Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Most thoracic aortic aneurysms arise from medial degeneration or from chronic aortic dissection. The overall incidence of thoracic aortic aneurysms (TAA) is approximately 10.4 per 100,000 person-years with a significant increase with advancing age. In contrast to abdominal aortic aneurysms (AAA) which predominantly affect men, TAAs arise equally in women. Fortunately, aneurysms that involve the aortic arch (15%) or thoracoabdominal aorta (10%) are relatively uncommon compared to isolated ascending (40%) or descending thoracic aneurysms (35%) as their surgical repair, with either open or endovascular means, can be quite complex. Traditional open repair of aortic arch aneurysms and thoracoabdominal aortic aneurysms (TAAA) carry substantial morbidity and mortality (see Ch. 79 , Thoracic and Thoracoabdominal Aneurysms: Open Surgical Treatment). A role for an open repair will likely always remain, but with advances in endovascular technology and techniques, catheter-based approaches to these complex aneurysms are now available. This chapter focuses on the various endovascular strategies to manage TAAAs and aortic arch aneurysms. Details related to endograft design, procedural planning, and operative conduct are described in Chapter 82 (Fenestrated and Branched Endograft Treatment of Juxtarenal, Paravisceral, Thoracoabdominal and Aortic Arch aneurysms: Device Selection and Technical Considerations).
The primary goal of treating patients with TAAA is to prolong meaningful life by reducing aortic-related mortality from rupture. For patients with intact, asymptomatic aneurysms, surgical intervention depends upon a balance between the risks of the procedure and that of nonoperative management. A detailed understanding of the morbidity and mortality related to repair, the diameter-associated risk of rupture, the interval growth rate, and the patient’s overall life expectancy and quality of life are necessary to make this decision.
Although more nuanced and individualized metrics are needed, aneurysm diameter is currently the most reliable predictor of aneurysm rupture and is therefore used as the most common indication for repair. The maximum diameter of the aorta from outer wall to outer wall in an orthogonal view should be used. The European Society for Vascular Surgery has released updated guidelines in 2017 for the treatment of patients with TAAAs. They recommend repair of TAAA for low to moderate surgical risk patients with TAAA greater than 60 mm (less for patients with connective tissue disorders), rapid growth (>10 mm/year), or with symptoms. A societal task force from the United States provided similar recommendations in 2010 using 60 mm diameter as the threshold to repair TAAAs. It is unclear, however, if utilization of a total endovascular repair of TAAAs will affect this diameter threshold over time, given that this treatment strategy may be associated with decreased procedural morbidity and mortality compared to traditional open repair.
Endovascular repair of TAAAs requires that the endograft span the visceral/renal segment of the aorta. Consequently, the endograft design and operative strategy must ensure preservation of perfusion to these critical aortic branch vessels. Fenestrations, circular openings in the endograft fabric, and/or branches, short cylindrical tubes attached to the endograft, can be constructed based on the patient’s preoperative computed tomography angiogram (CTA) imaging. If properly aligned, these two constructs permit flow into the visceral and renal target vessels that can be secured with bridging covered stents extending from the fenestration or branch to the target vessel. Fenestrated and/or branched endovascular repair (F/BEVAR) has been used over the past two decades for treating complex aortic pathology. Much like the perioperative advantages seen with thoracic endovascular aortic repair (TEVAR) and abdominal endovascular aortic repair (EVAR), F/BEVAR has been shown to effectively treat TAAAs with relatively decreased morbidity and mortality compared to traditional open repair. As with all endovascular procedures, there is a steep learning curve with F/BEVAR and durability remains an issue.
A variety of F/BEVAR devices exist which include physician-modified endografts (PMEG), as well as custom-made devices (CMD) and off-the-shelf designs manufactured by endograft companies.
