Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anesthesia for pediatric orthopedic surgery encompasses the entire age and medical spectrum of pediatrics—the newborn through adolescent, the otherwise normal, the critically ill, the chronically ill, the child with multiple complex congenital anomalies, the traumatically injured, and the “frequent flyer” patient. Pediatric orthopedic surgeons operate on virtually every area of the body, from the cervical spine to the pelvis to the toes. In many instances, the perioperative anesthetic plan for pediatric orthopedic patients depends more on their ages, on the site and emergent nature of surgery, and on the need for perioperative analgesia and sedation than it does on the specific underlying disease or the procedural details of the surgery. In other cases, the underlying medical condition, associated anomalies, pathophysiology, and surgical procedure dictate the anesthetic plan. Positioning the patient on the operating room (OR) table may be difficult because of deformities or contractures or the nature of the injury. Oftentimes, patients require special operating tables or frames to achieve the best posture for surgery.
Frequently, the anesthesiologist is faced with a child with an unusual syndrome that has both obvious orthopedic implications as well as systemic manifestations that impact the anesthetic plan. Conditions that are commonly encountered in pediatric orthopedic surgery and their anesthetic implications are listed in Table 36.1 , but the list is by no means comprehensive ( ; ). As an example, there are over 400 skeletal dysplasias identified with extreme phenotypic variability ( ). The Online Mendelian Inheritance in Man (OMIM) website ( http://www.ncbi.nlm.nih.gov/omim ) is an excellent initial source for more information about a specific syndrome. Regardless of the underlying condition, almost all orthopedic surgical procedures have recurring anesthetic concerns, involving positioning, airway management, blood loss and fluid replacement, conservation of body temperature, prevention of surgical site infection, and postoperative pain and sedation management ( ).
| Disease | Surgical Interventions | Anesthetic Implications |
|---|---|---|
| Congenital Malformations | ||
| Amniotic band constriction | Soft tissue release | May have facial clefts |
| Clubfoot | Tendon lengthening, release | Dictated by associated malformations |
| Klippel–Feil syndrome | Release, scoliosis | Hemi- or fused vertebra; limited C-spine mobility; possible difficult intubation; heart defects |
| Radial dysgenesis | Tendon lengthening, pollicization release | Episodic thrombocytopenia; congenital heart disease |
| Sprengel deformity | Only associated with Klippel–Feil syndrome | |
| Trisomy 21 (Down syndrome) | Cervical spine fusion | Large tongue; usually easy intubation; bradycardia on inductionin-line stabilization during intubation; congenital heart disease; opioid sensitivity |
| Acquired Conditions | ||
| Charcot–Marie–Tooth disease | Tendon transfer | Questionable use of nondepolarizing muscle relaxants |
| Legg–Calvé–Perthes disease | Osteotomies, pinning | None known |
| Osteomyelitis | Culture, aspiration | Systemic bacterial infection |
| Septic arthritis | Culture, irrigation | Systemic bacterial infection |
| Slipped femoral capital epiphysis | Pinning | Obesity |
| Tumors, benign | Excision, curettage | Possible significant blood loss; pathologic fracture |
| Tumors, malignant | Radical excision, amputation | Blood loss; metastasis: CNS, lung; chemotherapy; cardiotoxicity |
| Syndromes, Inherited Conditions | ||
| Apert and Crouzon syndrome | Syndactyly repair; craniosynostosis; hypertelorism | Airway obstruction common, but occasional mandibular hypoplasia and possible difficult mask ventilation and/or intubation; cervical fusion, cardiac defect |
