Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Injury is the leading cause of fatality worldwide, causing more than 5 million deaths—9% of the world's deaths—each year. According to the Centers for Disease Control and Prevention (CDC), trauma accounted for nearly 230,000 U.S. deaths in 2018, costing over $400 billion in health care and lost productivity. Trauma is the most frequent cause of fatality in those aged 1 to 44 years, accounting for 54% of deaths in age group 1 to 9 years, 85% in the age group 10 to 24 years, and 57% in the age group 25 to 44 years. The disease of trauma disproportionately affects younger age groups, with trauma responsible for over 36% of potential years of life lost in people younger than 65 years old.
During the past decades, mortality trends have continued to decrease as care for the severely injured patient has improved. Emergent care for the severely injured is aggregated at designated trauma centers, which are independently verified by strict criteria stipulated by the American College of Surgeons. The most specialized trauma centers, designated as Level I, have the ability to deliver 24-hour specialized multidisciplinary care. Trauma care delivered at Level I trauma centers decreases overall mortality risk by 25% when compared with nontrauma centers. Anesthesia providers, in particular, play a vital role in the acute resuscitation and early management of severely injured patients. In this chapter, the basics of trauma care for anesthesia providers will be discussed.
Physiologic derangements in patients who have suffered trauma-induced injuries depend on the mechanism and severity of injury. Most commonly, hypotension in trauma is the result of severe blood loss or hemorrhagic shock, which is the main cause of fatality in critically injured patients. After sources of hemorrhagic shock are investigated, the following causes of shock must also be considered when encountering hypotension in the trauma setting: relative hypovolemia from obstructed venous return (e.g., from tension pneumothorax or cardiac tamponade), cardiogenic shock, and neurogenic shock.
The initial presenting arterial blood pressure values of a trauma patient may be misleading in early hemorrhage. The degree of hemorrhage can be masked by compensatory reflexes via the sympathetic nervous system, carotid sinus and aortic arch baroreceptors, and other low-pressure receptors. The renin–angiotensin system and vasopressin secretion from the pituitary play a later compensatory role. These responses allow sympathetic vasoconstriction of the arterioles to increase total peripheral resistance, venoconstriction to increase venous return, and an increase in heart rate. With extreme hypoxia and acidosis, the central nervous system also provides additional sympathetic stimulation.
Hemorrhagic shock can generally be divided into a compensated and progressive phase. Each phase has different characteristics depending on the acuity and volume of blood lost ( Table 43.1 ).
| Class I | Class II | Class III | Class IV | |
|---|---|---|---|---|
| Blood loss a (mL) | Up to 750 | 750–1500 | 1500–2000 | >2000 |
| Blood loss (% blood volume) | Up to 15% | 15%–30% | 30%–40% | >40% |
| Pulse rate (BPM) | <100 | 100–120 | 120–140 | >140 |
| Systolic BP | Normal | Normal | Decreased | Decreased |
| Pulse pressure | Normal or increased | Decreased | Decreased | Decreased |
| Respiratory rate | 14–20 | 20–30 | 30–40 | >35 |
| Urine output (mL/h) | >30 | 20–30 | 5–15 | Negligible |
| CNS/mental status | Slightly anxious | Mildly anxious | Anxious, confused | Confused, lethargic |
a For 70-kg adult. Modified from the Advanced Trauma Life Support (ATLS) program. BP, Blood pressure; BPM, beats per minute; CNS, central nervous system.
In compensated hemorrhage, physiologic compensatory mechanisms that are intact may be adequate to sustain systemic perfusion without clinical intervention. About 10% to 15% of blood loss may be adequately compensated for by physiology alone. As blood loss continues, hemorrhagic shock progresses. If inadequate perfusion persists, generalized tissue and cellular necrosis, cardiac dysfunction, and metabolic acidosis occur. This can ultimately lead to multiorgan failure.
Hemorrhagic shock and tissue hypoperfusion subsequently lead to complex interactions between inflammatory factors, intrinsic anticoagulants, and other cellular dysfunctions that can cause an acute traumatic coagulopathy after injury. This coagulopathy is attributed to factor deficiency, hyperfibrinolysis, and platelet dysfunction. Iatrogenic factors of resuscitation can further disrupt the coagulation process. These factors include hemodilution, hypocalcemia, hypothermia, and acidosis. This is known as trauma-induced coagulopathy . All of these processes lead to a positive feedback loop that eventually ends in death. Hypothermia, coagulopathy, and acidosis are commonly termed the triad of death or lethal triad ( Fig. 43.1 ). Hemorrhagic shock may cross a threshold at which it becomes irreparable despite blood transfusions and other therapies owing to severe, irreversible multiorgan failure.