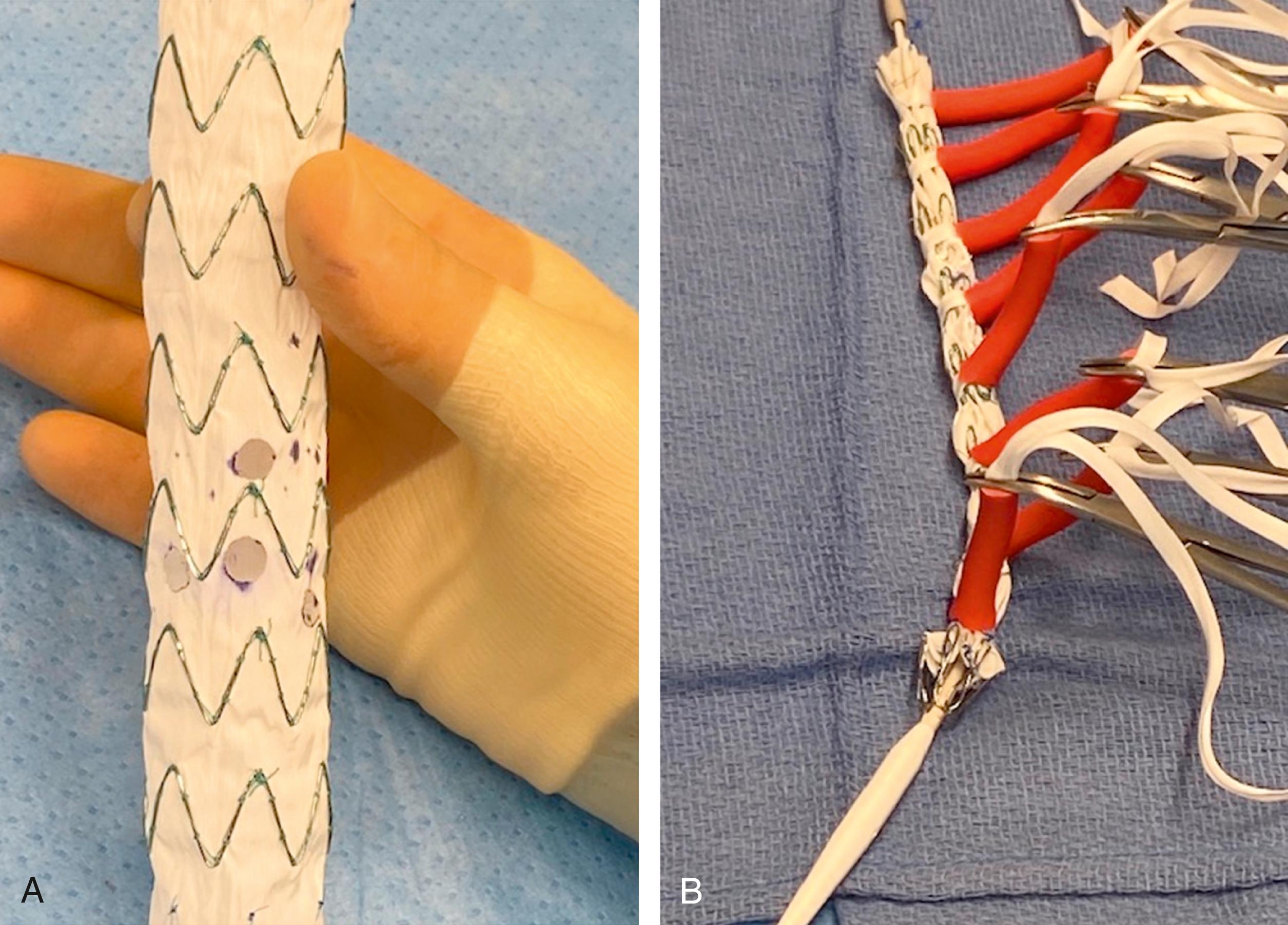
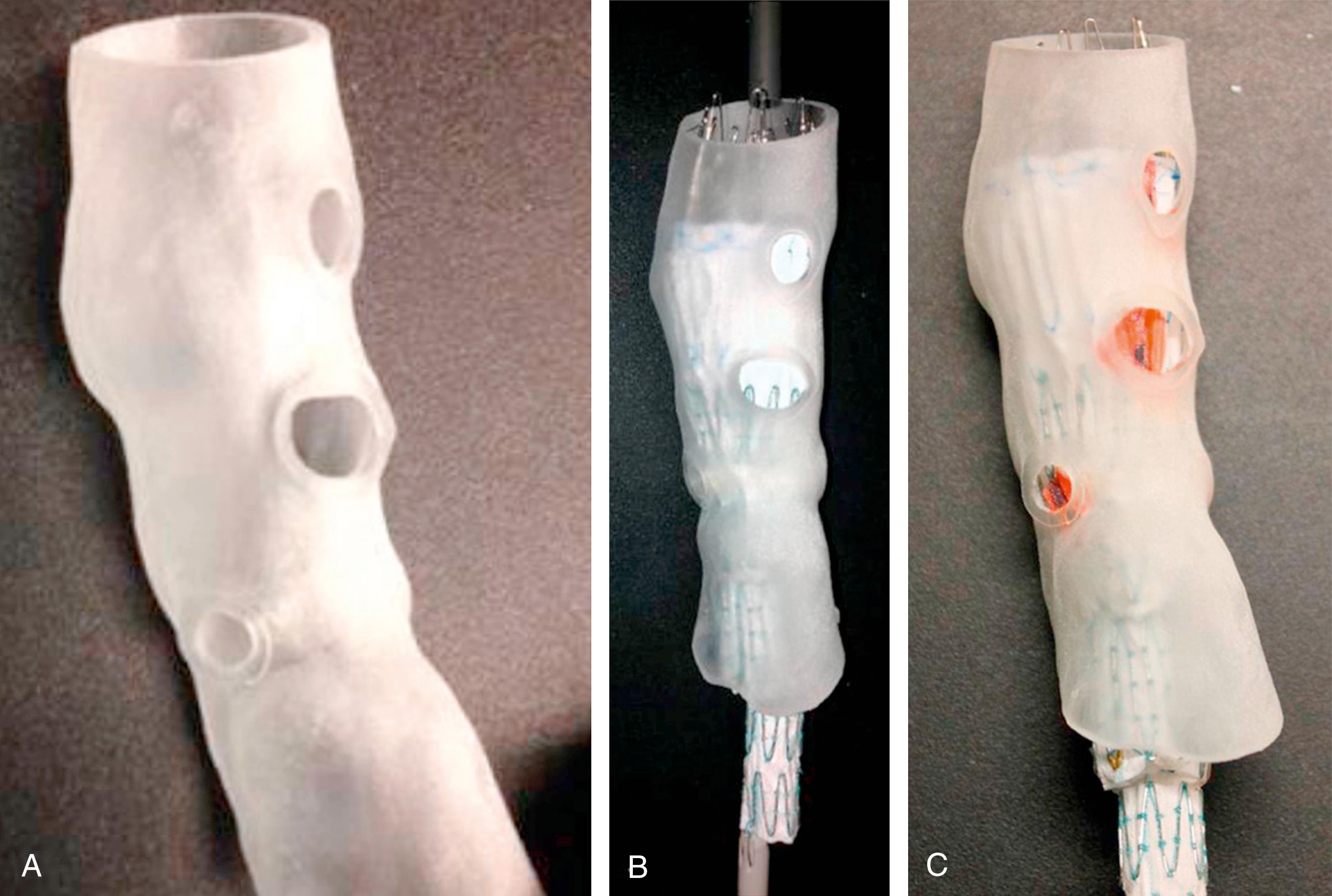
A PMEG requires a physician to make modifications to a commercially available endograft on a sterile back-table prior to implantation. Using CTA-derived preoperative centerline reconstruction measurements, the physician will add custom fenestrations or branches to the endograft for the visceral and renal aortic branches using a bovie cautery ( Fig. 81.1A ). The fenestrations are then reinforced with a radiopaque marker, such as a gooseneck snare or an embolization coil. Circumferential or posterior constraining sutures are added and then the device is reloaded into the delivery system using sequential peel-away sheaths ( Fig. 81.1B ). There are some data demonstrating the utility of three-dimensional printing in positioning these modifications more accurately as there can be a moderate amount of interobserver variability using centerline measurements ( Fig. 81.2 ). , A variety of endografts have been modified including, but not limited to, the Cook TX2, Alpha, and Zenith platforms (Cook Medical, Bloomington, IN), Medtronic Endurant system (Medtronic, Santa Rosa, CA), and the Terumo Aortic Treo (Bolton Medical, Inc, Sunrise, FL). Each system has its advantages and disadvantages, which must be balanced with the patient’s anatomy as well as the surgeon’s comfort level and knowledge of the stent–graft system. A PMEG construct is certainly advantageous for those patients with symptomatic TAAAs who cannot wait for the production of a CMD or entry into a clinical device trial. In situ laser fenestration is a relatively new technique whereby an