| Ellis–van Creveld syndrome | Polydactyly | Cardiac defects; bronchial collapse |
| Holt–Oram syndrome | Tendon lengthening, pollicization release | Cardiac defects (ASD, VSD) |
| Jeune syndrome (asphyxiating thoracic dystrophy) | Chest reconstruction, scoliosis | Respiratory failure, prolonged mechanical ventilation; renal failure |
| Marfan syndrome | Scoliosis | Cardiac (AI, MR), aortic aneurysm |
| Möbius syndrome | Syndactyly | Micrognathia; cleft palate; cranial nerve palsy |
| Osteogenesis imperfecta | Pathologic fractures, scoliosis | Fractures on positioning or intubation; hypermetabolic fever, platelet dysfunction; blood pressure cuff may cause fractures |
| VATER (vertebral, anal, tracheal esophageal fistula, renal, cardiac) | Tendon lengthening, pollicization release | Cardiac defects; tracheoesophageal fistula |
| Short-Stature Syndrome | ||
| Achondroplasia | Spinal fusion, cervical decompression, Ilizarov | Poor cervical mobility, difficult arterial catheterization |
| Morquio–Ullrich disease | Cervical spine fusion | Poor cervical mobility, difficult airway |
| Mucopolysaccharidoses (Hurler, Hunter, Morquio syndromes) |
Kyphoscoliosis, bony abnormalities | Very difficult intubation; unstable neck; respiratory failure perioperatively |
| Systemic Disease | ||
| Juvenile rheumatoid arthritis | Varies | TMJ ankylosis; C-spine immobility or instability; carditis; occasional pulmonary involvement; possible difficult airway |
| Neurofibromatosis | Scoliosis | CNS tumors; occasional pheochromocytoma |
| Sickle cell anemia | Osteomyelitis, Legg–Calvé–Perthes disease, pathologic fracture | Anemia; vaso-occlusive crisis; acute chest syndrome; stroke; hypothermia; hypoxia; hypovolemia; immunocompromised host; avoid tourniquet when possible |
| CNS Diseases | ||
| Arthrogryposis multiplex | Tendon releases (multiple congenital contractures), scoliosis | Difficult intubation (TMJ ankylosis, C-spine immobility); GE reflux; postoperative upper airway obstruction; congenital heart disease, difficult vascular access |
| Cerebral palsy | Tendon releases | GE reflux; postoperative upper airway obstruction |
| Myelomeningocele | Lower extremity tendon releases, scoliosis, kyphosis | Hydrocephalus |
| Werdnig–Hoffmann disease | Scoliosis | Respiratory insufficiency; bulbar involvement—poor secretion handling; succinylcholine-induced hyperkalemia |
| Myopathies | ||
| Duchenne muscular dystrophy | Tendon releases, scoliosis | Respiratory insufficiency; cardiomyopathy; succinylcholine-induced hyperkalemia, anesthesia-induced rhabdomyolysis |
| Myotonia dystrophica | Tendon releases | Succinylcholine-induced myotonic spasm; cardiac conduction system involvement; avoid direct muscle stimulation |
A common feature of children with orthopedic diseases, particularly patients with congenital and genetic musculoskeletal disorders, is the significant disability that affects their everyday lives. Many of these children must undergo multiple hospitalizations, anesthetics, and surgical procedures. A single adverse perioperative experience can have long-lasting negative effects not only on a child’s future orthopedic care but on their overall well-being and future health. Thus an individualized approach to patient management is necessary. Sometimes, these children are overwhelmingly fearful and may be completely terrorized by the hospital experience. Simply approaching these children in hospital clothing may elicit these fears. Others are “frequent flyers” and have very specific desires about who should treat them and how they are to be anesthetized. For example, some may prefer an intravenous (IV) induction versus a mask induction or have specific sites in which they want their IV placed, or even specify who places their IV or how it should be placed. Thus the anesthesiologist must review the child’s perioperative history with them and be as accommodating as possible to their preferences.