Successfully managing a patient who has suffered a major trauma requires a coordinated systematic approach to history, mechanism, examination, diagnosis, and treatment. Furthermore, these processes must run swiftly and in parallel. Often initial management is commenced before a definitive diagnosis has been established.
Each patient has a unique constellation of injuries and mechanisms, and when combined with their premorbid status, there are an immeasurable number of potential presentations. To prepare for the unpredictability of trauma, many of the initial assessment and management processes are standardized, and clinicians must be familiar with their local institution's policies and guidelines.
This section will focus on the initial management of a major trauma patient, focusing primarily on the time in the emergency department. The initial management can significantly influence intraoperative care. The components of a mature trauma system can be divided into prearrival, the trauma bay, adjuncts, and definitive care.
Preparation for the arrival of an intensely injured patient enables the trauma team to deliver rapid, effective care, which is essential for a positive outcome to occur. This involves more than just confirming that essential equipment is present and functioning. Although these checks are very important, organizational and patient-specific preparations also need to be considered.
Caring for a major trauma patient requires the mobilization and deployment of a large and diverse range of health care resources to a single point. Preparations include considering the following questions: (1) Is there a designated trauma bay in the emergency department? (2)Who attends the trauma call, and how are they notified? (3) What are the policies and protocols for activation of emergency radiology services, emergency operating room (OR) use, massive blood transfusion, and patient transport? (4) What are the referral pathways to internal and external providers?
These issues should be addressed before the arrival of a critically ill patient. Because of the unpredictable nature of trauma, a novel set of circumstances can overwhelm or bypass an organization's existing preparation. In these situations, the clinicians involved—as members of a learning organization—must notify and alert the individuals who can provide the required resources.
This should occur immediately before the arrival of an individual major trauma patient. Information regarding the patient's injury and status should be provided to emergency department staff by the ambulance service to facilitate resource mobilization.
Most ambulance services around the world use a standardized handover tool to provide essential information in a succinct and efficient manner. An example of this tool is IMIST, a mnemonic for I dentification of the patient, M echanism/medical complaint, I njuries/information relative to the complaint, S igns (including vital signs and Glasgow Coma Scale [GCS] score), and T reatment and trends/response to treatment ( Box 43.1 ). With this information , the health care team can anticipate the patient's clinical needs and prepare accordingly.
| I dentification of the patient | Age Gender Name (if known) |
| M echanism/medical complaint | What happened? |
| I njuries/information relative to the complaint | Known/suspected injuries |
| S igns (vital signs and Glasgow Coma Scale score) | Presence of breath sounds Tracheal deviation |
| T reatment and trends/response to treatment | Vital signs Drugs Fluids Splints |
The purpose of this briefing is to optimize team efficiency and performance. This enables all members of the team to introduce themselves, develop group situational awareness about the known condition of the patient, and assign appropriate team roles.
Once the patient arrives, the focus of the team shifts to rapid and simultaneous diagnosis and treatment of life-threatening conditions.
The Advanced Trauma Life Support (ATLS) approach was developed by the American College of Surgeons Committee on Trauma. ATLS was first introduced in 1980 and has since been widely adopted throughout the world. It is structured into primary, secondary, and tertiary surveys. This chapter will only address the primary survey. The ATLS course is highly recommended as an introduction to trauma management. Most importantly, it provides a common language and framework to organize thinking required for optimal individual and team performance.
The purpose of the primary survey is to identify and treat immediately life-threatening injuries. It is organized into the ABCDE mnemonic ( Box 43.2 ).
Establishment of a patent airway is of paramount importance to ensure a positive outcome for the patient. Rapid assessment is most easily achieved by asking the patient some simple questions. If the patient can speak, then the airway at the time of assessment is usually patent. Intervention may still be required, but there is time to plan the safest treatment.
