Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Echocardiography plays a pivotal role in decision making in patients with aortic stenosis (AS) and contributes to decision making for aortic valve replacement (AVR), as guided by the 2014 American Heart Association (AHA)/American College of Cardiology (ACC) Guideline for the Management of Patients with Valvular Heart Disease and its 2017 Focused Update, as well as the 2017 American College of Cardiology Appropriate Use Criteria Task Force/American Association for Thoracic Surgery/American Heart Association/American Society of Echocardiography/European Association for Cardio-Thoracic Surgery/Heart Valve Society/Society of Cardiovascular Anesthesiologists/Society for Cardiovascular Angiography and Interventions/Society of Cardiovascular Computed Tomography/Society for Cardiovascular Magnetic Resonance/Society of Thoracic Surgeons (ACC/AATS/AHA/ASE/EACTS/HVS/SCA/SCAI/SCCT/SCMR/STS) 2017 Appropriate Use Criteria for the Treatment of Patients With Severe Aortic Stenosis. AS is relentlessly progressive. Although there is a long period when patients remain asymptomatic as disease progresses, when symptoms occur, AS becomes a malignant disease. The classic symptoms are those of heart failure, typically dyspnea and, to a lesser degree, evidence of low cardiac output, angina, and syncope or presyncope, the latter typically with exertion. The negative prognostic impact of symptoms was first described by Ross and Braunwald in 1968 in a group of patients with predominantly rheumatic and bicuspid AS whose symptoms onset in the patients’ early 60s. Importantly, this observation was subsequently confirmed in the Partner I (Placement of Aortic Transcatheter Valves I) trial, in which inoperable patients who did not undergo transcatheter aortic valve replacement (TAVR) had a 1-year all-cause mortality rate greater than 50%. A survival benefit with surgical aortic valve replacement (SAVR) or TAVR has been a consistent finding. Thus, risk stratification and appropriate selection of patients for intervention is clinically important. The role of echocardiography is in identifying patients who are candidates for AVR. First, it establishes the diagnosis of severe AS, a topic covered in Chapters 78 and 79 . The focus of this chapter is on patients with classical severe high-gradient AS (mean gradient ≥40 mm Hg, peak velocity ≥4 m/s, AVA ≤1.0 cm 2 ).
The guidelines ( Table 81.1 ) assign a class I indication for patients with severe AS and left ventricular (LV) systolic dysfunction defined as a left ventricular ejection fraction (LVEF) less than 50%. Echocardiographic methods for calculating LVEF are discussed in Chapters 23 and 24 along with the ASE Recommendations for Cardiac Chamber Quantitation with Echocardiography in Adults. However, it has been noted that reduced LVEF (<50%) is uncommon in AS (0.4%) in the absence of symptoms that also carry a class I indication for intervention. It has been shown that patients with LVEFs of 50% to 59% are at increased risk versus those with LVEFs 60% or greater, and it has therefore been argued that a cutoff of 60% would be more appropriate.
