Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Surgeons manage infections that require invasive intervention (e.g., complicated intra-abdominal infections [cIAIs] and skin/soft tissue infections [cSSTIs]), but also surgical patients afflicted by nosocomial infections. Therefore, the surgeon must be concerned with the prevention and treatment of all infections that affect surgical patients, including surgical site infections (SSIs), central line–associated bloodstream infections (CLABSIs), complicated urinary tract infections (cUTIs), and hospital-acquired or ventilator-associated bacterial pneumonia (HABP/VABP). Trauma patients are particularly vulnerable to infections of injured tissue as well as nosocomial infections related to environmental factors (e.g., hypothermia), host immunosuppression (e.g., inadequate glycemic control, transplant immunosuppression), and therapeutic interventions (e.g., incisions, catheters, blood transfusion).
Recognizing and minimizing risk goes hand in hand with an aggressive approach to diagnosis and treatment. Infection is preventable to some degree, and every acute care surgeon must do their utmost toward prevention. An ensemble of tactics is required, because no single method, including antibiotic prophylaxis, is effective itself. Infection control is paramount. Surgical illness and injury are inherently immunosuppressive, as are many critical care therapies. Surgical incisions and traumatic wounds must be handled gently, inspected daily, and dressed if necessary using strict asepsis. Drains and catheters must be avoided if possible and removed as soon as practicable. Whether prophylactic or therapeutic, and either empiric or directed against a known infection, antimicrobial agents must be prescribed according to the principles of antibiotic stewardship so as to minimize antibiotic selection pressure and the development of multidrug-resistant (MDR) pathogens.
Pharmacokinetics (PK) describe the principles of drug absorption, distribution, and metabolism. Dose-response relationships are influenced by dose, dosing interval, and route of administration. Plasma and tissue drug concentrations are influenced by absorption, distribution, and elimination, which in turn depend on drug metabolism and excretion. Relationships between local drug concentration and effect are described by pharmacodynamic (PD) principles (see following discussion).
Bioavailability , the percentage of drug dose that reaches the systemic circulation after oral administration, is affected by absorption, intestinal transit time, and hepatic metabolism. Half - life (t 1/2 ), the time required for the serum drug concentration to reduce by one-half, reflects both clearance and volume of distribution (V d ). The V d is used to estimate the plasma drug concentration achievable from a given dose. V d varies substantially according to pathophysiology; reduced V d may cause a higher plasma drug concentration for a given dose, whereas fluid overload and hypoalbuminemia (which decrease drug binding) increase V d , making dosing more complex.
Clearance refers to the volume of fluid from which drug is eliminated completely per unit of time, regardless of the mode of elimination (e.g., metabolism, excretion, or dialysis); knowledge of drug clearance is important to determine the dose of drug necessary to maintain a steady-state concentration ( Fig. 1 ). Most drugs are metabolized by the liver to polar compounds for eventual renal excretion, which may occur by filtration or either active or passive transport. In general, if 40% or more of active drug (including active metabolites) is eliminated unchanged in the urine, a dosage adjustment is required if renal function is decreased.
PD is unique for antibiotic therapy, because drug–patient, drug–microbe, and microbe–patient interactions must be accounted for. Microbial physiology, inoculum characteristics (i.e., size, quorum sensing, presence of a device-related biofilm), microbial growth phase, mechanisms of resistance, the microenvironment (e.g., local pH), and the host’s response must be considered. Because of microbial resistance, mere administration of the “correct” drug may not be microbicidal if an adequate dose/concentration is not achieved. In vitro results may be irrelevant if bacteria are inhibited only by drug concentrations that cannot be achieved clinically.