off-the-shelf endograft can be quickly modified to treat TAAAs in emergency situations. With this technique, the target visceral/renal vessels are initially stented with noncovered stents to permit ease of aligning the laser catheter using a steerable sheath. After creating a laser fenestration, the opening is progressively dilated with a cutting balloon and then a covered bridging stent is placed into the target vessel.
In the United States, access to CMDs is limited to select centers with access to an industry-sponsored clinical trial or a physician-sponsored investigational device exemption (PS-IDE) trial. These custom-made devices can combine almost any combination of fenestrations and branches, which may be oriented upward, downward, or helical. The decision of when to utilize fenestrations or branches remains provider-specific, but most would agree that directional branches are favorable in anatomy where there will be moderate distance to bridge from the endograft to the target vessel orifice (typically if the aortic lumen is >35 mm). These systems can be designed with preloaded catheters, which permit ease of fenestration/branch cannulation. In addition, these devices can be significantly tapered, which can be useful in patients with chronic dissection where the true lumen is relatively compressed. Finally, CMD devices can be manufactured to be “low profile” to fit in an 18-French sheath by utilizing a thin polyester fabric and nitinol stents. Despite rather limited availability in the United States, there is a tremendous amount of experience in the country and globally with their safe and effective implantation.
Several off-the-shelf F/BEVAR devices are now in clinical trial in the United States. These devices are advantageous as they avoid the 6- to 8-week manufacturing period that is required for CMDs. Despite a finite number and/or spacing of fenestrations or branches, their design has been reported to accommodate the anatomy in 50%–60% of patients with a TAAA. , The current clinical trials in this arena include the Cook “p-Branch”, Cook “t-Branch”, and Gore Excluder Thoracoabdominal Branch Endoprosthesis (“TAMBE”). Additional detail surrounding these devices is provided in Chapter 82 (Fenestrated and Branched Endograft Treatment of Juxtarenal, Paravisceral, Thoracoabdominal and Aortic Arch Aneurysms: Device Selection and Technical Considerations).
Only a brief overview of the operative technique for F/BEVAR implantation will be provided here as this is covered in great detail in the following chapter. F/BEVAR of TAAA is usually performed in a “hybrid” operating room under general anesthesia after adequate arterial and central venous access has been obtained. Preoperative insertion of a CSF drain is often employed, most commonly when extensive aortic coverage will occur. The use of fusion imaging, albeit not a requirement, is instrumental during these complex cases and has been shown to decrease contrast volume, procedure time, and radiation exposure. Femoral arterial access is always required for F/BEVAR insertion and deployment. Additional arterial access from the contralateral femoral artery and/or the brachial or axillary artery is dependent upon the preoperative case plan with regards to ease of target vessel cannulation.