The trauma patient may require a definitive patent airway (a cuffed endotracheal tube) for many reasons ( Box 43.3 ). Induction of anesthesia before tracheal intubation may be a high-risk and dangerous procedure. The top priority is always to maintain adequate tissue oxygenation. If a patent airway is maintained with simple airway maneuvers, then there is time to optimize the patient's physiology and properly prepare for the intubation attempt. If the clinical situation allows, performing a focused neurologic assessment before anesthesia induction can provide invaluable information that would be difficult to obtain in the sedated, endotracheally intubated patient.
Maxillofacial trauma
Major hemodynamic instability
Low Sa o 2
Burns
Head injury
Intoxicated/behavioral/safety issues
Transport (radiology/OR/ICU/external)
There are several differences in the approach to intubating the trachea in a trauma patient in the emergency department as compared with an elective surgical patient in the OR.
Preoxygenation in the patient who has injuries from trauma can be challenging. The objective of preoxygenation is to “denitrogenate” the lung, thus providing a reservoir of oxygen in the patient's functional residual capacity (FRC) to prevent desaturation (hypoxemia) during the apneic phase of tracheal intubation. However, many injuries sustained by trauma patients prevent this process from being effective. Specifically, any injury that reduces FRC or creates a shunt (lung units that are perfused but not ventilated) will increase the likelihood of desaturation despite technically adequate preoxygenation. Examples of such injuries include direct lung parenchymal injury, hemothorax or pneumothorax, pulmonary aspiration of blood or gastric contents, intraabdominal bleeding, diaphragmatic injury, and rib fractures. Addition of an alternative oxygen source to provide apneic oxygenation throughout the peri-intubation period has been evaluated in the emergency department literature. Despite a theoretical benefit, as a result of all the issues with preoxygenation in trauma patients, no benefit has been demonstrated in experienced laryngoscopists' hands. The best defense against desaturation is reducing total apneic time.
All trauma patients should be assumed to have a “full stomach” even when many hours have elapsed since their last oral intake. As such, a rapid-sequence induction (RSI) is considered standard practice. The use of cricoid pressure is common clinical practice but may worsen the view at laryngoscopy. A review of the evidence is presented in Chapter 16 .
Major trauma patients who require emergent tracheal intubation are often the most critically ill patients in the hospital. The justification for endotracheal intubation and the impact of that physiologic insult on their response to laryngoscopy must be clearly defined. For example, if the reason for endotracheal intubation is respiratory distress from a major lung injury, then optimal preoxygenation may still result in rapid desaturation after apnea occurs.
The hemodynamic response to anesthetics given to induce anesthesia is often exaggerated for two main reasons. First, acute intravascular volume loss from bleeding results in an inability to maintain arterial blood pressure and cardiac output in the face of the vasodilatory effects of anesthetic drugs. Second, sympathetic stimulation caused by pain and distress can mask the true intravascular volume state; if so, induction of anesthesia can cause marked hemodynamic instability. This can usually be anticipated by the appropriate intravascular administration of fluids (i.e., crystalloids, blood, colloids) and the availability of vasopressors.
The choice of drugs to induce anesthesia in the critically ill patient is an area of much controversy (also see Chapter 8 ). Propofol and etomidate are often the primary choice, with some opting for ketamine in certain situations. The hemodynamic impact and stability are more a reflection of drug dosing rather than the choice of agent. Most important is for the clinician to be very familiar with the anesthetic drugs they plan to use rather than using a new, unfamiliar drug. In general, the dose of anesthetic drug should be decreased because of a relatively reduced volume of distribution of drugs in a very ill or hypovolemic patient. Preferential perfusion to essential organs, such as the brain, heart, and kidneys, occurs in such patients. A vasopressor should be immediately available to manage any transient hypotension caused by the anesthetic drugs.
