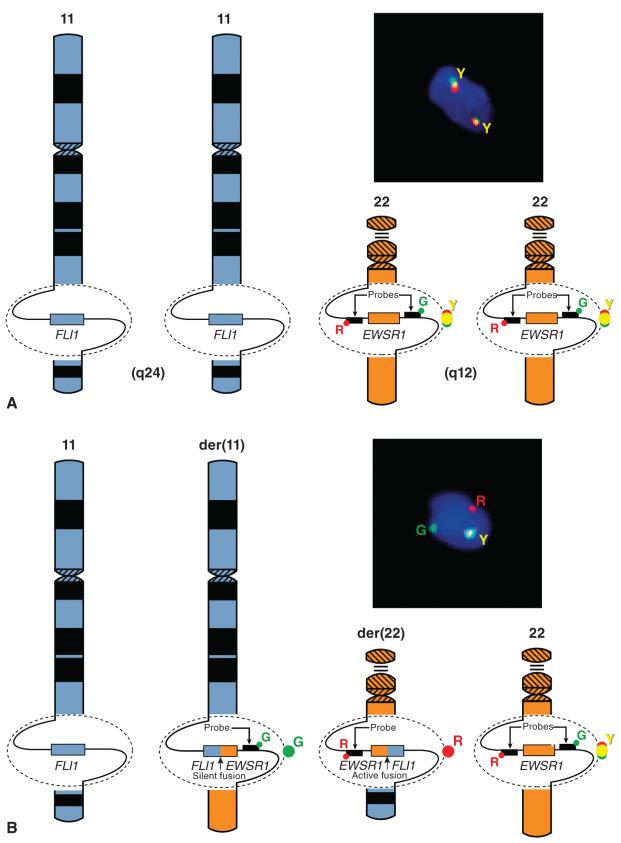
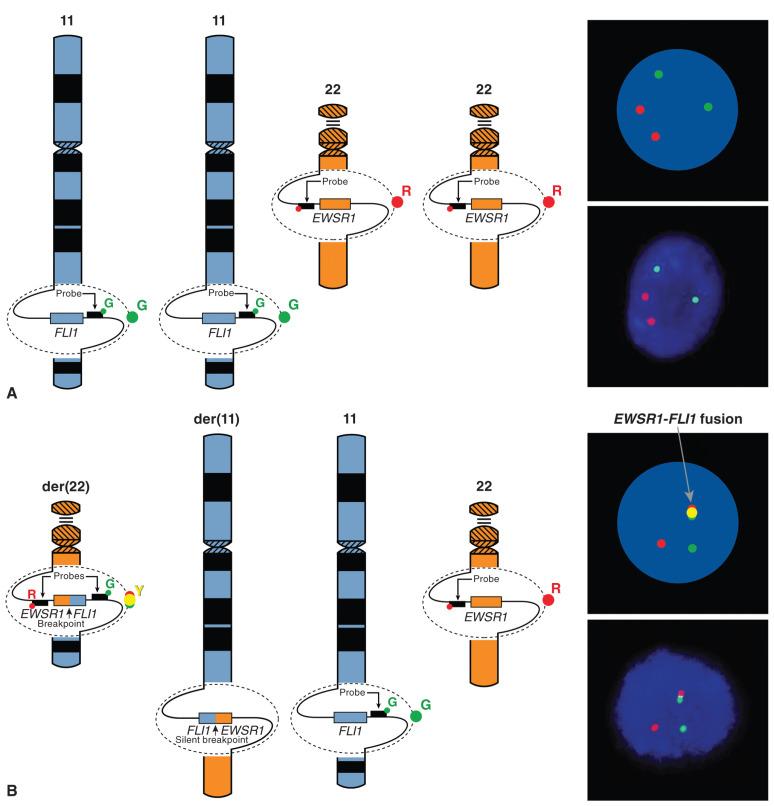
FISH can detect chromosomal translocations and also amplifications of a genetic locus. FISH relies on the ability to design DNA probes that specifically hybridize to a unique site on DNA. These probes are labeled with fluorescent dyes, often red and green, so that they can be distinguished from one another. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole (DAPI) which fluoresces blue, so that one can determine which probes are hybridized to the DNA content of an individual cell nucleus. The probes are designed to hybridize to the DNA immediately flanking the adjacent centromeric and telomeric borders of a gene or locus of interest. These regions are in close enough proximity that the overlap of the emission spectra of the green and red probes imparts a single yellow signal. There are normally paired chromosomes, each containing the gene or locus; therefore two yellow signals are observed. In an aneuploid nucleus with chromosomal gains or losses, more or fewer signals can be seen. When the probed locus is rearranged in a balanced translocation, the centromeric probe is retained and the telomeric probe is transferred to the reciprocal chromosome. At this point, the two probes are no longer restricted in close physical proximity, and the red and green probes are separately visualized because they reside on separately segregated chromosomes. This is known as a break-apart FISH probe strategy, which can be used on either interphase nuclei or in cytogenetic metaphase preparations. This technique is illustrated for the example of Ewing sarcoma in Fig. 18.6 . It is important to note that although this technique demonstrates that a probed locus is rearranged, it does not provide information regarding the identity of the reciprocal chromosome or fusion gene partner. This can be valuable in the case of Ewing sarcoma, because a number of alternative genes can recombine with EWSR1, and FISH detects all of them. However, because it shows an identical pattern of rearrangement for any fusion partner of EWSR1 , it is not specific for Ewing sarcoma versus the other sarcomas that harbor EWSR1 gene rearrangements, as illustrated in Fig. 18.5 and Table 18.4 . Thus careful correlation with other pathologic features and the clinical setting of any particular case is required to avoid misinterpretation of non–Ewing sarcoma cases, in which EWSR1 can also be rearranged. This potential pitfall can be avoided using the combination probe strategy, where the two loci are separately probed with red and green hybridization probes, such that a normal cell has two red probes and two green probes, and a cell harboring the rearrangement has one yellow (combined) signal along with single red and green signals ( Fig. 18.7 ). Although this technique has great specificity, a different set of probes is required for each potential binding partner. Virtually all commercially available probes for sarcomas are of the break-apart type.
