Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intravascular placement of a foreign body with its nonendothelial surface activates the clotting mechanism, leading to thrombus formation. The exposure of blood to the synthetic surface leads rapidly to deposition of a fine layer of plasma components, mostly protein, followed by platelet deposition. The intrinsic coagulation cascade is initiated along with the extrinsic coagulation cascade: the inflammatory response, including leukocyte activation; the complement system; and fibrinolysis.
Fibrinogen is one of the major plasma proteins, often the first that is deposited on these artificial surfaces. Once the layer of fibrinogen is absorbed onto the surface, platelets can adhere to the fibrinogen. Although surfaces vary greatly in their tendency to promote thrombosis, the reactivity of most materials to blood can be significantly increased if they are first exposed to fibrinogen. Other proteins also are deposited, including fibronectin (a surface protein of many cells), von Willebrand factor (a glycoprotein essential for the adhesion of platelets to subendothelial tissue), thrombospondin (a platelet protein secreted by activated platelets), and factor XII (Hageman factor, the primary activator of the intrinsic coagulation system).
Once platelets attach to the protein layer and spread out on the artificial surface, materials present in the platelet intracellular granules are secreted, including β-thromboglobulin, which inhibits prostacyclin production; platelet factor 4, which neutralizes heparin sulfate in the endothelium; and serotonin, adenosine triphosphate (ATP), and adenosine diphosphate (ADP). Synthesis of prostaglandins E and F is evident as well, suggesting that endoperoxide metabolism has taken place along with formation of thromboxane A2 from platelet arachidonic acid. Serotonin, thromboxane A2, and endoperoxide are potent vasoconstrictors and platelet stimulatory factors. Finally, platelet aggregation follows platelet adhesion, probably by ADP and serotonin secretion from the adherent platelets. Fibrinogen and thromboxane A2 are key in this step.
The coagulation cascade is initiated either by reaction of plasma proteins with the artificial surface to form enzymatically active components such as factor XII (intrinsic system) or by introduction of thromboplastin through exposure of subendothelial tissue to the surface (extrinsic system). Figure 83-1 is a schematic diagram of the clotting cascade. Activation of factors XIIa and XIa initiates the intrinsic system, leading to activated factor Xa. Platelets provide the phospholipid surface for this reaction. Activated factor XIIa also initiates the kininogen-kallikrein system, and kallikrein provides positive feedback for the contact activation. Kallikrein cleaves factor XII to convert it to factor XIIa, thereby accelerating contact activation. Bradykinin is also released when kallikrein cleaves high-molecular-weight kininogen. Activated high-molecular-weight kininogen can then bind more prekallikrein and factor XI to the activating surface, which further increases the reaction. In the final common pathway, prothrombin is converted to thrombin, and fibrinogen is converted to fibrin. Thrombin recruits more platelets, creating more adhesion and aggregation. A fibrin platelet clot is formed, and thrombosis occurs.