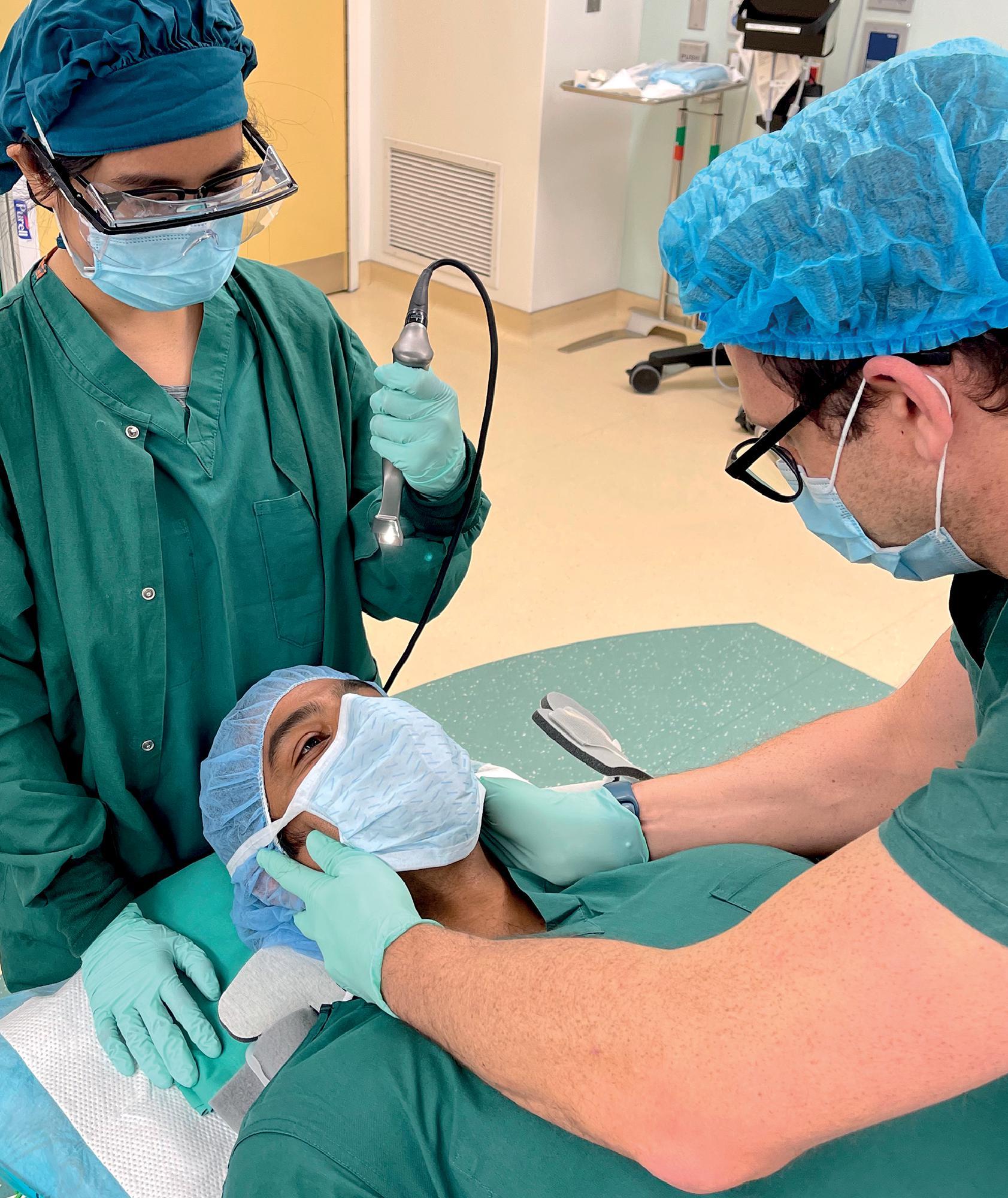
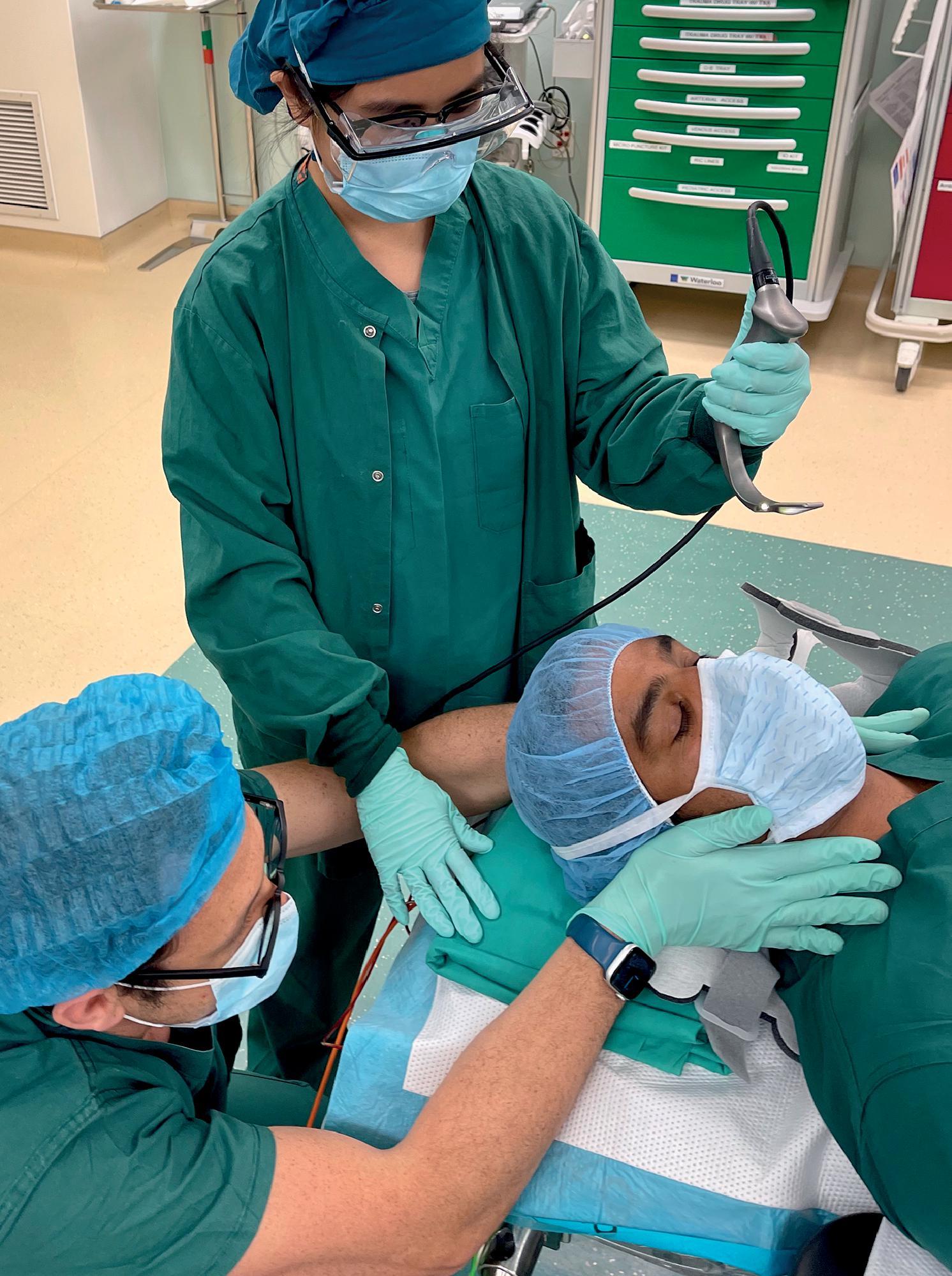
The process of laryngoscopy can produce an unacceptable amount of force through the cervical spine. Attempts should always be made to reduce this force. Any patient with a suspected spinal injury should be placed in a hard collar. The front of the collar should be opened at the time of laryngoscopy and the head and neck stabilized by an additional clinician. Ideally, the manual in-line stabilization (MILS) of the neck is performed by a second provider standing at the patient's torso, so as to not interfere with the laryngoscopist ( Fig. 43.2 ). When the intubation and laryngoscopy are done in the OR with the patient prepped and draped, MILS needs to be performed with the person stabilizing the neck standing (or squatting) directly next to the laryngoscopist ( Fig. 43.3 ). In this case, the person attempts to stabilize the neck while allowing the optimal position for the laryngoscopist. The objective is to minimize movement of the cervical spine during laryngoscopy. A pragmatic approach is required during MILS because a failed endotracheal intubation attempt presents a much greater immediate risk to the patient (i.e., hypoxia) than the risk of small neck movements. In the event of poor visualization of the glottic structures, consideration should be given to relaxing MILS to facilitate endotracheal intubation before replacing the hard cervical collar. The use of video laryngoscopy may facilitate adequate intubating conditions while minimizing unnecessary neck movement and should be considered as a first-line device in these patients where available.