Although anesthesiologists play an important role in preventing perioperative infection in all patients, the orthopedic patient is of particular concern. Treating deep wound or joint infections, osteomyelitis, or having to remove infected hardware are significant medical management challenges. A bundle approach of hand hygiene, provider education, chlorhexidine skin preparation, environmental decontamination, aseptic technique when administering intravenous drugs (particularly propofol), and the timely and appropriate administration of perioperative antibiotics can make a significant difference in reducing hospital-acquired infection ( Table 36.2 ) ( ). Indeed, the Surgical Care Improvement Project (SCIP) was developed in 2006 to reduce surgical site infections (SSIs), focusing on the timely and appropriate administration of antibiotics before skin incision ( ; ; ). Adherence (or failure to adhere) to these infection prevention strategies affects patient outcome as well as provider and hospital reimbursement. The patient, environmental-healthcare equipment, and anesthesia provider routines can affect the incidence of hospital-acquired infections. Bacterial reservoirs are also important sources of pathogens ( ; ; ). Techniques for drawing up drugs (e.g., failure to cleanse a rubber dam with alcohol), accessing IVs (failure to decontaminate hands and wear gloves), and cleaning IV port sites contribute to SSIs and all are within the purview of anesthesia care ( ; ). Finally, over the past decade, anesthesiologists are now responsible for the timely administration and appropriate choice of prophylactic antibiotics ( Table 36.2 ) ( ; ). Specifically, SCIP measures call for the administration of appropriate antibiotics in a window that precedes surgical incision, but not by more than 60 minutes (120 minutes for vancomycin or