Another application of FISH is to demonstrate amplification of a gene or chromosomal region. Here, one colored probe is used to hybridize to the centromere, and a second probe is used to hybridize to the potentially amplified region on the same chromosome. The centromeric probe controls for chromosomal copy number. If the ratio of probe to the potentially amplified locus is in excess of the centromeric probe, then amplification is demonstrated. An application of this technique is demonstrating amplification of the 12q13-15 region in well-differentiated or dedifferentiated liposarcoma (see later discussion).
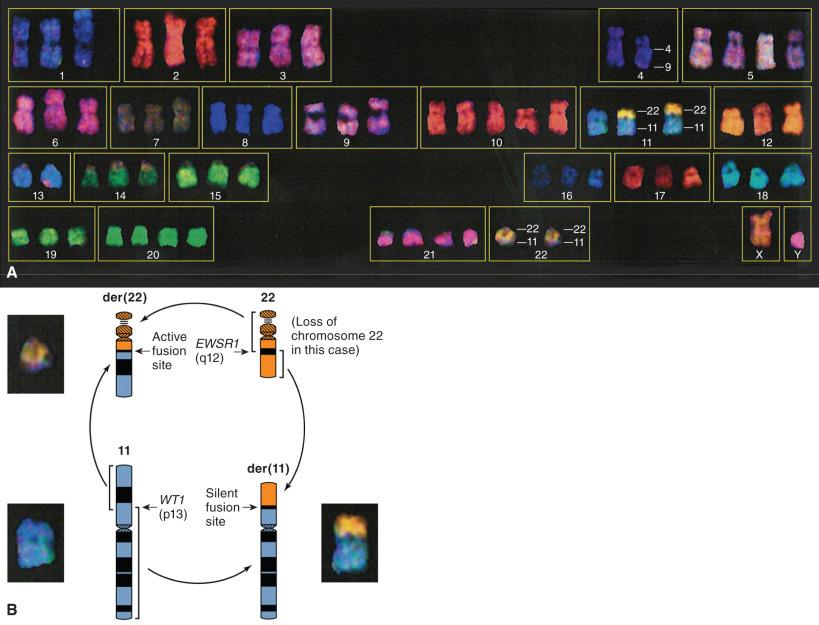
FISH is applicable to standard formalin-fixed paraffin-embedded (FFPE) sections, but it can also be used on touch preparations, karyotype metaphase preparations, and disaggregated nuclei from thick FFPE sections. A modification of the FISH technique allows whole chromosomal painting by numerous individual probes to each chromosome, so that each one is identified by a unique color (spectral karyotyping). A computer system using pseudocoloration enables the interpretation of complex karyotypes, as depicted for desmoplastic small round cell tumor in Fig. 18.8 . This technique is basically an unbiased enhancement of the traditional karyotyping method; although not usually necessary, it can be very helpful in complex cases ( Box 18.3 ).
Can be used to show rearrangement of a particular locus, such as EWSR1 or FUS
Can provide evidence for a particular fusion variant
Can provide evidence and rough quantification of the amplification of a locus such as 12q13-15 in well-differentiated liposarcoma
FISH , Fluorescence in situ hybridization.
Reverse transcription polymerase chain reaction (RT-PCR) makes use of the precise specificity of PCR primers and the enhanced detection afforded by amplification. Since the completion of the Human Genome Project, the sequences of the intron and exon segments of all human genes are known. Once the two genes involved in a specific translocation have been identified, primers can be designed to amplify the fusion types, which can sometimes be numerous, as for Ewing sarcoma (see Fig. 18.4 ). Genes are primarily composed of relatively large segments of nontranscribed intronic DNA. It is within these introns that the chromosomal breaks occur, allowing recombination in the nontranscribed DNA, so that when introns are transcribed to RNA, the proper reading frame is maintained, allowing for translation to an active chimeric protein. Because the introns are large, ranging from several to many kilobases, it is not practical to design primers to detect the actual recombination point in the genomic DNA. Recall that only about 1.5% of the ~3 billion base pairs within the human genome is exonic, comprising the ~20,000 genes ultimately transcribed to messenger RNA (mRNA) and then translated to protein. Furthermore, there is a limit to the length of an amplicon that can reliably be produced by PCR. This is further compounded by the fact that genomic DNA is sheared into relatively small fragments because of the cross-linking activity of formalin fixation. For PCR amplification to be consistent in this setting, amplicons cannot be larger than 150 to 200 base pairs, and smaller ones (<100 base pairs) are more reliable. Thus PCR-based strategies take advantage of mRNA instead of DNA because introns are spliced out when mRNA is produced. This juxtaposes the exon from one gene directly to an exon from the other gene when the fusion is present. For PCR to function, one must use a reverse transcriptase (RT) enzyme to convert the extracted RNA to DNA, known as complementary DNA (cDNA); thus the technique is known as RT-PCR. As depicted in Fig. 18.9 , one is able to amplify a product only if the primers extend toward each other in an intact fusion transcript. If no fusion gene is present, there is no amplification product. A housekeeping gene such as β-actin is usually also amplified to confirm that the cDNA is of sufficient quality and quantity to allow amplification (see Fig. 18.3B ). A variety of techniques can be used to confirm the identity of the amplicon, with direct sequencing being the most specific; but real-time detection, restriction fragment analysis, or other techniques can also be used.