Video laryngoscopy has transformed the way airways are managed in the emergency department and OR. The advantages of video laryngoscopy include the following: (1) gives an adequate tracheal intubating view with reduced pressure and force; (2) provides group situational awareness of progress and difficulty of laryngoscopy; (3) improves visualization of the larynx in more difficult patients without the need to change equipment; and (4) allows supervisors and trainers to provide dynamic feedback throughout the laryngoscopy.
Failed endotracheal intubations are rare. Nevertheless, management of the unanticipated difficult airway is more likely in the trauma patient. Several factors contribute to the increased difficulty of airway management: cervical spine precautions, blood or foreign body in the airway, and the stress experienced by the person performing the intubation. Plans for failed intubation must be made explicit, such as what equipment will be needed, its location, and the immediate availability of staff who are assisting with intubation before induction of anesthesia. Staff who work outside the OR environment may not be familiar with airway equipment such as laryngeal masks or surgical airway kits, and their comfort with using this type of equipment should be known in advance.
Endotracheal intubation is a mechanism for providing physiologic support—it is not therapeutic treatment itself. The focus of the emergency department team needs to remain on the patient's transition to definitive care. Several issues need to be managed immediately after intubation ( Box 43.4 ).
Ongoing sedation—postintubation hypertension is common and should be avoided because of its effect on uncontrolled bleeding and intracerebral physiology
Ventilator and settings
Disposition—where is the patient going next? To the computed tomography (CT) scanner, the operating room (OR), or the intensive care unit (ICU)?
Additional intravenous or arterial access, or both—these procedures should not delay any movement to definitive care
Several special circumstances need to be considered when making a decision on the management of the trauma patient's airway:
Airway burns: Patients with airway burns require expedited management of their airway. Within a very short time, they can progress from minimal or no respiratory distress to a completely occluded airway because of edema expansion. Warning signs of potential airway burns include facial burns, soot in mouth/nose, carbonaceous sputum, explosive injuries to the upper body, and stridor.
Oral trauma: Blood is often found in the upper airway of trauma patients. Bleeding can range from a minor nuisance to a life-threatening hemorrhage. It is important to recognize this situation (i.e., blood in the airway) because induction of anesthesia can result in the rapid loss of a patent airway. Video laryngoscopes and fiber-optic scopes do not perform well when blood obscures the field of view. An additional suction source is mandatory, and a surgical airway team should be immediately available.
Direct airway injury: Although uncommon, tracheal trauma should be suspected in any patient with direct penetrating or blunt trauma to the neck. Warning signs such as stridor and subcutaneous emphysema may be present. These airways should be managed only by experienced clinicians with early involvement of a head and neck surgeon. Advancing the cuff of the endotracheal tube past a confirmed or suspected lesion is a critical concept in these scenarios.
Adequate circulation and perfusion need to be reestablished to ensure sufficient oxygen delivery to essential organs. The first priority is to stop any bleeding. This can be achieved through a combination of interventions performed in the emergency department (direct pressure, suturing wounds), surgical intervention, or angioembolization. Simultaneously, the anesthesia provider must ensure adequate oxygen delivery to essential organs such as the brain and heart. Damage control resuscitation (DCR) is the term given to a resuscitative strategy that provides circulatory support sufficient to prevent permanent end-organ damage while avoiding the pitfalls of excessive resuscitation. Hypothermia, acidosis, and hypocalcemia represent additional clinical issues that often arise in managing the hypotensive trauma patient.
The assessment of the neurologic system should identify potentially catastrophic injuries that require prompt management. This rapid assessment is based on the GCS score, pupillary response, and gross limb function. Intubation is usually required for patients with a GCS score less than 8 ( Box 43.5 ).
Eyes (E)
4—Open spontaneously
3—Open to voice
2—Open to pain
1—Do not open
Verbal (V)
5—Oriented
4—Confused
3—Inappropriate words
2—Incomprehensible sounds
1—No sounds
Motor (M)
6—Obeys commands
5—Localizes to pain
4—Withdraws to pain
3—Abnormal flexion to pain
2—Abnormal extension to pain
1—No response
Total score = best responses for eyes, verbal, and motor
E = 4
V = 5
M = 6
Total GCS score = 15
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here