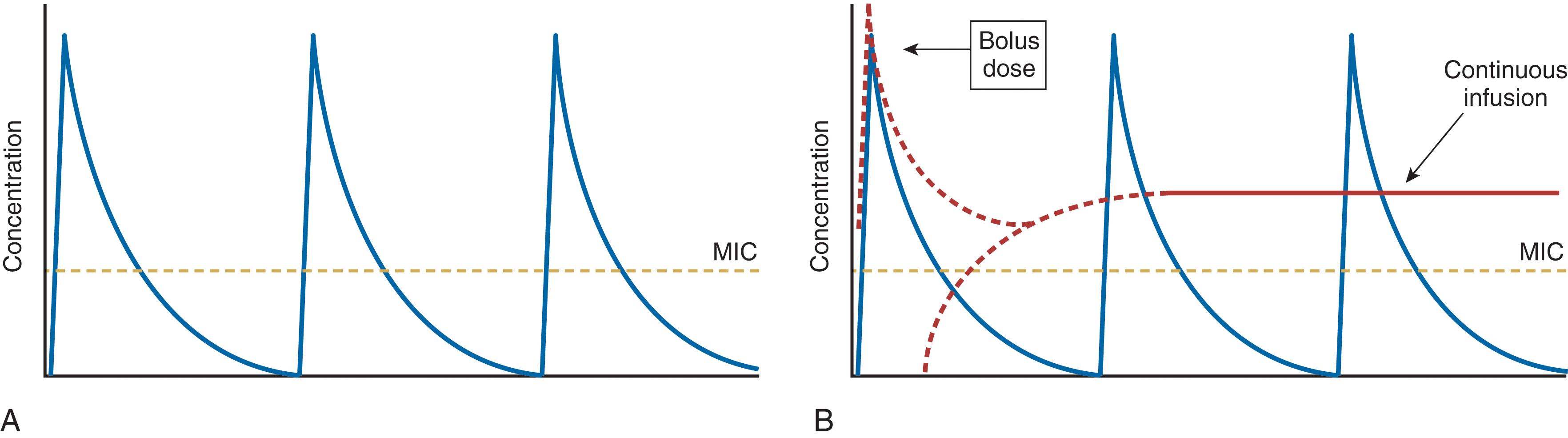
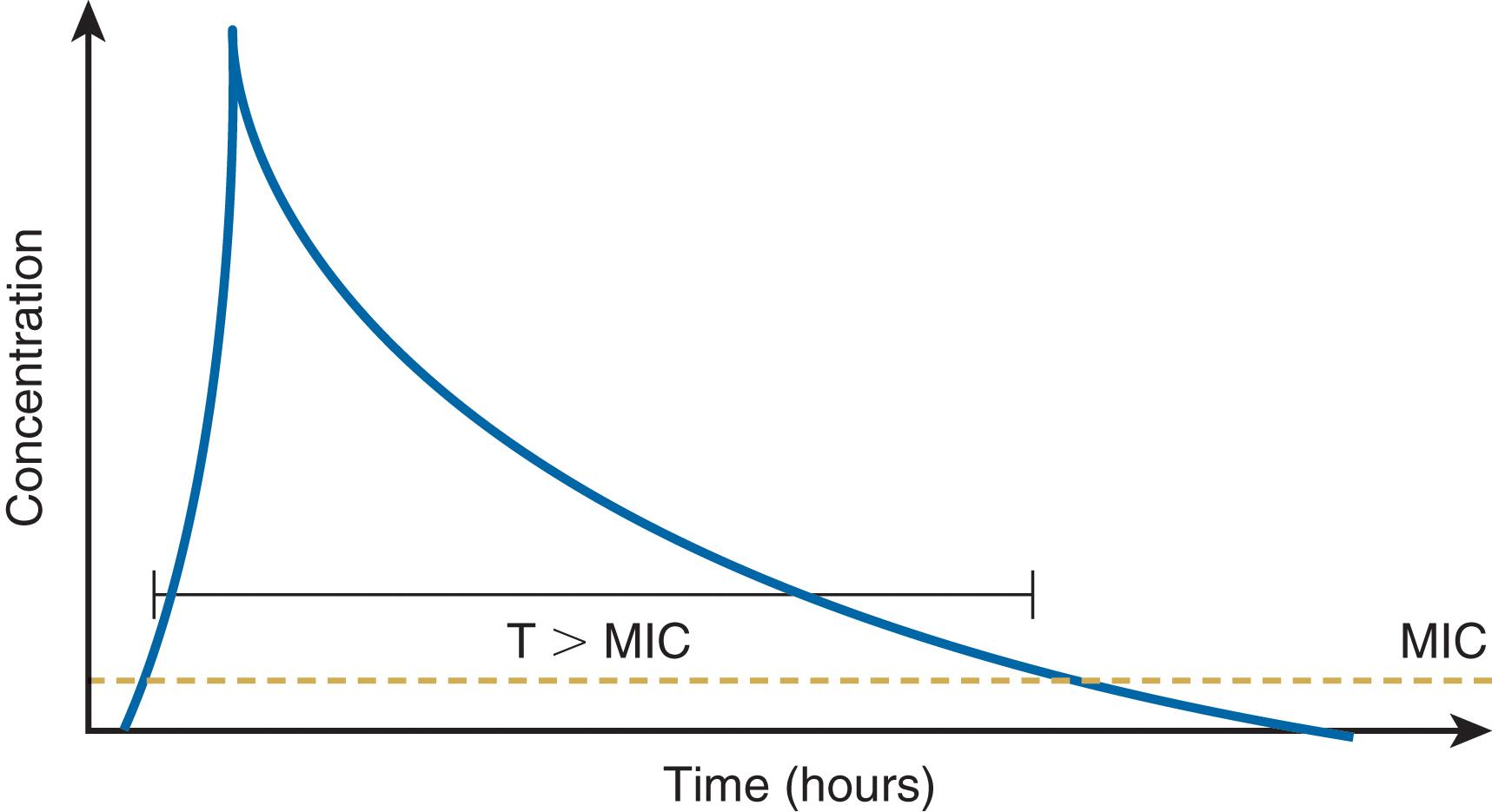
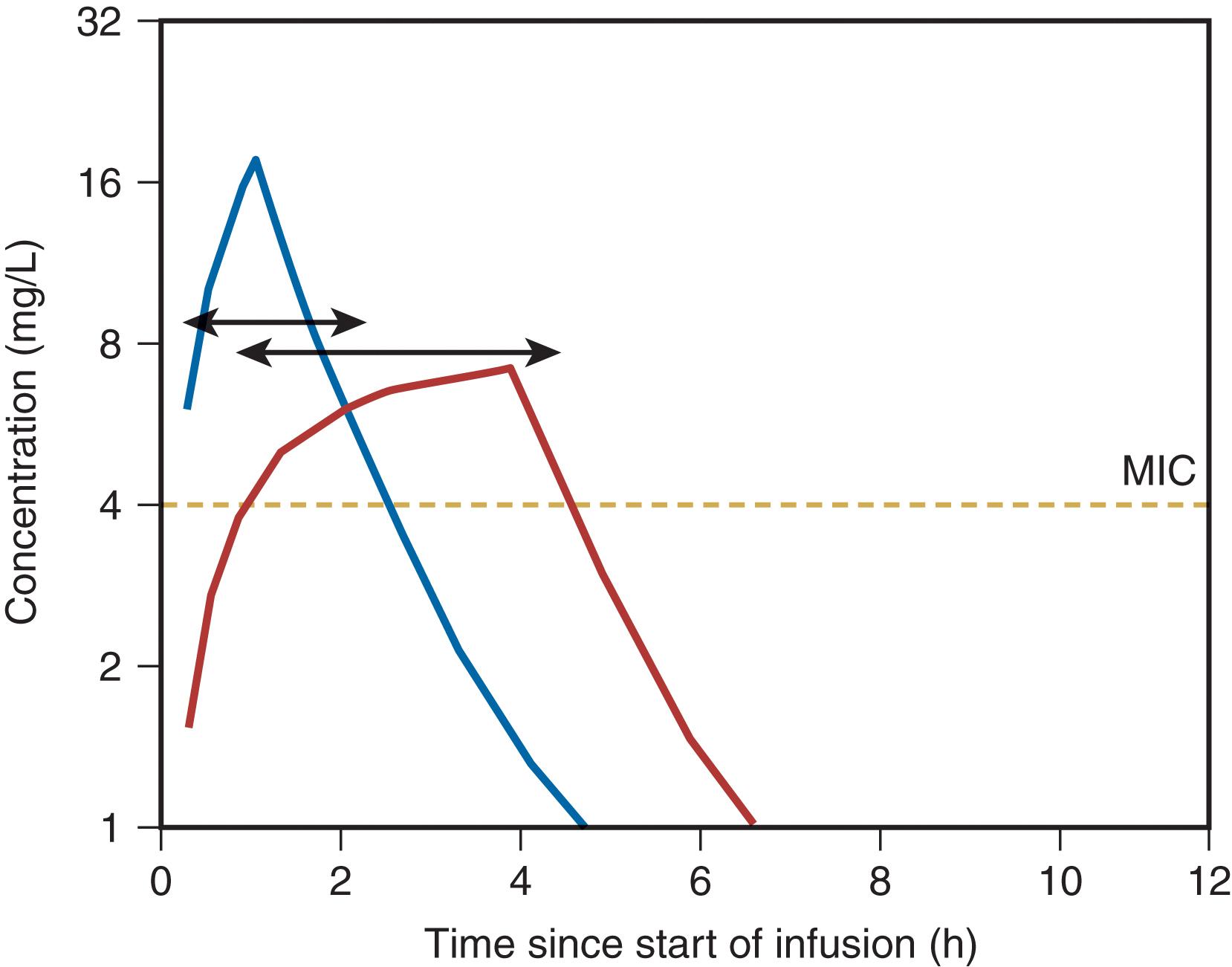
Antibiotic PD parameters determined by laboratory analysis include the minimal inhibitory concentration (MIC), the lowest serum drug concentration that inhibits bacterial growth (MIC 90 refers to 90% inhibition) ( Fig. 2 ). However, some antibiotics may suppress bacterial growth at subinhibitory concentrations ( postantibiotic effect , PAE). Sophisticated analytic strategies use both PK and PD, for example, by determination of the peak serum concentration:MIC ratio, the duration of time (fT) that plasma concentration remains above the MIC (fT > MIC), and the area of the plasma concentration-time curve above the MIC (the area under the curve , or AUC) ( Fig. 3 ). Accordingly, aminoglycosides exhibit concentration-dependent killing and exert optimal bactericidal activity when the peak concentration:MIC is ≥10. By contrast, efficacy of β-lactam agents is determined by fT > MIC, which should be at least 40% of the dosing interval (longer is better). For β-lactam antibiotics with short t ½ , administration by continuous infusion is logical, although prolonged (e.g., 3–4 hours) intermittent infusion accomplishes the same while maintaining vascular access for other medications. Some agents (e.g., fluoroquinolones, vancomycin) exhibit both properties; bacterial killing increases as drug concentration increases up to a saturation point, after which the effect becomes concentration-independent. A 24-hour AUC:MIC > 125 is associated with optimal effect for such drugs.