PCR amplification of genomic DNA, followed by Sanger sequencing, can be used to search for mutations in introns such as the deletions, insertions, duplications, and point mutations seen in the KIT gene in GIST. These are discussed further later in the chapter. In using Sanger sequencing, it is important to have relatively pure tumor DNA, because there will usually be one nonmutated copy (allele) of a gene in addition to the mutated gene in a tumor cell. The limit of detection of such methods is theoretically about one tumor cell per five total cells (20% tumor; 10% mutated DNA) or better, but for the Sanger method to be reliably effective, tumor should ideally constitute 50% of the sample or greater. Microdissection may be helpful in samples where there is an abundance of nonneoplastic tissue. Alternative methods such as pyrosequencing or mass spectrometry, which have higher sensitivity, can also be used to evaluate for specific point mutations. The advent of NGS into clinical practice is dramatically reducing the 20% tumor needed as input for reliable results, as noted in the following text.
Use of RT-PCR to detect gene fusions is applicable to fresh and FFPE samples. An advantage of this technique is that the fusion product can be detected even if the tumor DNA is not enriched in the sample (unlike the demonstration of gene mutations in genomic DNA discussed earlier); therefore small samples such as needle biopsies can be used. When there are multiple or numerous different possible fusion types combining various exons between two genes, multiple primer pairs may be required. Similarly, when there are a variety of genes that can recombine in a particular tumor type, at least one primer pair for each of these must be used. As most RT-PCR assays are designed to detect the most common fusions, rare fusions can be missed. Finally, PCR is extremely sensitive, and contamination can be a problem. Thus it is critical to use negative controls and rigorously maintain strict separation of pre-PCR sample preparation and the PCR amplification/postamplification processes to minimize potential contamination.
Massively parallel DNA sequencing, commonly referred to as NGS, allows a sequencing examination of many genes in parallel. Although it is possible to sequence the entire genome or exome of a cancer sample, most clinical panels range from 25 to 500 genes considered important for cancer. In appropriately designed panels, it is also possible to obtain copy number data in addition to sequence. Many of the broad gene panels are developed based on common mutational events in carcinomas and melanoma; thus they are not optimized for sarcoma. Although some sarcomas have characteristic gene sequence mutations, such as KIT or PDGFRA in GIST or CTNNB1 (encoding β-catenin) in desmoid-type fibromatosis, many sarcomas lack specific gene sequence mutations that are helpful diagnostically and rather harbor translocations and/or copy number alterations. These single gene mutations can also be readily assessed by more traditional sequencing methods. However, most complex karyotype sarcomas are characterized more by extensive segmental copy number alterations rather than mutations, although TP53 , ATRX , and RB1 inactivating mutations are often encountered. Because of the broad coverage, NGS can also discover mutations that are rare in sarcomas but occur in potentially targetable genes such as BRAF or PIK3CA . Characteristic copy number alterations such as the punctuated amplification of the 12q13-15 interval in well-differentiated and dedifferentiated liposarcomas can also be demonstrated on appropriately designed NGS panels. NGS can be readily applied to FFPE specimens. It has additional advantages in that the amounts of DNA needed for sequencing are relatively small and the percent tumor nuclei is lower than that needed for Sanger and other forms of sequencing. Though very powerful, NGS is more complex than traditional sequencing; a germline source of DNA is often needed for comparison to the tumor sample (particularly in large gene panels), and extensive bioinformatic support is needed for definitive interpretation. A full discussion of NGS is beyond the scope of this chapter.
Another exciting application of NGS in sarcoma is RNA sequencing (RNA seq) to demonstrate characteristic gene fusion events in the simple karyotype sarcomas. Like RT-PCR, this method is aided by splicing out the large introns of genes so that only the linked exons remain; thus the junction between two different genes can be more readily detected ( Fig. 18.10 ). A more in-depth description of this technique is beyond the scope of this chapter. Although the entire transcriptome (all coding RNA) can be sequenced, it is usually more practical to examine relevant RNA subsets. Methods have been developed that employ primers based on the known break point of one fusion gene and random primers that will detect any second gene involved in the fusion. Such methods can be very effective for promiscuous genes, which can have many partners and break points and which are challenging to address with RT-PCR due to the many primer sets required. The use of panels that can detect fusions with many genes allows a single test to be used for the detection of many different fusions and can also lead to the discovery of unexpected or novel fusion in clinical cases. Gene expression signatures can also be obtained through RNA seq in sarcoma and, combined with copy number data, can convey prognostic information, but clinical implementation remains a future goal.