| Recommendation | Class of Recommendation /Level of Evidence |
|---|---|
| AVR is recommended for symptomatic patients with severe AS | I/B |
| AVR is recommended for asymptomatic patients severe AS (stage C2) when LVEF <50% | I/B |
| AVR is reasonable for asymptomatic patients with very severe AS (stage C1) (aortic velocity ≥5 m/s) and low surgical risk | IIa/B |
| AVR is reasonable in symptomatic patients with low-flow/low-gradient severe AS with reduced LVEF (stage D2) with a positive low-dose dobutamine stress study (increase in aortic velocity ≥4 m/s and aortic mean gradient ≥40 mm Hg, with a valve area <1 cm 2 ) | IIa/B |
| AVR is reasonable in symptomatic patients with low-flow/low-gradient severe AS (stage D3) with LVEF ≥50% if clinical, hemodynamic, and anatomic data support AS as the most likely cause of symptoms | IIa/C |
| AVR is reasonable for patients with moderate AS (stage B) who are undergoing other cardiac surgery | IIa/C |
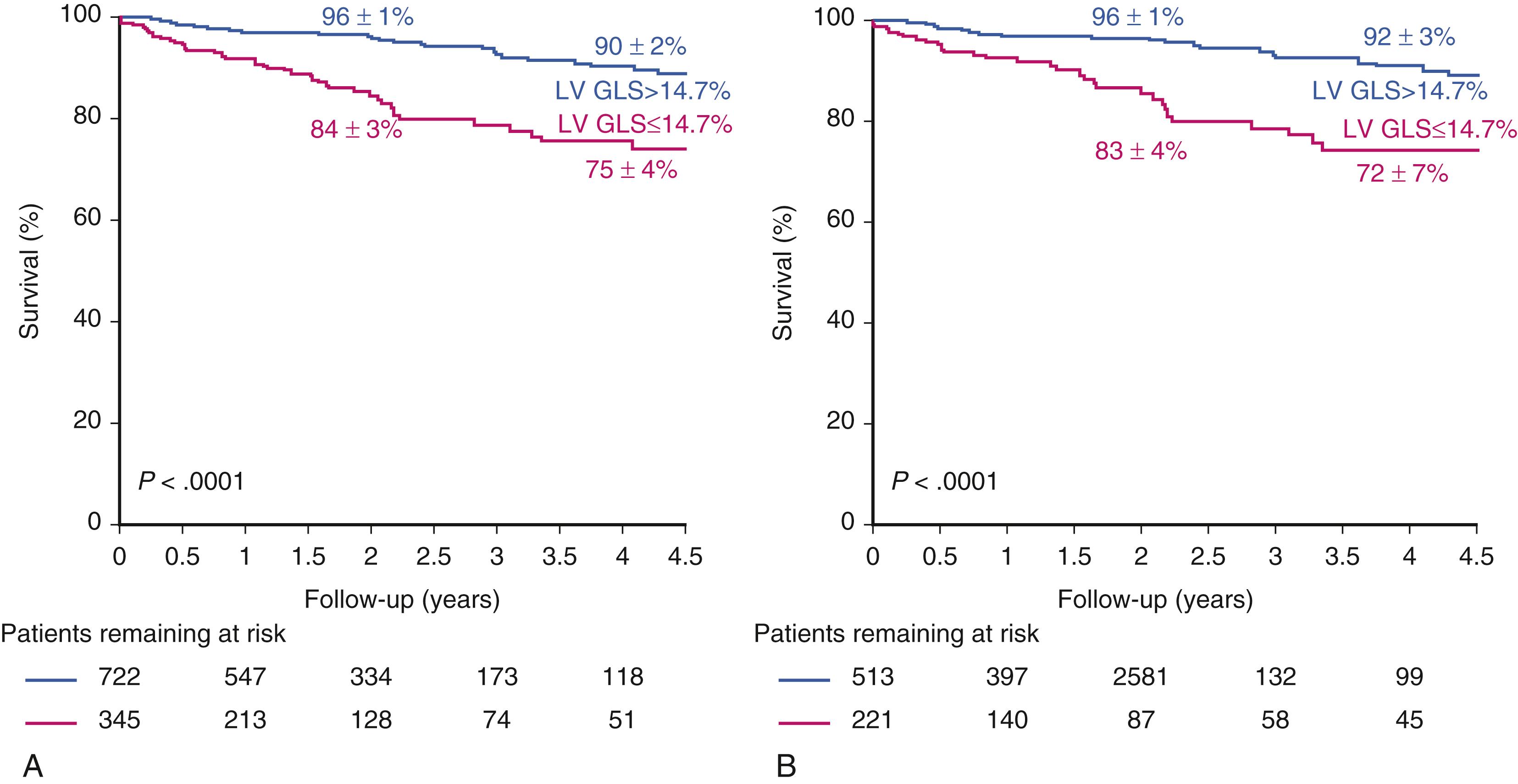
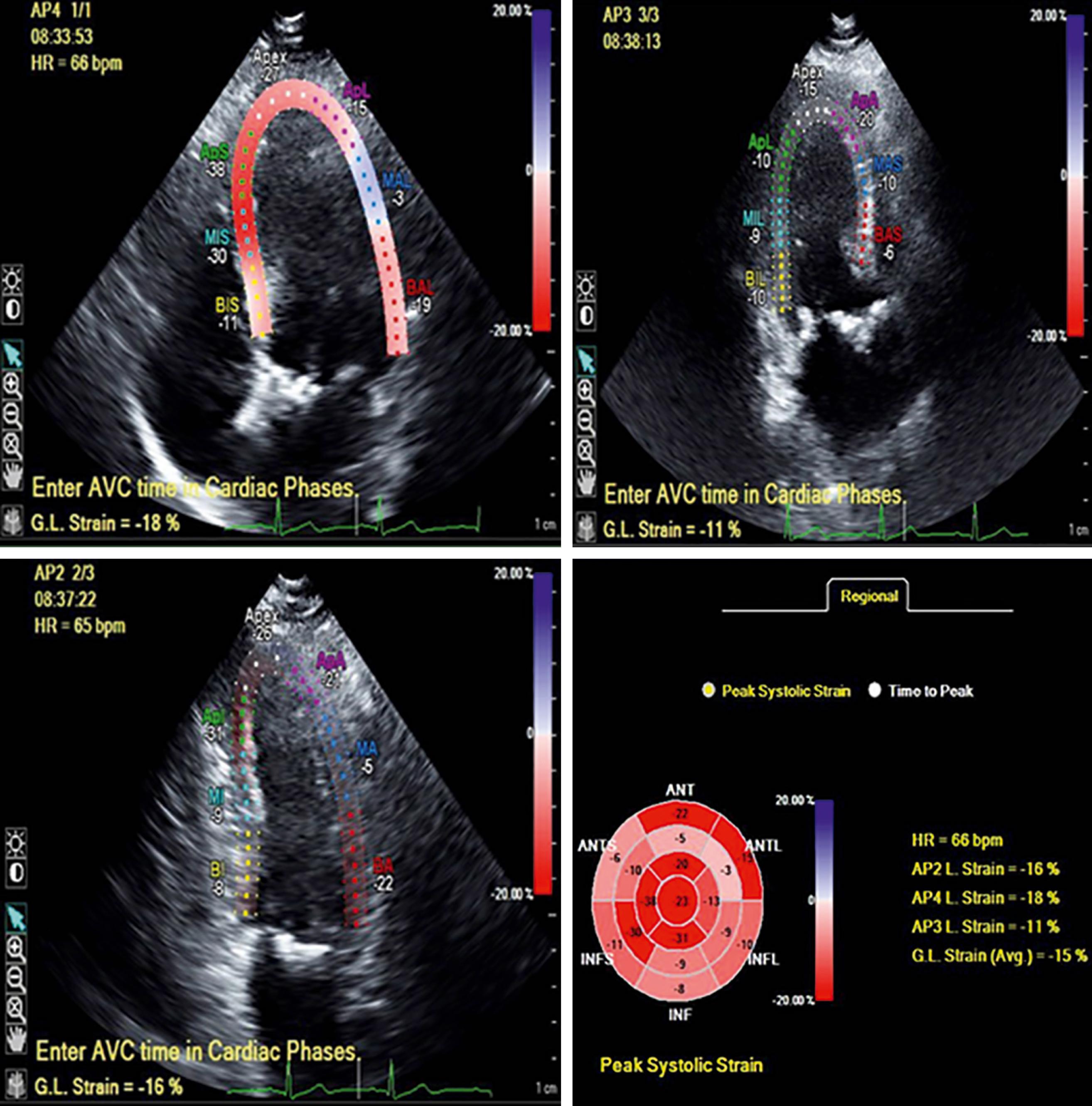
Furthermore, since global longitudinal strain (GLS) was recognized to be a more sensitive index of LV systolic dysfunction than LVEF, a number of studies have explored its value in AS. Indeed, although abnormal LV strain has not yet transitioned to a guideline indication for intervention, it is important to note the expanding literature that speaks to the prognostic value of GLS in patients with severe AS. In a recent meta-analysis of 10 studies with 1067 individual participants with severe AS, all asymptomatic and with LVEF greater than 50%, Magne and coworkers reported that abnormal GLS was predictive of survival at 2- and 4-year follow-up ( Fig. 81.1 ). An empiric cut-off of −14.7% best identified low- and high-risk groups, and the prognostic importance of GLS was particularly strong in those with LVEF of 60% or greater. An example of this is shown in Fig. 81.2 in a patient with severe AS and LVEF of 62% with an abnormal GLS. These data support the general concept that LVEF below 50% is an insensitive marker of LV decompensation and that GLS has incremental value as a tool for risk stratification and perhaps identifying those who would benefit from intervention.