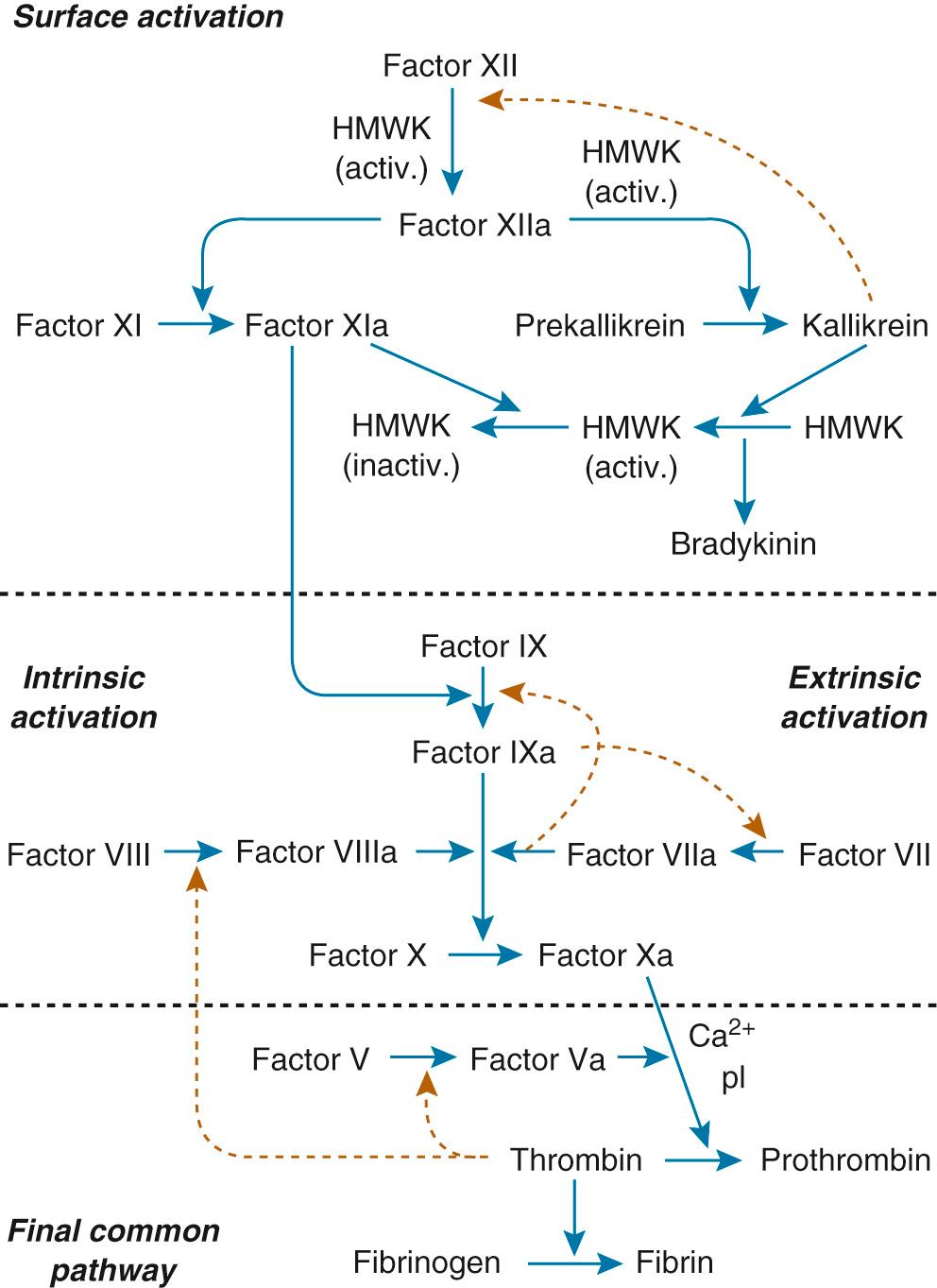
Activation of the clotting cascade on the surface of an artificial device occurs similarly whether it is on the cardiac valve, cardiopulmonary bypass system, vascular graft, extracorporeal membrane oxygenation (ECMO) circuit, mechanical assist device, or vascular catheter. It will produce thrombus formation, and macroscopic and microscopic platelet-fibrin emboli occur commonly as well. Factor XII, kallikrein, and plasmin activate the complement system and activate neutrophils, and kinin formation mediates vasodilation, vascular permeability, and white blood cell migration. Normally, a delicate balance is maintained between these two systems so that uncontrolled clotting or hemorrhage does not occur. The coagulation cascade is initiated, and factor XII and kallikrein initiate clot lysis with conversion of plasminogen to plasmin. Antiplasmins in the circulating blood, particularly α 2 -antiplasmin, rapidly neutralize most of the circulating plasmin; however, plasmin also is incorporated into the clot during clot formation. The fibrin meshwork protects plasmin from antiplasmin once the plasmin is activated to plasmin, allowing fibrin degradation in the clot. In fact, many natural inhibitors offset activated procoagulant protein. Protein C, heparin, antithrombin III, protein S, thrombomodulin, prostacyclin, and plasmin all counter steps in the coagulation cascade.
Clinically useful drugs that block the clotting cascade fall into the following primary groups: orally administered vitamin K antagonists; natural anticoagulants, such as the heparin–antithrombin III system; direct thrombin inhibitors, antiplatelet drugs, factor Xa inhibitors; and fibrinolytic agents.