Prophylactic antibiotics are used most often to prevent SSIs, for which the benefit is demonstrated. However, if administered improperly, antibiotic prophylaxis is ineffective and may even be harmful. Antibiotic prophylaxis helps to prevent SSI only when the incision is open/vulnerable, thus single-dose prophylaxis, given prior to the skin incision, is sufficient in most circumstances. Intraoperative redosing of prophylactic agents with short T 1/2 (e.g., cefazolin, cefoxitin) during prolonged operations is more important than postoperative administration. The prescribing physician must be mindful of both appropriate timing of administration as well as prompt discontinuation to minimize the risk of development of MDR bacteria and Clostridioides difficile infection. Other than SSI, antibiotic prophylaxis does not prevent postoperative nosocomial infections, which actually occur at an increased rate after prolonged prophylaxis.
Four principles guide the administration of antimicrobial agent(s) for prophylaxis: (1) safety; (2) an appropriate narrow spectrum of coverage of relevant pathogens; (3) little or no therapeutic use; and (4) timely administration prior to incision and for a defined, brief period of time thereafter (no more than 24 hours [48 hours for cardiac surgery]; ideally, a single dose). The optimal time for administration is within 1 hour prior to incision or within 2 hours for vancomycin (which must be administered by a minimum 1-hour infusion of a 1-g dose [longer for higher doses] and fluoroquinolones). Agents given sooner, or after the incision is closed, are ineffective. Antibiotics with a short half-life should be redosed every 3 (e.g., cefoxitin) or 4 hours (e.g., cefazolin) during prolonged or bloody operations.
Most SSIs are caused by gram-positive cocci, therefore prophylaxis should be directed primarily against staphylococci for clean cases and high-risk clean-contaminated upper abdominal surgery (e.g., gastric surgery). A first-generation cephalosporin is preferred in most circumstances, with clindamycin as an alternative for patients with a history of anaphylactoid allergy to penicillin or cephalosporins. If gram-negative or anaerobic coverage is required (e.g., penetrating abdominal trauma), cefoxitin, or cefazolin plus metronidazole are the regimens of first choice. Vancomycin prophylaxis is discouraged, except perhaps in institutions where the incidence of methicillin-resistant Staphylococcus aureus (MRSA) infection is high (more than 20% of all SSIs caused by MRSA).
Skin closure of a contaminated or dirty incision increases the risk of SSI, but few good studies evaluate the multiplicity of wound closure techniques used by surgeons. “Open abdomen” techniques of temporary abdominal closure as an adjunct to “damage control” for management of trauma or severe peritonitis are used increasingly. Antibiotic prophylaxis of the open abdomen is not indicated, although an inability to achieve primary abdominal closure is associated with several nosocomial infections (e.g., pneumonia, bloodstream infection, SSI) and increased cost (prolonged length of stay), but not higher mortality.