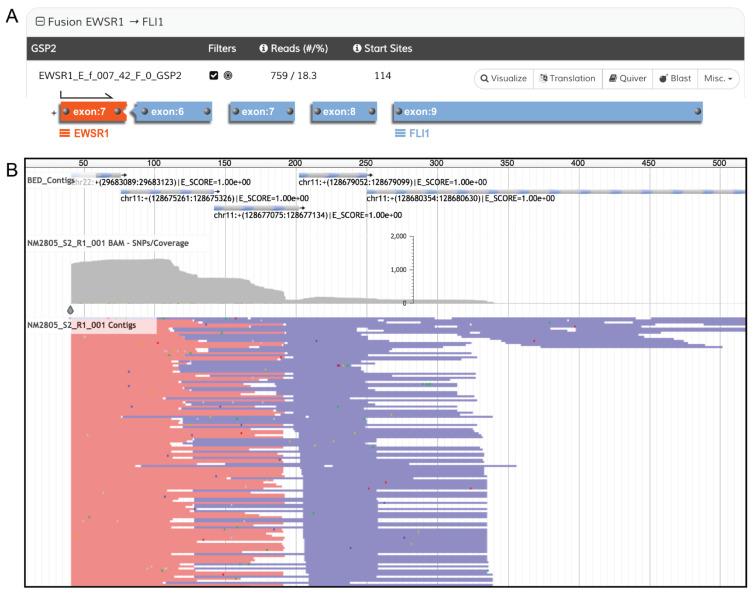
Both RNA seq and DNA NGS testing are transforming the molecular diagnostics landscape, but they require complex bioinformatic processing to support the interpretation of the sequencing results. As many different genes will be sequenced, DNA or RNA (cDNA) libraries incorporating tumor DNA must be constructed. To adequately assess sequence variants and mutations, normal or germline DNA must usually also be sequenced for comparison. Thus the increased sequence information that one gains is earned through more complex, time-consuming, and expensive procedures and extensive bioinformatic interpretation. However, the costs are dropping rapidly, and NGS is quickly becoming the sequencing platform of choice for many applications in molecular testing.
Definitively demonstrates a specific fusion variant and type
Detects only the specific fusion variants and types for which it was designed
Care must be taken to avoid contamination yielding false positive results
Technique works well in both fresh and formalin-fixed paraffin-embedded tissue
RNA from blocks older than 5 years of age or decalcified blocks can be often unreliable due to RNA degradation
From the preceding discussion, it is clear that there are multiple levels at which evidence of a gene fusion event can be identified ( Fig. 18.11 ). Cytogenetics can reveal chromosomal features using a traditional karyotype. FISH allows a more focused look at a specific genetic locus, and spectral karyotyping (essentially whole-genome FISH) provides an unbiased and enhanced view of the entire chromosomal complement. Although translocations can be revealed at the genomic DNA level, these are difficult to detect within large introns and require long-range PCR or NGS that tiles across entire introns. Such approaches are amenable to research applications but are rarely practical for clinical diagnostic work. Transcription to RNA avoids this problem and, using reverse transcription, fusion transcripts can readily be detected. RNA seq is gaining popularity as a sequencing method for fusion detection. Fusion transcripts are ultimately translated into proteins, which, in some cases, can be detected by immunohistochemistry, although the available antibodies are often not completely specific. Finally, many of the soft tissue tumors associated with translocations have unique histologic features. These features are presumably strongly linked to the action of the expressed fusion gene. Thus there are many levels of biologic evidence that directly or indirectly suggest the presence of the fusion gene.

Currently emerging technologies are being applied to sarcomas. These include gene expression arrays, which examine RNA and microRNA production from genes; comparative genomic hybridization, which demonstrates DNA copy numbers of chromosomal regions; DNA methylation studies, which can reveal transcriptionally active and inactive regions of the genome; and proteomic approaches, which examine protein expression and state of activation by a variety of techniques. NGS approaches to characterize genomes and transcriptomes have also provided fascinating insights into biology and are increasingly incorporated into the clinical workflow, as discussed earlier. DNA methylation studies are also of increasing interest. These approaches have provided critical insights into the classification and biology of these tumors. These techniques are beginning to be applied to sarcoma for diagnosis and prognosis. Traditional molecular studies are critically important for sarcoma diagnosis and clinical management. Embracing new technologies where appropriate will further assist diagnosis and treatment of this group of diseases.
This section contains a discussion of particular soft tissue sarcomas for which molecular features may be evaluated as a diagnostic adjunct. Although numerous chromosomal alterations and, less commonly, DNA mutations are found in sarcomas, this discussion focuses on those that are diagnostically relevant. This is followed by a discussion of specific differential diagnostic conundrums where molecular diagnostics may be particularly helpful. In keeping with the approach of this book, the focus is on practical applications of molecular characterization; the many recent and exciting research advances for many of these tumors are not discussed unless they are currently germane to diagnostic or prognostic molecular analysis. Future trends in diagnostic testing for certain tumors are presented when relevant. The clinical and morphologic features and diagnostic criteria for these entities are presented in previous chapters; thus the reader is referred there for additional information.