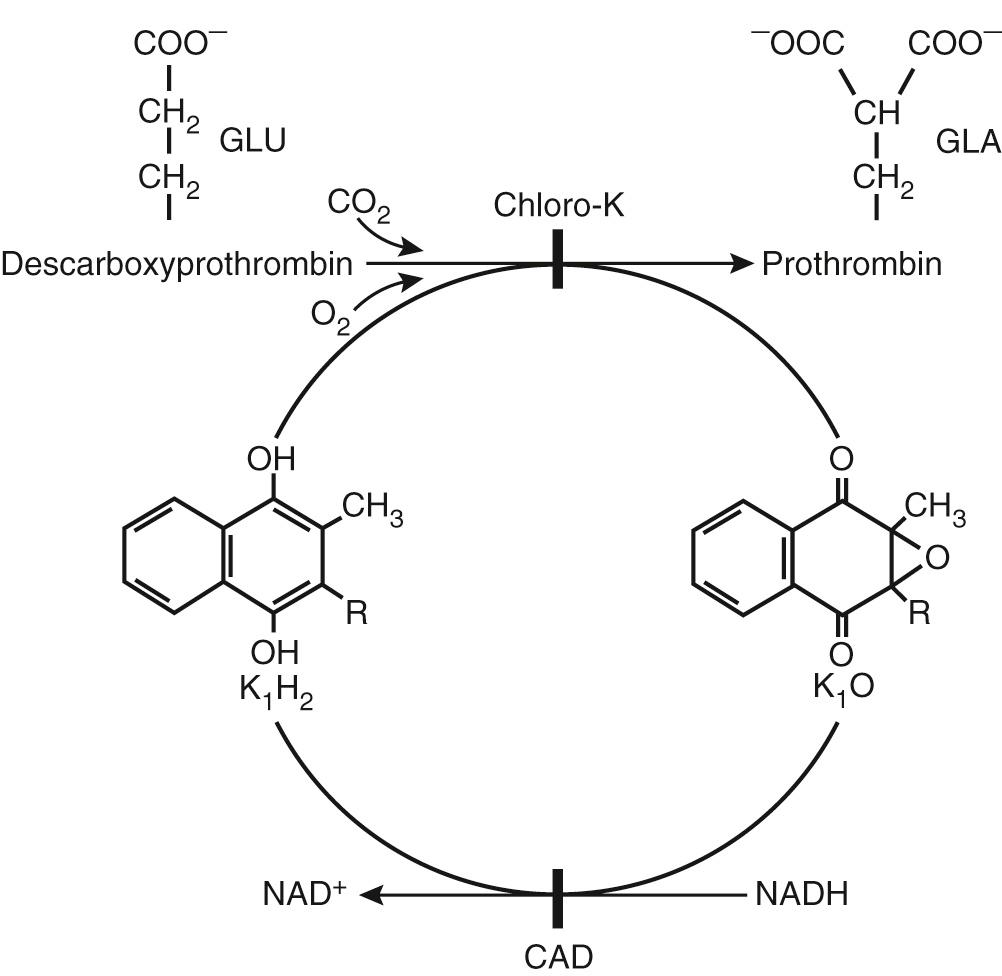
Warfarin sodium remains the most popular orally administered vitamin K antagonist used today in the United States. It blocks the formation of the four vitamin K–dependent clotting factors (prothrombin and factors VII, IX, and X), creating a buildup of their precursors. Warfarin sodium blocks the vitamin K cycle at the regeneration of reduced vitamin K, which is the active form of vitamin K ( Fig. 83-2 ).
Heparin's anticoagulant effect is fairly complex and not completely understood. Heparin sulfate is a glycosaminoglycan that binds to antithrombin III and activates this serine protease inhibitor. Heparin and antithrombin III occur naturally in humans, are secreted by endothelial cells, and are required to produce their anticoagulant effect. Antithrombin III binds to thrombin and blocks the enzymes of the intrinsic coagulation cascade, including thrombin and factors IXa, Xa, XIa, and XIIa. Both unfractionated heparin and low-molecular-weight heparins work via the previously mentioned mechanisms.