For trauma, antibiotic prophylaxis may be required for operative management or prevention of infection of traumatic wounds. Moreover, many surgical procedures performed for trauma are likely to be in a contaminated field (e.g., penetrating trauma, open reduction/internal fixation of open fractures). The evidence is robust for penetrating abdominal trauma, for which no more than 24 hours of prophylaxis with a second-generation cephalosporin (or equivalent) is recommended, even for colon injury. Likewise, although most traumatic wounds are contaminated somewhat, and the likelihood of infection increases when contamination is greater, prolonged antibiotic prophylaxis is not indicated, notably for facial fractures, whether closed or open.
Antimicrobial therapy is a mainstay of the treatment of infections, but widespread overuse and misuse of antibiotics have led to an alarming increase in MDR pathogens ( Table 1 ). New agents and innovative ways to administer existing antibiotics may allow shorter courses of therapy, which is desirable for cost savings and microbial ecology. Effective therapy with no toxicity requires a careful but expeditious search for the source of infection and an understanding of the principles of PK (see previous discussion).
| Initial Therapeutic Agent | Emergent Resistant Bacteria | Treatment of Resistant Bacteria |
|---|---|---|
| Fluoroquinolones, cephalosporins | MRSA | Vancomycin, ceftaroline, daptomycin, linezolid, eravacycline, tigecycline |
| Numerous agents, multiple classes | MDR gram-negative bacilli * | Carbapenem or carbapenem BLIC or cefiderocol or ceftolozane-tazobactam or polymyxin or eravacycline/tigecycline (not for Pseudomonas ) |
| Numerous agents, multiple classes | Clostridioides difficile | Vancomycin or fidaxomicin or metronidazole (no longer recommended as first-line therapy) |
| Vancomycin ± cephalosporin, daptomycin | VRE | Eravacycline, tigecycline, or linezolid |
| Vancomycin | VISA | Ceftaroline, eravacycline, tigecycline, linezolid, daptomycin |
| C. difficile | Vancomycin or metronidazole or fidaxomicin |
* MDR gram-negative bacilli include producers of extended-spectrum β-lactamases, metallo-β-lactamases, and carbapenemases.
Fever is usually the trigger for an evaluation for the presence of infection (hence, “fever workup”). However, some infected patients (e.g., elderly patients; those on immunosuppression; those with open abdominal wounds, end-stage liver disease, hypothyroidism, or chronic kidney disease; and patients taking anti-inflammatory or antipyretic drugs) do not manifest fever and may even be hypothermic (which is adverse prognostically). Absent fever, any symptom or sign of hypotension, tachycardia, tachypnea, confusion, rigors, skin lesions, hypoxemia, oliguria, lactic acidosis, leukocytosis, leukopenia, immature neutrophils (i.e., bands greater than 10%), or thrombocytopenia may indicate the need for an evaluation for infection and immediate empiric therapy. Moreover, fever in the early postoperative period (before postoperative day 4) often has a noninfectious cause and hence does not conflate with infection ( Table 2 ). In addition, noninfectious and infectious causes of fever may coexist, and an infected patient may harbor more than one discrete focus of fever or infection.
|
The only mandatory intervention for fever is physical examination; all other decisions are predicated on physical findings and clinical context. Antibiotics should not be given until evaluation has occurred, including the collection of specimens for culture/susceptibility testing. Therefore, evaluation must be expeditious, as delay in antibiotic administration, particularly if accompanied by unstable hemodynamics, is associated with an increased risk of death. Inspection of all incisions is mandatory. If an incision is opened and cultured, a deep culture specimen should be collected rather than swabbing the open wound superficially. There is no probative value of collecting fluid from drains (the likelihood of colonization is high). A chest radiograph is optional for evaluation of postoperative fever unless mechanical ventilation, physical examination, abnormal blood gases, or pulmonary secretions suggest a high yield. Urinalysis/urine culture is not mandatory in the early postoperative period unless there is reason by history or examination to suspect a urinary tract infection. However, on or after postoperative day 4, nosocomial infection becomes much more likely; evaluation should be comprehensive, and the threshold for empiric therapy decreased. Central lines should all be assessed.
Empiric antibiotic therapy must be administered judiciously and expeditiously. Injudicious therapy could result in undertreatment of established infection or unnecessary therapy in the setting of sterile inflammation or bacterial colonization; either may be deleterious. Inappropriate therapy (e.g., delay more than 1 hour in cases of septic shock, therapy misdirected against usual pathogens, failure to treat MDR pathogens) leads unequivocally to increased mortality.