Given the slow progression of AS, it is not surprising that symptoms may be insidious and that patients subconsciously slow down or otherwise restrict activity to avoid symptoms. Additionally, symptoms of AS may be erroneously attributed to aging or a comorbid condition. Thus, exercise stress testing, preferably with echocardiography is considered an essential step in the evaluation of patients with severe “asymptomatic” AS. Indeed it carries a class IIA indication in the current ACC/AHA guidelines for patients with classic severe AS (peak velocity ≥4 m/s and mean gradient ≥40 mm Hg) and is an important consideration in many of the high-gradient AS scenarios in the appropriate use criteria. The implication of an exercise stress echocardiography (ESE) result that is positive, as defined by symptoms, is that the patient is considered to be symptomatic and therefore a candidate for AVR. Thus, exercise-induced symptoms carry class I indications for surgery in both the ACC/AHA and ESC/EACTS Guidelines. Other ESE outcomes including a decrease or a less than 20 mmHg increase in systolic blood pressure (BP), , mean gradient increase of greater than 18 10 or 20 12 mmHg, ventricular arrhythmias, , 2 mm or greater ST-segment depression, , and exercise-induced decrease in LVEF have also been shown to be predictive of spontaneous symptom onset, symptom-driven AVR, , and sudden cardiac death. However, the link between ESE results other than symptoms and outcomes has not been entirely consistent, and death has been a rare event typically occurring only after patients have become symptomatic. , In particular, electrocardiographic (ECG) changes have been reported to be inadequately discriminating, particularly in women. ECG ST-segment changes are not specific when AS is present, with significant ST-segment depression seen in 80% of adults with AS regardless of whether coronary artery disease is present. Thus, these ESE measures of test positivity carry no (ECG changes, decrease in EF, ventricular arrhythmias) or only class IIA (decrease in BP) , , or IIB (>20 mm Hg rise in gradient) indications for AVR.
Although exercise-induced chest pain, presyncope, and syncope are fairly clear-cut, the interpretation of dyspnea is more difficult. For example, the dyspnea of patients with AS and concomitant lung disease may be caused by pulmonary rather than cardiac limitations, and even healthy individuals become short of breath with high-intensity or prolonged exercise, making the workload at which dyspnea occurs an important consideration. Indeed, Masri and colleagues reported the incremental prognostic utility of reduced exercise tolerance (as defined by <85% age-gender predicted metabolic equivalents [METs] achieved) and slower 1-minute postexercise heart rate recovery after symptom-limited treadmill ESE with a primary outcome of all-cause mortality.
Although the authors used age- and gender-predicted METS achieved using the approaches of Morris and coworkers for men and Gulati and coworkers for women, it is worth noting that these references include few participants in the very old (older than 80 years) age groups in whom AS is typically encountered. Indeed, the nomograms provided in these references do not extend beyond age cut-offs of 70 to 75 years. Thus, there are challenges in establishing benchmarks for normal exercise performance in the typical patient with AS.
Recognizing the advanced age of many patients with AS, Naughton, modified Bruce, or manual protocols are often used. Physician presence for test supervision is essential. BP must be monitored carefully, and failure to augment BP is considered a positive response. Although concomitant coronary artery disease is not unusual, the default would be to attribute the chest pain to AS pending coronary angiography, although regional wall motion abnormalities and localizing ST-segment elevation may be helpful.
One-third of patients with severe AS who are “asymptomatic” in fact have exercise-limiting symptoms detected during exercise testing. Although exercise testing has been found to be relatively safe in asymptomatic patients, symptomatic patients with severe AS should not undergo exercise testing because of the high risk of complications, including syncope, ventricular tachycardia, and death.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here