fluoroquinolones) ( ; ). Many of the antibiotics used for SSI prophylaxis, including the beta lactams (e.g., cephalosporins, penicillins) have short half-lives and must be given frequently to maintain their bactericidal effect ( ; ). They have little postantibiotic effect, so operations taking more than two drug elimination half-live hours require redosing or administering as a continuous infusion ( Table 36.2 ) ( ; ). On the other hand, the glycopeptides, quinolones, metronidazole, and the aminoglycosides have significant postantibiotic effect and do not require continuous infusions or frequent redosing ( ).
| A. Antibiotic | B. Class | C. Sensitivity | D. Preoperative Dose Half-life (t1/2 β ) | E. Redose (Using Dose from Column D) Intraoperatively Every: | F. Intraoperative Replacement Dose for Blood Loss |
|---|---|---|---|---|---|
| Ampicillin | Amino penicillin (beta lactam) | GPC, GN, enterococcus, anaerobes | 50 mg/kg up to 2000 mg t1/2β = 1 hour | 2 hours | 25 mg/kg up to 1000 mg |
| Ampicillin/sulbactam | Penicillin + beta lactamase inhibitor | GPC, GN, enterococcus, anaerobes, MSSA, bacteroides | 50 mg/kg (ampicillin) up to 2000 mg t1/2β = 1 hour | 2 hours | 25 mg/kg (ampicillin) up to 1000 mg |
| Azithromycin | Macrolide | GPC, GN, anaerobes, chlamydia, mycoplasma | 10 mg/kg to 500 mg IV | No redose | N/A |
| Cefazolin Noncardiothoracic | Cephalosporin (beta lactam), first generation | GPC, some GN aerobes: Escherichia coli Proteus mirabilis | 30–40 mg/kg up to 2000 mg (if ≥120 kg, dose is 3000 mg) t1/2β = 1.8 hour | 3–4 hours | 15 mg/kg up to 1000 mg (if ≥120 kg, dose is 1500 mg) |
| Cefazolin Cardiothoracic | Cephalosporin (beta lactam), first generation | GPC, some GN aerobes: Escherichia coli Proteus mirabilis | 40–50 mg/kg up to 2000 mg (≥120 kg, dose is 3000 mg) t1/2β = 1.8 hour | 3–4 hours and/or within 1 hour after coming off bypass | 20 mg/kg up to 1000 mg (if ≥120 kg, dose is 1500 mg) |