Antibiotic choice is based on several interrelated factors ( Table 3 ). Paramount is activity against identified or likely (for empiric therapy) pathogens, presuming infecting and colonizing organisms can be distinguished and that narrow-spectrum coverage is always desired. Important factors include the disease process believed responsible, whether the infection is health care associated or community or hospital acquired, and whether MDR organisms are present, or likely to be. Local knowledge of antimicrobial resistance patterns is essential. Patient-specific factors of importance include age, debility, intrinsic organ function, immunosuppression, prior allergy or other adverse reaction, and recent antibiotic therapy. Institutional factors of importance include guidelines, formulary availability, outbreaks of infections caused by MDR pathogens, and antibiotic stewardship programs.
|
Numerous agents are available for therapy ( Table 4 ). Agents may be chosen based on spectrum, whether broad or targeted (e.g., antipseudomonal, antianaerobic), in addition to the aforementioned factors. If a nosocomial gram-positive pathogen is suspected (e.g., wound or SSI, CLABSI, HABP/VABP) or MRSA is endemic, empiric vancomycin (or linezolid) is appropriate. Some authorities recommend dual-agent therapy for serious Pseudomonas infections (i.e., an antipseudomonal β-lactam drug plus an aminoglycoside), but evidence of enhanced efficacy is lacking, and the risk of nephrotoxicity is increased. Regardless of the choice that is made, initial empiric therapy of any infection caused potentially by either a gram-positive or gram-negative bacterium (e.g., HABP/VABP, hospital-acquired cIAI) must include activity against all likely pathogens.
| Antipseudomonal Agents |
|
| Targeted-Spectrum Agents |
|
| Broad-Spectrum Agents |
|
| Anti-Anaerobic Agents |
|
| Anti-MRSA Agents |
|
Conventional antibiotic dosing may not apply to the critically ill or injured patient. Higher doses may be required for MDR isolates, increased extracellular volume, decreased serum albumin concentration, or increased glomerular filtration rate (e.g., burns, traumatic brain injury, multiple trauma). Underdosing of antibiotics is a major factor for the development of resistance during therapy and failure thereof. By contrast, lower doses may be required with multiple-organ dysfunction syndrome, acute kidney injury, and chronic kidney disease. Dosing of vancomycin and aminoglycosides may be monitored via measurement of drug concentrations in serum.
Continuous or prolonged infusion of β-lactam agents may be the optimal way to administer these drugs. Lengthened fT > MIC is achieved, increasing the likelihood of therapeutic success, especially against organisms with higher MICs, and minimizing the development of resistance. Continuous-infusion vancomycin has been described but is not recommended currently.
Vancomycin has been a mainstay of therapy of infections of critically ill patients, but MICs for vancomycin against MRSA have been increasing, even within the susceptible range. As a result, MIC cut-points for resistance of S . aureus have been revised downward to minimize the chance of ineffective therapy, and higher doses of vancomycin are recommended, although at a greater risk of nephrotoxicity. Vancomycin therapy should be undertaken with caution in patients with MRSA infections caused by isolates with MICs between 1 and 2 μg/mL. Adequate vancomycin therapeutic concentrations may not be achievable safely for S . aureus isolates with MICs > 2 μg/mL, so alternative therapy should be considered. Linezolid retains generally excellent activity, but resistance to daptomycin is being reported.
Aminoglycoside therapy experienced a resurgence owing to formerly limited options for treatment of MDR pathogens, including extended-spectrum β-lactamase (ESBL)-producing strains and the emergence of carbapenemases. Aminoglycoside use as part of an empiric therapy regimen remains debated, but is waning after the introduction of several new β-lactam/β-lactamase inhibitor combination (BLIC) drugs (see later). Single daily-dose aminoglycoside therapy ensures that an optimal peak serum concentration:MIC > 10 μg/mL will be achieved. A dose of gentamicin or tobramycin (7 mg/kg) or amikacin (20–30 mg/kg) is administered and a trough concentration is determined at 23 hours after the dose (some protocols call for an intermediate determination as well, at ∼ 16 hours after the dose, so that the elimination curve can be determined with greater precision). A trough concentration of 0.5 to 1.0 μg/mL is sought for gentamicin or tobramycin, whereas a trough concentration of 5 to 10 μg/mL is ideal for amikacin. Higher trough concentrations suggest that the dosing interval is too short or the dose is too high and are associated with increased toxicity, whereas an undetectable trough concentration suggests the converse. Outcomes of single daily-dose aminoglycoside therapy are comparable or better compared with conventional dosing, with decreased toxicity, but it has not been validated for children, during pregnancy, with burns, in patients older than age 70, or for treatment of bacterial endocarditis.