Direct thrombin inhibitors and factor Xa inhibitors have emerged as alternative agents to warfarin for oral anticoagulation. Dabigatran is an orally administered direct thrombin inhibitor, whereas rivaroxaban and apixaban are factor Xa inhibitors. Dabigatran received U.S. Food and Drug Administration (FDA) approval for prevention and treatment of venous thromboembolic disease and treatment of nonvalvular atrial fibrillation based primarily on data from the RE-LY trial. Several intravenous direct thrombin inhibitors exist, including lepirudin, desirudin, argatroban, and bivalirudin. These agents are used primarily as alternatives to heparin when heparin-induced thrombocytopenia is suspected or confirmed with appropriate diagnostic studies.
Rivaroxaban is an orally administered competitive, direct factor Xa inhibitor. Noteworthy is that this agent is contraindicated in patients with creatinine clearance of less than 15 mL/min or patients on hemodialysis. Rivaroxaban was studied in the ROCKET-AF trial and found to be non-inferior to warfarin in the prevention of stroke in patients with atrial fibrillation. This agent also was studied for prevention of venous thromboembolism (VTE) during orthopedic surgery and showed efficacy.
The various antiplatelet drugs have different mechanisms of action, making them more or less useful as therapeutic anticoagulation agents. Figure 83-3 is a schematic diagram of the actions of antiplatelet drugs. Aspirin inhibits platelet aggregation by irreversible acetylation of platelet cyclooxygenase, hence blocking the synthesis of prostaglandins and thromboxane A2. Aspirin prolongs the bleeding time, although variable responses to aspirin's anticoagulant effect exist. Aspirin inhibits platelet aggregation for the life span of the platelet, which is typically 7 to 10 days.
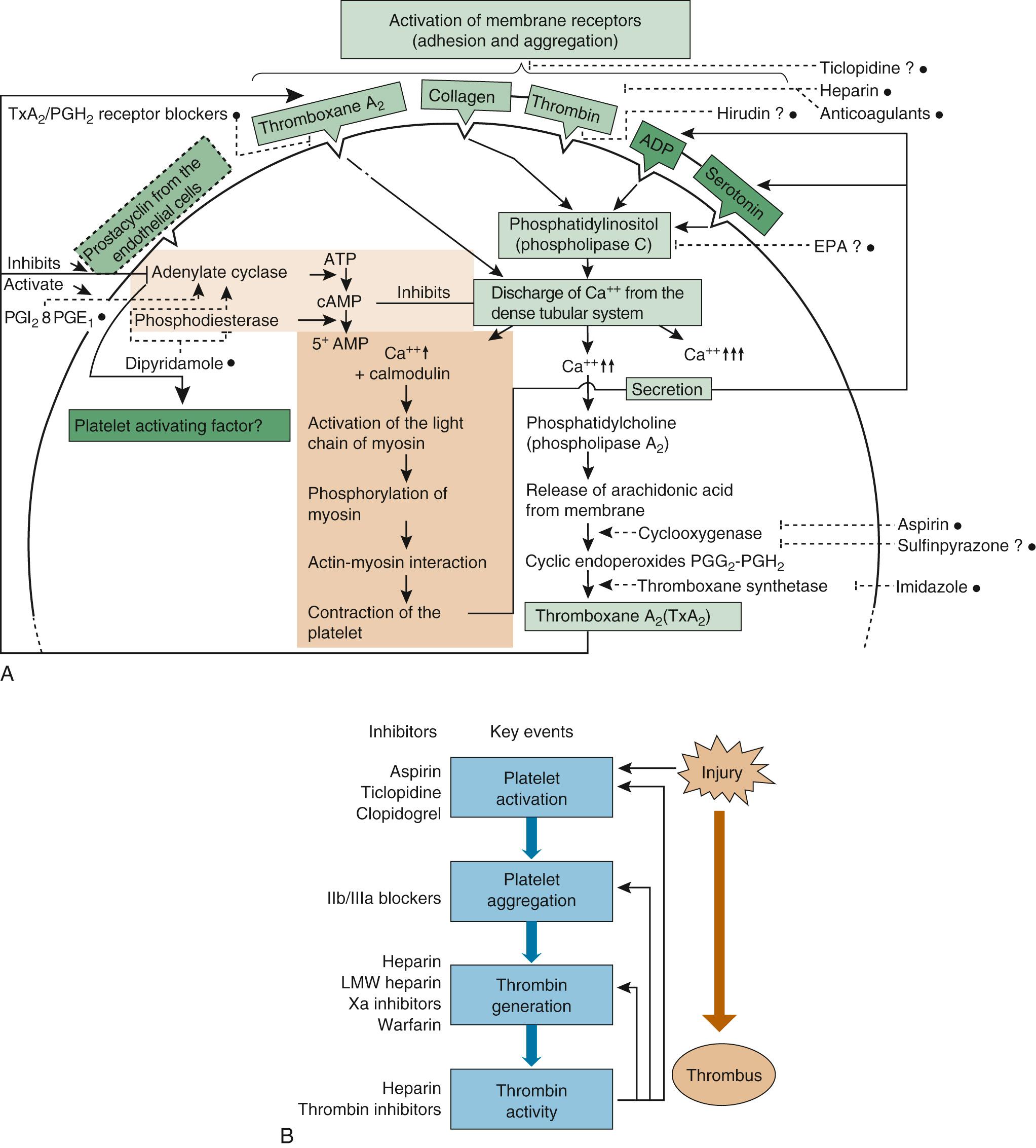
Four adenosine diphosphate P2Y12 (ADP PY212) receptor antagonists are FDA approved. These agents reduce platelet aggregation mediated by the binding of fibrinogen to activated glycoprotein (GP) IIb/IIIa receptors on platelets. The thienopyridines are in widespread use as potent antiplatelet agents and are typically used in dual antiplatelet therapy following percutaneous coronary intervention. Clopidogrel in vitro does not affect platelet aggregation. However, in vivo, clopidogrel is metabolized by the liver to several