| Cefotaxime | Cephalosporin (beta lactam), third generation | GPC, GN, enterococcus | 50 mg/kg up to 2000 mg t1/2β = 0.6–1 hour | 2 hours | 25 mg/kg up to 1000 mg |
| Cefoxitin | Cephalosporin (beta lactam), second generation | GPC, some GN | 40 mg/kg up to 2000 mg t1/2β = 0.6–1 hour | 2 hours | 20 mg/kg up to 1000 mg |
| Ceftazidime | Cephalosporin (beta lactam), third generation | GPC, GN, anaerobes | 50 mg/kg/dose up to 2000 mg t1/2β = 1.9 hour | 4 hours | 25 mg/kg up to 1000 mg |
| Ceftriaxone | Cephalosporin (beta lactam), third generation | GPC, GN, Citrobacter, Serratia | 50 mg/kg up to 2000 mg t1/2β = 6–9 hour | No redose | 25 mg/kg up to 1000 mg |
| Ciprofloxacin | Fluoroquinolone | GN | 10 mg/kg up to 400 mg t1/2β = 3–5 hour | No redose | 5 mg/kg up to 200 mg |
| Clindamycin | Lincosamide | GPC, GN, MRSA, anaerobes, used | 10 mg/kg up to 900 mg t1/2β = 2.5–3 hour | 5–6 hours | 5 mg/kg up to 450 mg |
Cephalosporins are the mainstay of surgical site prophylaxis strategies. Unfortunately, due to prevalent misconceptions, patients labeled as having a penicillin allergy often receive alternate and less effective antibiotics, usually vancomycin, placing them at risk of a variety of adverse effects including increased morbidity (“red man syndrome”) and higher risk of surgical site infection. Current evidence refutes the misconception of cross-reactivity between penicillins and cefazolin, and there is no clear evidence of an increased risk of anaphylaxis in cefazolin-naive, penicillin-allergic patients ( ; ) (see Chapter 54 : Special Pediatric Disorders, Fig. 54.2 ). Consultation with the patient’s allergist, when appropriate, is useful in alleviating the family’s concerns.
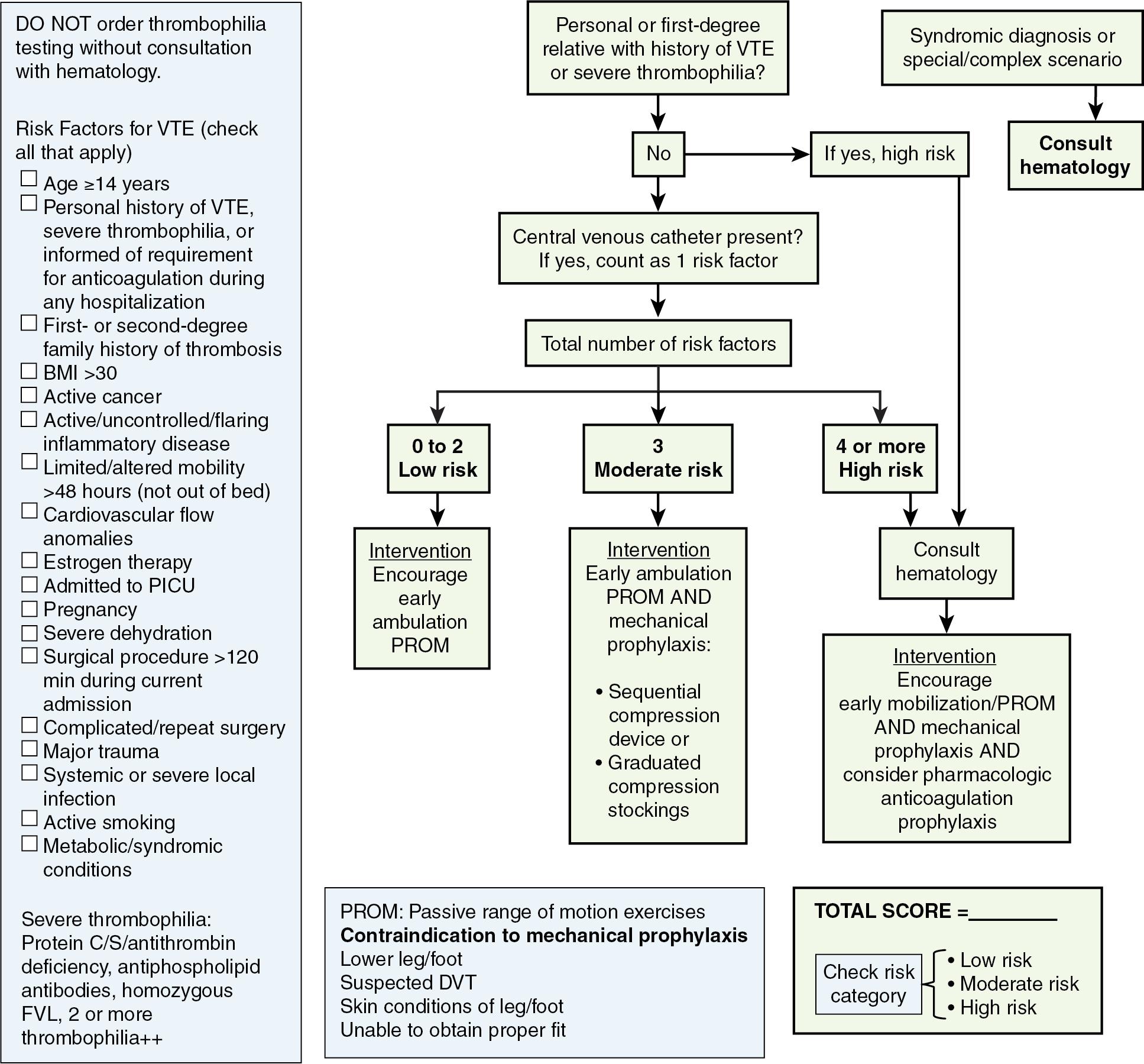