Infections caused by highly resistant (“pan-resistant”) gram-negative bacilli pose a major problem. Carbapenems, eravacycline, tigecycline, and polymyxins (see later) offer useful activity against ESBL-producing organisms. MDR nonfermenting gram-negative bacilli (e.g., Pseudomonas aeruginosa , Acinetobacter spp., Stenotrophomonas spp.) and carbapenemase-producing Enterobacteriaceae may require therapy with one of the newer BLIC agents, a high-dose carbapenem by prolonged or continuous infusion, a polymyxin, or an unusual combination of agents that have demonstrated synergy in vitro but remain of uncertain clinical utility.
The endpoint of antibiotic therapy is largely undefined, because quality data are few. In general, a date certain for discontinuation must be defined at the initiation of therapy; open-ended courses of therapy are inadvisable. If cultures are negative, empiric antibiotic therapy should be stopped in most cases after no more than 48 to 72 hours. In general, few surgical infections require more than 7 days of therapy, with notable exceptions being S. aureus bacteremia (which requires a minimum of 2 weeks of therapy) and select solid-organ abscesses (e.g., liver, brain). Unnecessary antibiotic therapy increases the risk of MDR infection; therefore, prolonged therapy with negative cultures is usually unjustifiable. The morbidity of antibiotic therapy also includes allergic reactions; development of nosocomial superinfections (e.g., fungal, enterococcal, and C. difficile –related infections); organ toxicity; reduced yield from subsequent cultures; vitamin K deficiency with coagulopathy or accentuation of warfarin effect; and occasionally bone marrow toxicity (e.g., neutropenia, thrombocytopenia).
If infection is evident, treatment is continued as indicated clinically, but the microbiology data should be examined to see if the antibiotic regimen can be deescalated to a narrower regimen (e.g., choosing a narrower-spectrum agent, changing multidrug therapy to monotherapy). If empiric therapy has been appropriate, escalation of therapy (e.g., choosing a more broad-spectrum agent, changing monotherapy to multidrug therapy, adding antifungal therapy) should be seldom needed.
It cannot be overemphasized that every decision to start antibiotics must be accompanied by an a priori decision regarding duration of therapy. A reason to continue therapy beyond the predetermined endpoint must be compelling. Bacterial killing is rapid in response to effective agents, but the host response may not subside immediately. Therefore, the clinical response of the patient should not be the sole determinant. There is increasing belief that shorter courses of antibiotic therapy, given appropriately, are equally effective with fewer side effects and that therapy should continue to a predetermined endpoint, after which it should be stopped.
Procalcitonin has been evaluated extensively as a biomarker to guide duration of therapy. Without exception, clinical outcomes were unchanged or length of stay was reduced. Also without exception, procalcitonin monitoring was antibiotic-sparing, reducing prescriptions of antibiotics by 32% to 72% in three trials and duration of therapy by 1.7 to 7.1 days in eight trials.
There are few data and no guidelines for the clinical approach to the patient who has failed a course of antibacterial therapy. There is not even a clear definition of what constitutes failure. Has the patient failed if the systemic inflammatory response persists after 3 days, or 5, or 7? When should antibiotics be changed? Is the antibiotic therapy well chosen, but the patient has inadequate source control and requires operation (or reoperation)? If a patient still has systemic inflammatory response syndrome (SIRS) at the predetermined endpoint, it is more useful to stop therapy and reevaluate for persistent or new infection, MDR pathogens, and noninfectious causes than to continue therapy uninformed or to add or change antibiotics without additional data.
Susceptibility testing of specific organisms is necessary for management of serious infections (including all nosocomial infections). This chapter will focus on the agents most useful for the treatment of serious/nosocomial infections; not every agent within the class will receive mention. Recommended agents for specific organisms are given as guidelines only, because in vitro susceptibilities may not correlate with clinical efficacy.
The β-lactam antibiotic group consists of penicillins, cephalosporins, monobactams, and carbapenems. Within this group, several agents have been combined with β-lactamase inhibitors to broaden the spectrum of activity. Several subgroups of antibiotics are recognized within the group, notably penicillinase-resistant penicillins and several “generations” of cephalosporins.