For FEVAR, the endograft is advanced into the thoracoabdominal aorta after open or percutaneous femoral artery access and then slowly unsheathed under fluoroscopy, paying careful attention to aligning the various fenestrations with the visceral/renal target vessels. This critical step is where fusion imaging is incredibly valuable. If a non-preloaded FEVAR device is being used, a large working sheath, which has been inserted through the contralateral femoral artery, is positioned within the distal aspect of the FEVAR device. The target vessels are then sequentially cannulated with a hydrophilic wire. Once the target vessel has been selected and confirmed, the hydrophilic wire is exchanged for a stiff wire over which the bridging stent will be subsequently deployed. A similar cannulation process can transpire from the brachial/axillary artery if the target vessel angulation is best suited for a cranial-to-caudal approach. For preloaded FEVAR devices, cannulation of the fenestrations is expedited by advancing a guiding sheath through the endograft delivery system over preloaded wires. This allows for the guiding sheath to be positioned outside of the fenestration and can ease target artery cannulation. For BEVAR devices or endografts that combine branches and fenestrations, the directional branches are typically cannulated from a brachial/axillary approach. However, with the introduction of steerable sheaths, it is becoming increasingly common to approach downward-oriented branches from a femoral approach. ,
Once all target vessels are cannulated and stiff wires inserted, the endograft is fully deployed and the diameter-reducing ties/sutures are released and the delivery system is removed. The bridging stents are now deployed into each target vessel with the proximal aspect of these stents extending 4–5 mm into the endograft lumen and then flared with a 10–12 mm balloon to obtain seal between the fenestration and the bridging stent.
Several different bridging stents can be used for F/BEVAR, but no stent thus far has been specifically approved for this indication. There is no consensus as to ideal stent or combination of stents, although a covered stent must be used in order to obtain TAAA exclusion. Ideally, the bridging stent needs to be accurate, flexible, resistant to mechanical stress, and relatively low profile. Both balloon-expandable and self-expanding stents are options that meet some but not all of the above attributes, hence the controversy amongst providers as to which stent is best. In addition, the ideal bridging stent selection may differ if mating the endograft to the target artery via a fenestration versus a branch.
If using a fenestration, a balloon-expandable covered stent is the most commonly used bridging stent although self-expanding covered stents can also be utilized. In addition, some will reline these covered stents with self-expanding nitinol stents. The most commonly used balloon-expandable covered stents include the Atrium iCAST (Atrium Medical Corporation, Hudson, NH), Gore Viabahn VBX (Gore, Flagstaff, AZ), and Bentley BeGraft (Bentley InnoMed, Hechingen, Germany), although the Bentley device is not currently available in the United States. The Gore Viabahn VBX, which has been introduced to the market recently, arguably offers more flexibility than other stents since the stent rows are not connected longitudinally. However, this attribute raises some concern over its applicability in fenestrations as it can be challenging to ensure in the operating room that the non-stented (unsupported) gap does not span the fenestration. An in vitro study demonstrated similar mechanical properties and resilience of the VBX compared to iCAST and BeGraft. However, additional data and experience using VBX with fenestrations are needed. It is worth noting that using a balloon-expandable stent in a target vessel that has tortuosity can worsen the angle of curvature as the stent causes the vessel to lengthen before the turn. If a kink develops in the vessel, this poses a risk for loss of long-term patency. In this scenario, most surgeons will extend a bare-metal stent distal to the covered stent, but there has been concern in the past that uncovered stents are prone to in-stent restenosis as well as fractures. , However, a recent comparison of covered bridging stents alone versus those which had a covered stent followed by a bare-metal extension showed no difference in mid-term outcomes.
If using a directional branch configuration for F/BEVAR, both balloon-expandable and self-expanding covered stents have been used. Again, multiple combinations of these stents with or without self-expanding nitinol based stents have been reported. Options include the same balloon-expandable options lists above as well as self-expanding covered stents such as Gore Viabahn (Gore, Flagstaff, AZ) and the Bard Fluency (Bard Medical Inc, Murray Hill, NJ). Since its introduction in 2017, there has been an increase in the utilization of the Gore Viabahn VBX for this indication. In contrast to its use with fenestrations, there have been already several reports showing excellent 2-year primary patency (98%) with equivalent freedom from branch instability compared to self-expanding options.