As previously mentioned, sarcomas generally fall into two cytogenetic classes: those with complex karyotypes and those with simple karyotypes, the latter often harboring diagnostically useful translocations or mutations. The neoplasms in the former category have complex karyotypes; some of these complex chromosomal alterations may have prognostic implications, although additional study is needed. Some of these tumors have also now been discovered to have recurrent chromosomal alterations or genetic mutations that can be diagnostically useful, as discussed earlier. A complex karyotype is presented in Fig. 18.1 . It is important to recognize that even neoplasms with characteristic genetic alterations may go on to develop complex karyotypes with tumor progression. On the basis of the clinical, morphologic, and immunohistochemical features of the tumor, such investigations can be performed using FISH, spectral karyotyping, or RT-PCR techniques. Soft tissue sarcomas within this group are listed in Table 18.1 . Most cytogenetically complex sarcomas have many more copy number alterations than classic activating or inactivating gene mutations. The critical genetic events driving the biology of these sarcomas are largely unknown.
Tumors with simple cytogenetic features often have characteristic molecular findings that can be exploited diagnostically. Although gene fusion producing a novel transcription factor with aberrant activity is the most common pathway, other mechanisms are also involved, as listed in Table 18.5 . These are discussed further in the following individual sections.
| Molecular Feature | Example(s) |
|---|---|
| Chromosomal Translocation/Gene Fusion | |
| Novel transcription factor with aberrant function | Ewing sarcoma |
| Synovial sarcoma | |
| Overproduction of a normal growth factor | Dermatofibrosarcoma protuberans |
| Change in subcellular localization of tyrosine kinase receptor | Inflammatory myofibroblastic tumor |
| Gene Mutation | |
| Ligand-independent activation of tyrosine kinase receptor | Gastrointestinal stromal tumor |
| Decreased degradation of a protein | Desmoid fibromatosis |
| Increased activation of a signaling molecule | Intramuscular/cellular myxoma |
Many sarcomas can be classified according to the cell type or tissue whose lineage they most closely resemble, such as leiomyosarcoma (smooth muscle), angiosarcoma (endothelium), and rhabdomyosarcoma (skeletal muscle). However, there are a group of sarcomas whose lineage is uncertain. Interestingly, chromosomal translocations and resultant fusion genes are especially common in the latter group, as depicted in Box 18.1 .
Tumors in the simple cytogenetic group are discussed further in alphabetical order to allow for ease of reference. Table 18.3 lists the tumors in this group, along with their relevant cytogenetic and molecular features. Principles of molecular diagnostics are discussed within these sections as relevant, followed by a discussion of the practical use of molecular testing to resolve examples of particularly problematic differential diagnostic situations.
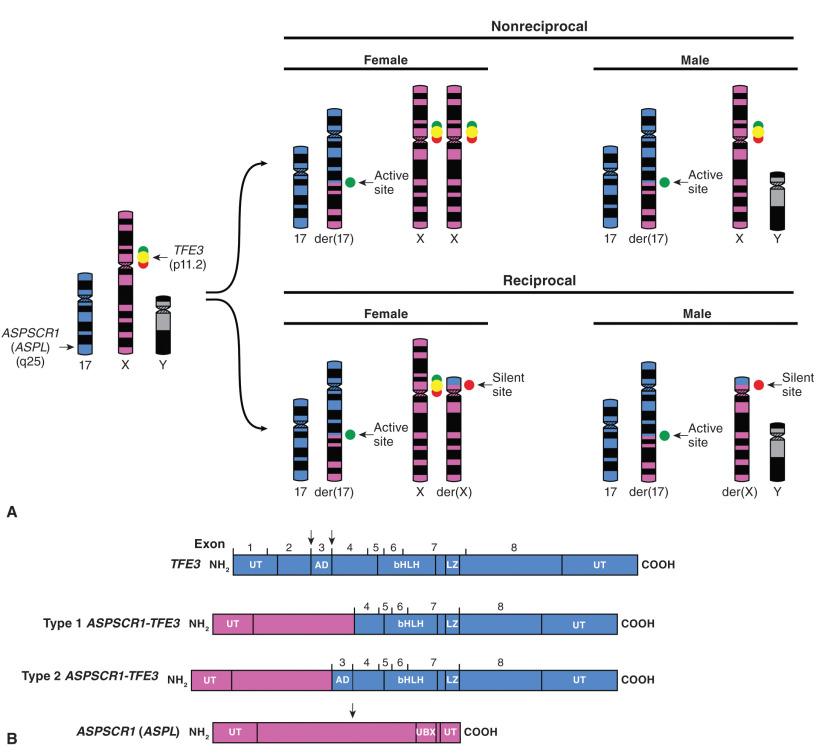
This rare soft tissue sarcoma (see Chapter 6 ) is associated with an unbalanced translocation der(17)(X;17)(p11;q25). Chromosome 17 contains a novel gene known as ASPSCR1 (formerly ASPL ), whereas chromosome X contains TFE3 , which provides the DNA targeting function of the fusion protein. Not much is known about the function of the protein encoded by ASPSCR1 , but TFE3 encodes a member of the helix-loop-helix superfamily of DNA-binding proteins, which is normally involved in immunoglobulin gene regulation. TFE3 is also rearranged with ASPSCR1 and other genes in rare forms of renal cell carcinoma, which are most common in children. Interestingly, the TFE3 - ASPSCR1 translocations in the renal cell carcinomas tend to be balanced, whereas the translocation is unbalanced in alveolar soft part sarcoma ( Fig. 18.12A ). TFE3 has also been found to be rearranged in a small subset of perivascular epithelioid cell tumors (PEComas) (see Chapters 6 and 16 ). Translocations can be demonstrated by FISH or RT-PCR. Break-apart FISH probes directed at TFE3 are generally used. TFE3 is located on chromosome X; therefore the FISH probe pattern changes depending on whether the patient is male or female and whether the translocation is the most common unbalanced type or a rare instance of a balanced translocation. By RT-PCR, two alternative transcripts are seen that differ by inclusion of one exon from TFE3 . The break point in ASPSCR1 is constant. Type 1 does not include exon 3 of TFE3 and constitutes approximately 75% of reported cases, whereas type 2 includes exon 3 and accounts for 25% of cases (see Fig. 18.12B ). Virtually all cases of alveolar soft part sarcoma show evidence of one of these two variant fusion transcripts. Tumors can be tested for reciprocity using PCR primers to the silent fusion site. This is negative in most cases of alveolar soft part sarcoma and positive in the subset of renal cell carcinomas that combine TFE3 and ASPSCR1 . Such information could potentially be useful if these two entities needed to be distinguished in a small biopsy, but this would be an uncommon differential diagnosis. Immunohistochemistry demonstrating nuclear TFE3 staining is strongly suggestive of the presence of the fusion transcript, although the available antibodies have been difficult to optimize and can show somewhat inconsistent results.
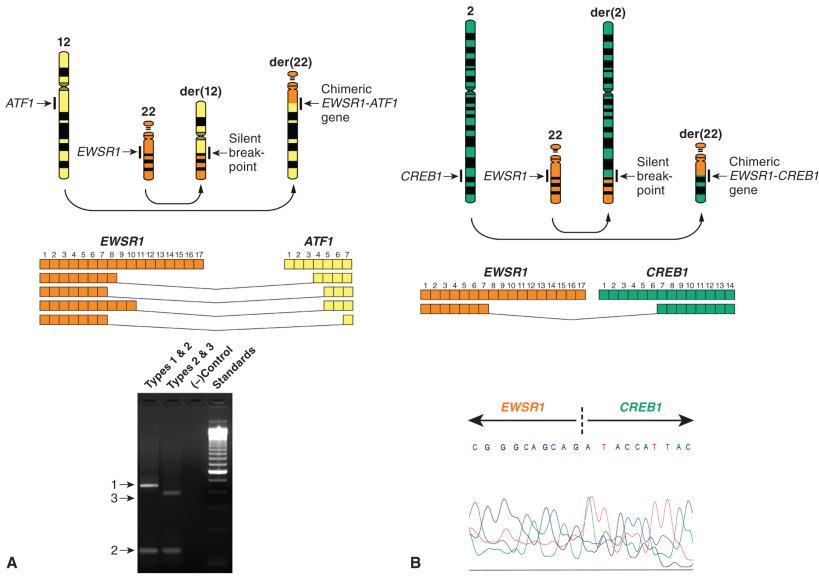
Angiomatoid fibrous histiocytoma (previously referred to as angiomatoid MFH) is a rare tumor of uncertain differentiation and very limited metastatic potential (see Chapters 3 and 10 ). Several chromosomal translocations have been identified in this neoplasm. The first demonstrated were t(12;16)(q13;p11) and t(12;22)(q13;q12), resulting in FUS - ATF1 and EWSR1 - ATF1 fusion genes, respectively. These findings were very surprising, because the EWSR1 - ATF1 fusion is precisely the same fusion variant seen in clear cell sarcoma, a much more aggressive tumor that will be discussed further in the following paragraphs. This indicates that some fusion genes are not diagnostic of a single entity and must therefore be interpreted in the context of clinical and histologic features. The substitution of EWSR1 and FUS is not surprising because these genes are homologous, and similar substitutions are seen in rare cases of Ewing sarcoma and myxoid liposarcoma (as well as other tumor types), as illustrated in Fig. 18.5 . More recently, it has become clear that t(2;22)(q33;q12), resulting in an EWSR1 - CREB1 fusion gene, is the most common genetic event in angiomatoid fibrous histiocytoma. Both ATF1 and CREB1 belong to the cyclic adenosine monophosphate (cAMP) response element binding a protein family of transcription factors and likely affecting the transcription of similar genes. Interestingly, a FUS - CREB1 fusion variant has not yet been described. The vast majority of angiomatoid fibrous histiocytomas contain one of these three gene fusions, but additional variants with other homologous genes may eventually be discovered. These rearrangements are readily detected by FISH. RT-PCR is also a viable option, because there is only one type of EWSR1 - CREB1 variant; the other fusion variants result in limited fusion types as well ( Fig. 18.13 ). Both EWSR1-CREB1 and EWSR1-ATF1 are also shared with other tumor types, most notably clear cell sarcoma. See the following section for more information regarding the fusion types and variants in this family.