Penicillinase-resistant semisynthetic penicillins include methicillin, nafcillin, and oxacillin. These agents are the treatments of choice for methicillin-susceptible S. aureus (MSSA) and other susceptible staphylococcal species, but not as empiric agents because of high rates of MRSA, and can be used for infections such as bacteremia, endocarditis, pneumonia, and soft tissue infections. Penicillins exhibit good penetration of the central nervous system, therefore providing coverage for meningitis caused by susceptible organisms. Virtually all enterococcal species are resistant, except rarely Enterococcus faecalis .
Except for carboxypenicillins and ureidopenicillins, penicillins retain little or no activity against gram-negative bacilli. Carboxypenicillins (ticarcillin and carbenicillin) and ureidopenicillins (azlocillin, mezlocillin, and piperacillin; sometimes referred to as acylampicillins) are no longer used unless complexed as a BLIC. Piperacillin-tazobactam retains useful activity against Enterobacteriaceae, and reasonable potency against P . aeruginosa , but emerging data suggest it may be synergistically nephrotoxic in combination with vancomycin. Ampicillin-sulbactam is unreliable against Escherichia coli and Klebsiella spp. (resistance rate ∼ 50%), and thus is no longer recommended to treat cIAI, but it has some activity against Acinetobacter spp. owing to the sulbactam moiety.
Newer BLIC agents include ceftolozane-tazobactam, ceftazidime-avibactam, imipenem/cilastatin-relebactam, and meropenem-vaborbactam. The newer β-lactamase moieties (avibactam, relebactam, and vaborbactam) differ structurally from their predecessors in that they do not have a β-lactam core and thus have enhanced activity against MDR gram-negative bacilli. Without exception, earlier BLIC agents (ampicillin-sulbactam, piperacillin-tazobactam, and ticarcillin-clavulanic acid and the oral formulation amoxicillin-clavulanic acid) have excellent antianaerobic activity (and thus do not need to be administered in tandem with metronidazole), as do the newer carbapenem-based BLIC agents, whereas the newer combinations with cephalosporins (ceftolozane-tazobactam, ceftazidime-avibactam) do not have reliable antianaerobic activity. See the following sections on cephalosporins and carbapenems for additional information about the newer BLIC agents.
First- and second-generation agents are used mainly for prophylaxis, for uncomplicated infections, or for deescalation when results of susceptibility testing are known. Recent evidence indicates that cefazolin can be used as first-line therapy for MSSA bacteremia, with lower risk of nephrotoxicity as compared with nafcillin. None of the cephalosporins are useful against enterococci.
Third-generation cephalosporins have enhanced activity against gram-negative bacilli, but only ceftriaxone is active against most gram-positive cocci (MRSA excepted), and none are effective against anaerobes. Ceftazidime and cefoperazone have antipseudomonal activity. The common place usage of third-generation cephalosporins requires caution, as these agents are known to induce resistance in gram-negative bacilli, with adverse effects on microbial ecology. Prudence suggests these agents should be limited to use when alternatives are not available. The BLIC ceftazidime-avibactam is relatively impervious to hydrolysis by several classes of β-lactamases and is useful for therapy of infections caused by MDR Enterobacteriaceae.
Cefepime, a fourth-generation cephalosporin, has antipseudomonal activity and activity against most gram-positive cocci, but not MRSA. The safety profile is comparable to that of third-generation agents, but the potential to induce resistance is less. Three newer cephalosporins, cefiderocol, ceftaroline, and ceftolozane, have been introduced, the latter only in combination with tazobactam as a BLIC. These drugs do not fit neatly into the cephalosporin classification paradigm, and microbiologists have resisted doing so. Cefiderocol, a siderophore cephalosporin, has a unique mechanism of action. Chelating iron molecules essential for bacterial metabolism to use the bacterial ion transport system to penetrate the cell membrane of gram-negative bacilli. Cefiderocol is U.S. Food and Drug Administration (FDA)-approved to treat cUTI (including pyelonephritis) and HABP/VABP caused by susceptible strains of Acinetobacter baumannii complex (the only FDA-approved drug to treat this organism for any indication) , Enterobacter cloacae complex . E. coli, Klebsiella pneumoniae, Proteus mirabilis, P. aeruginosa, and Serratia marcescens, depending on the indication. Ceftaroline has anti-MRSA activity unique among cephalosporins while retaining modest activity against gram-negative bacilli comparable to first-generation agents and has been FDA-approved for treatment of skin and skin structure infections and community-acquired bacterial pneumonia (CABP). Ceftolozane-tazobactam has multiple indications including cIAI, cUTI, and HABP/VABP, but has notable activity against MDR P. aeruginosa , which may define its niche in the armamentarium.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here