Several technical issues are pertinent to applying F/BEVAR in patients with TAAA with chronic dissection. These issues involve the location of proximal and distal seal zones, compression of the true lumen (TL), aortic branch vessels emanating from either the TL or false lumen (FL) or both, and presence of intercostals in the FL. In cases of chronic dissection, the main endograft is almost always deployed in the TL. As such, it is critical to correctly identify the TL. Intravascular ultrasound (IVUS) can be very helpful in doing so, but others prefer to use different colored masks of the TL and FL based on the preoperative fusion imaging. If there is difficulty in navigating the wire into a highly compressed TL from a transfemoral approach, it can often be achieved in an antegrade fashion from the brachial or axillary approach and then snared from below. Fenestrations are usually preferred over branches if dealing with a compressed TL as there is concern that the TL might lead to branch collapse. Tapered endograft design and pre-loaded delivery systems for the renal fenestrations are two additional design characteristics that can effectively facilitate working within a compressed TL.
Identifying intimal tears in the dissection flap across the visceral segment of the aorta on preoperative CT imaging is critical for cannulating target vessels that emanate from the FL. An intimal tear in the dissection flap is almost always located at the level of the target vessel that arises from the FL. If so, this tear is cannulated from the TL and then dilated with a 12-mm angioplasty balloon. If no tear is identified, a tear can be created in the dissection flap by penetrating it with a trans-septal needle or laser fiber and then dilating the track with an angioplasty balloon. The distance that the bridging stent must travel from the tear in the flap to the target vessel must be considered as longer distances may contribute to type III endoleaks.
Early endoleaks are common after F/BEVAR for TAAA with chronic dissection as there are often intercostal and lumbar arteries that arise from the FL. In addition, with chronic dissection it is much more likely to see retrograde FL perfusion from dissection tears in the great vessels or iliac arteries. Despite up to 50% of patients having a type II endoleak, they were not associated with an increase in aneurysm sac diameter. Because post-F/BEVAR access to these vessels can be challenging, preemptive embolization of large lumbars/intercostals or inferior mesenteric arteries can be considered in these cases, which may also provide some spinal cord protection via preconditioning. Secondary embolization procedures of the FL of the innominate, common iliac, and external iliac arteries have been reported with large embolization devices (e.g., candy-plugs) and other techniques.
Given the associated procedural complexity, F/BEVAR require increased fluoroscopy times compared to conventional infrarenal EVAR. CT fusion technology with image overlay has helped with technical feasibility of F/BEVAR and reduced operative times, fluoroscopy use, and contrast volume, but these procedures still rely on conventional two-dimensional fluoroscopy imaging. As such, there is a need for imaging modalities that do not rely on ionizing radiation. IVUS is one such modality that has been used as an adjunct in EVAR, TEVAR, and F/BEVAR for some time. Endovascular robotic catheter is another new technology in various iterations of development which permits the operator to remotely control endovascular catheters and wires reducing their radiation exposure in addition to providing more stable and precise catheter navigation. A feasibility study in F/BEVAR demonstrated that >80% of target vessels were cannulated within 15 minutes using a robotic catheter system. However, there remains limited clinical data on their use in complex endovascular aortic surgery and there is debate over their cost and functional utility. Another innovation in nonionizing radiation imaging involves the use of electromagnetic navigation systems. These systems combine electromagnetic tracking technology from a local magnetic field that has been created around the patient with preoperative CT imaging, thereby providing real-time 3D imaging of a patient’s anatomy along with tracking of catheters and wires within the anatomy. This technology has been successfully used for F/BEVAR tasks in several phantom models and in a porcine model, but its clinical use in humans is just now emerging. , Most recently, imaging systems using fiberoptic real shape technology (FORS) are being investigated, which leverage the use of catheters and wires with embedded fiberoptic cables. Deformation of the fiberoptic cable enables real-time 3D visualization of the full shape of devices inside the body without the need for fluoroscopy. Although in their infancy, these aforementioned disruptive technologies will be transformative in advancing the safety and efficacy of F/BEVAR.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here