active metabolites—with these metabolites irreversibly inhibiting the P2Y 12 platelet receptor. Clopidogrel supplanted ticlopidine principally because ticlopidine is associated with aplastic anemia and thrombotic thrombocytopenic purpura. The thienopyridine prasugrel converts to active metabolites more efficiently than does clopidogrel; as such, the bleeding risk with this agent is increased compared with other agents in this class. Lastly, ticagrelor is a novel thienopyridine that directly antagonizes the P2Y12 receptor. This drug is also reversible in its inhibition of the receptor. However, it requires twice-daily dosing in contrast with the other thienopyridines. It is presently a Class I recommendation to discontinue all P2Y12 inhibitors before cardiac surgery. The exact duration in days of discontinuation cannot be specified as the safe interval between discontinuation and cardiac surgery is unknown. In general, the range for cessation is between 3 and 7 days.
Dipyridamole is a reversible platelet agent, a weak vasodilator, and a weak inhibitor of the enzyme phosphodiesterase, which degrades cyclic adenosine monophosphate (cAMP) to 5′-AMP. With this block, more cAMP is available to inhibit platelet aggregation. Sulfinpyrazone appears to reversibly block platelet prostaglandin synthesis and is another fairly weak anticoagulant. At clinical dosages, neither dipyridamole nor sulfinpyrazone prolongs the bleeding time.
Finally, fibrinolytic therapy has a small but definite place in the management of thrombosis of artificial devices. Streptokinase and urokinase act similarly and induce rapid thrombolysis by activating plasminogen and subsequently forming plasmin. Plasmin causes degradation of the fibrin, reducing thrombus size. Unfortunately, streptokinase and urokinase also induce a generalized plasma proteolytic state as well as local fibrin degradation in the thrombus, and this can lead to uncontrolled hemorrhage. Newer agents (second-generation plasminogen activators) such as recombinant tissue plasminogen activator were developed to prevent induction of this generalized plasma proteolytic state by making these agents fibrin specific. However, this function appears to depend on the dose, and clinical use has not confirmed the decrease in potential for hemorrhage that was hoped for with these drugs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here