Angiosarcoma typically has complex karyotypes. However, recent studies have found recurrent mutations including PTPRB and PLCG1 in a subset of angiosarcomas. Another series revealed recurrent CIC gene abnormalities including rearrangements as well as mutations in PLCG1 and KDR . Some of these mutations were associated with site and prognosis. Finally, amplification of MYC and sometimes FLT4 is seen in those tumors that are radiation-associated. This amplification can be detected by FISH or by immunohistochemical studies.
These common cutaneous tumors (see Chapter 15 ) have been found to harbor translocations involving the regions encoding for the membrane components of LAMTOR1 (11q13), podoplanin ( PDPN ; 1p36), CD63 (12q12-13), and the catalytic domain of the protein kinase C isoforms PRKCD (3p21) or PRKCB (16p12). The fusion proteins are thought to result in aberrant activated kinases in the cell membrane. Another study identified a case of aneurysmal fibrous histiocytoma that harbored the translocation t(3;11)(p21;q13), resulting in two fusions LAMTOR1 - PRKCD and NUMA1 - SFMBT1 . Although the vast majority of cases are benign and molecular confirmation is not needed for diagnosis, there are rare cases that metastasize (termed by some as benign metastasizing fibrous histiocytoma); these are often deep-seated and large. Tumors with aggressive behavior have increased chromosomal aberrations with frequent gains of 7 and 8q and loss of Xq in metastatic cases, recurrent losses in 9 and 22 in atypical fibrous histiocytoma, and isolated loss of 5q and gains in 20 in cellular benign fibrous histiocytomas. Although previously considered to be a histologic variant of fibrous histiocytoma, the majority of epithelioid fibrous histiocytomas have recently been identified to have ALK (2p23) rearrangements. This finding suggests that they may be unrelated to conventional fibrous histiocytomas. Immunohistochemical study for ALK can be useful in these tumors and correlates strongly with ALK rearrangement demonstrated by FISH.
This rare neoplasm harbors a PAX3 - MAML3 gene fusion in over half of cases. PAX3 - NCOA1 and PAX3 - FOXO1 fusions have also been identified; tumors with such rearrangements sometimes show focal rhabdomyoblastic differentiation. Both PAX3 break-apart FISH and RNA seq focused on PAX3 are reasonable diagnostic approaches.
This neoplasm was previously also known as melanoma of soft parts owing to the presence of melanocytic differentiation and in some cases melanosomes and melanin production (see also Chapter 3 ). In the clear cell sarcomas that occur in the distal extremities, the predominant chromosomal translocation is t(12;22)(q13;q12), which is detected in almost all such cases. There are four described fusion types, with types 1 and 2 constituting about 95% of reported cases. Interestingly, a mixture of both type 1 and type 2 can be detected in many cases by RT-PCR, perhaps indicating alternative splicing of the fusion gene (see Fig. 18.13A ). The biologic significance of this finding is unclear, but this phenomenon has not been clearly documented in other fusion genes, including other variants that involve the EWSR1 gene. More recently, a clear cell sarcoma–like tumor of the gastrointestinal tract has been described, which is often associated with t(2;22)(q33;q12), resulting in an EWSR1-CREB1 fusion gene (see Fig. 18.13B ) (see also Chapter 16 ). This translocation was initially described in a gastrointestinal clear cell sarcoma–like tumor and subsequently identified in angiomatoid fibrous histiocytoma (see previous section). Only a single type of this fusion gene variant has been described. Interestingly, clear cell sarcoma–like tumors of the gastrointestinal tract, with either EWSR1-ATF1 or EWSR1-CREB1, lack melanocytic differentiation, although they show S-100 protein and SOX10 expression by immunohistochemistry. The majority of such tumors show histologic features distinct from conventional clear cell sarcoma, suggesting that they may represent a unique tumor type (see Chapter 16 for details). The EWSR1-CREB1 fusion gene has also been reported rarely in distal extremity clear cell sarcoma.
It is intriguing that precisely the same fusion genes can be encountered in both the highly malignant clear cell sarcoma and the indolent angiomatoid fibrous histiocytoma. One model of mesenchymal tumor histogenesis suggests that the fusion gene drives the biology of the tumor type with which it is associated, perhaps by occurring in a relatively primitive mesenchymal cell ( Fig. 18.14A ). This model breaks down in the case of angiomatoid fibrous histiocytoma and clear cell sarcoma (see Fig. 18.14B ). Based on the recent work of Capecchi and colleagues on alveolar rhabdomyosarcoma and synovial sarcoma, it seems that the precise cell in which a fusion gene occurs and the differentiation state of that cell are critical factors in tumorigenesis. Even within a defined cell lineage, a certain cellular compartment may be permissive to tumorigenesis, whereas a closely related cell may find the fusion gene to have little or no effect or even to be lethal (see Fig. 18.14C ). Furthermore, although fusion genes appear to be very early events in tumorigenesis, additional genetic events may also be required. This is an area of active research.