Although not as common as in adult orthopedic surgery, deep vein thrombosis (DVT) does occur in the pediatric orthopedic patient, with an estimated incidence of 0.05% and with a case fatality rate of 5.4% ( ). Identified risk factors for DVT include postoperative electrolyte abnormalities; dehydration and hypernatremia; obesity; older age; trauma (particularly lower extremity trauma); and infection, particularly osteomyelitis ( ; ; ). Prevention has become a universal aspect of modern orthopedic postoperative care ( ). The pathophysiology and prophylactic strategies have not changed dramatically in years. In addition to mechanical prophylaxis and early ambulation, low molecular weight heparin (LMWH) has become the mainstay of pharmacologic prophylaxis ( ). The relatively low incidence of DVT must be balanced against the risk of bleeding from anticoagulation. Scoring systems that stratify risk take into account factors like age >14, presence of a central line, limited ambulation, and obesity. Fig. 36.1 is a sample algorithm ( ).
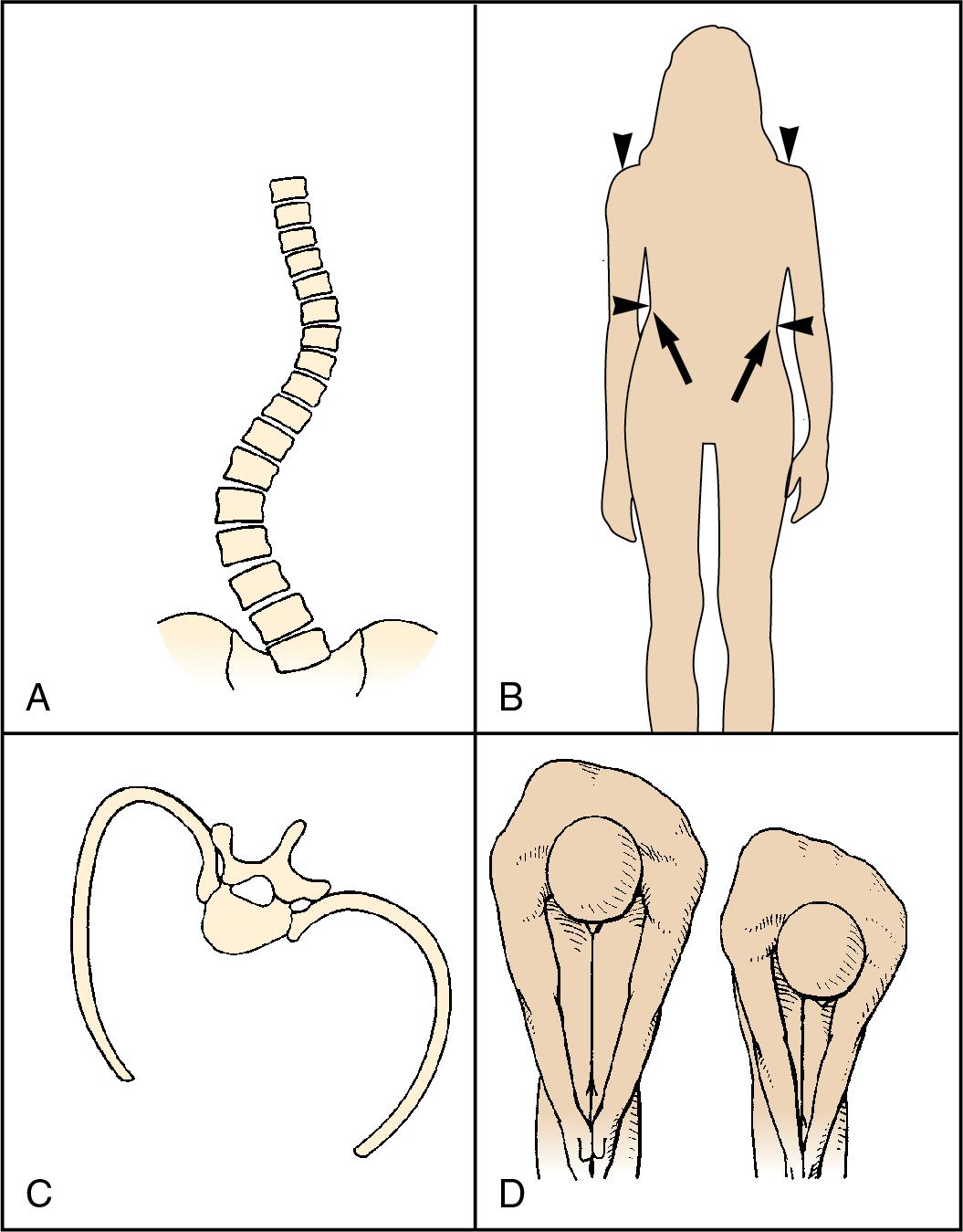
Scoliosis, derived from the Greek root meaning “crooked,” is a lateral and rotational deformity of the thoracolumbar spine. With progression of the lateral spinal curvature, the spinous processes rotate toward the concave side of the curve. The ribs on the convex side are pushed posteriorly by the rotating spine, forming the characteristic gibbous deformity. The ribs on the concave side become prominent anteriorly and are crowded together. Occasionally, scoliosis is associated with kyphosis (“humpback”) or lordosis (“bent backward”) ( Fig. 36.2 ).
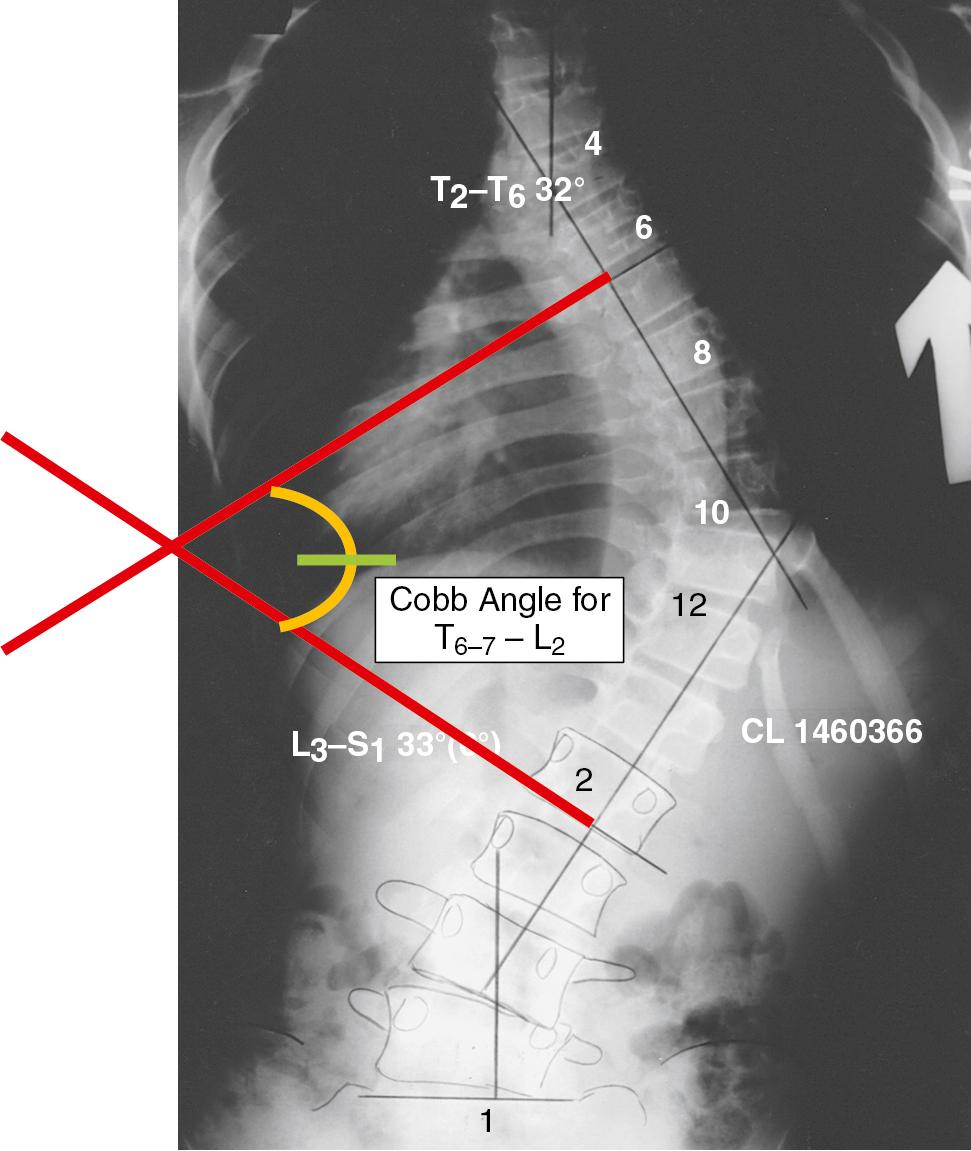