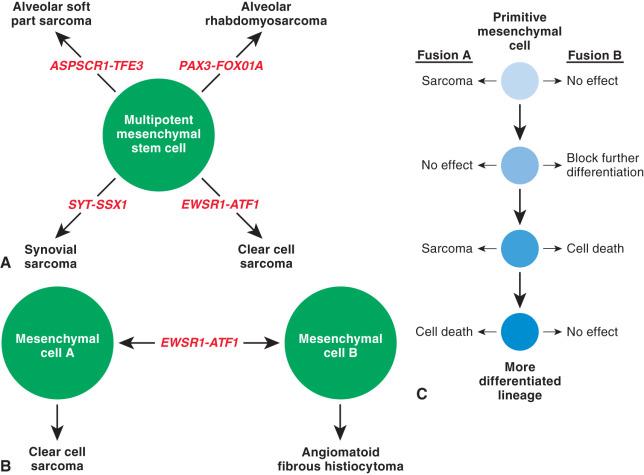
The lack of fusion variant specificity has diagnostic implications as well. Multiple fusion genes involve EWSR1 or other loci such that detection of rearrangement by break-apart FISH, which does not provide the specific fusion partner, can be a diagnostic pitfall (see Fig. 18.5 ). In addition, the exact same fusion gene variant and type can be detected in two distinct sarcoma types. The EWSR1-ATF1 fusion transcript has also been identified in primary pulmonary myxoid sarcoma, hyalinizing clear cell carcinoma of the salivary gland, clear cell odontogenic carcinoma, and recently a novel myxoid mesenchymal tumor with predilection for intracranial locations, further demonstrating the lack of tumor specificity ( Box 18.4 ). This can add complexity and nuance to the interpretation of molecular testing in certain instances.
Clear cell sarcoma
Angiomatoid fibrous histiocytoma
Malignant gastrointestinal neuroectodermal tumor (clear cell sarcoma–like tumor of the gastrointestinal tract)
Hyalinizing clear cell sarcoma of the salivary gland
Clear cell odontogenic tumor
Primary pulmonary myxoid tumor of lung
Novel myxoid mesenchymal tumor with predilection for intracranial location
This superficial dermal sarcoma is associated with overexpression of platelet-derived growth factor-β (encoded by PDGFB ), driven by the strong promoter of COL1A1 , the gene encoding collagen type 1. This fusion gene is formed by a usually unbalanced der(17)(17;22)(q12;q12) and is often expressed as supernumerary ring forms of duplicated segments of chromosomes 17 and 22, resulting in amplification of the fusion gene. This fusion gene functions differently than those described previously; the COL1A1 ultimately provides a promoter function as the amino terminus of the fusion protein is proteolytically processed and the remaining carboxy terminus dimerizes to produce “wild type” PDGF-β ( Fig. 18.15 ). Thus this mechanism serves to overproduce a normal protein rather than producing a novel fusion protein with aberrant function. COL1A1 comprises 52 exons, and the break points are scattered within any of the intervening introns. The break point in PDGFB is invariant, occurring in the intron preceding exon 2, thus allowing for production of the full-length protein after further processing. Because of the many different exons that can be donated by COL1A1 , RT-PCR primer design is challenging and requires multiple multiplexed amplification reactions. Focused RNA seq is a good potential solution that overcomes the primer design and multiplexing issues. Break-apart FISH probes for the PDGFB locus are also used. Interpretation can be a bit challenging, because the centromeric probes can be amplified, sometimes prominently, in the ring chromosomes.
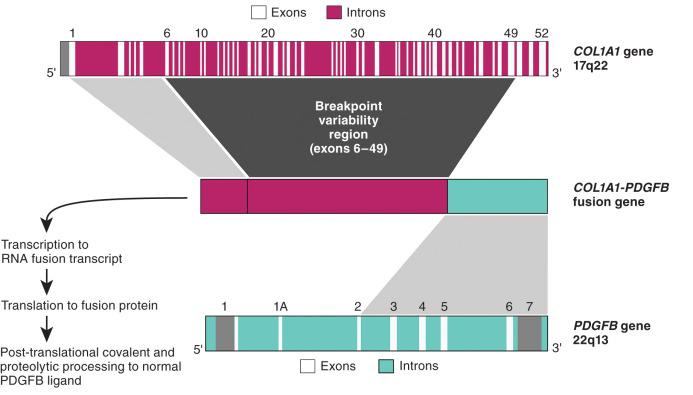
Direct demonstration of the COL1A1-PDGFB fusion gene is usually not required diagnostically, given the characteristic morphology and diffuse CD34 immunoreactivity in dermatofibrosarcoma protuberans (DFSP) (see Chapter 15 ). However, the fibrosarcomatous variant and the pediatric giant cell fibroblastoma can be diagnostically challenging. In addition, sometimes the evolution to higher grade in DFSP can adopt unusual morphologic features resembling undifferentiated pleomorphic sarcoma. Copy gains of COL1A1 - PDGFB can be seen in the fibrosarcomatous variant, and generally more copies are seen in DFSP than in giant cell fibroblastoma. Although DFSP is usually a surgical disease, tumors not amenable to resection can be treated with imatinib mesylate; in this setting, demonstration of the fusion event may be desirable. Imatinib mesylate blocks the kinase function of the PDGF receptor-β and thus inhibits the function of the overproduced ligand, PDGF-β.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here