Historically, the progression of scoliosis and the severity of its systemic manifestations correlate with the angle of curvature measured by the Cobb method ( Table 36.3 )—that is, the angle between the upper surface of the “top-end” vertebra and the lower surface of the “bottom-end” vertebra. The end vertebrae are those that are maximally tilted. Perpendicular lines are extended from these end vertebrae to the center of the curve. The angle formed by the intersecting perpendiculars determines the angle of curvature ( Fig. 36.3 ). The curve is defined as facing to the right or to the left, depending on the convexity of the curve. A lateral curve of >10 degrees is abnormal. Respiratory impairment rarely occurs with a curvature of <60 degrees. The Cobb angle only accounts for two dimensions; the Lenke classification, among others, accounts for the three-dimensional aspect of the scoliosis and is useful in predicting the risk of massive transfusion during corrective surgery ( ; ).
| Angle of Curvature | Significance |
|---|---|
| <10 | Normal |
| >25 | Echocardiographic evidence of increased pulmonary artery pressures |
| >40 | Surgical intervention |
| >65 | Restrictive lung disease |
| >100 | Symptomatic lung disease, dyspnea on exertion |
| >120 | Alveolar hypoventilation |
The overall prevalence of spinal deformities in the North American population is between 1% and 2%, and scoliosis represents approximately 30% of children undergoing orthopedic surgical procedures annually ( ; ). Curves can be described on the basis of their anatomic configurations, age of onset, and associated pathology. In the past, polio or tuberculosis infection was the most common cause. Today, most cases of scoliosis are classified as idiopathic because the basic pathophysiology remains unknown ( ). Pedigree analysis suggests that adolescent idiopathic scoliosis is polygenic and multifactorial with variable penetrance; at-risk females are three times more likely to have scoliosis than their male counterparts. Neuromuscular scoliosis, which includes spinal muscular atrophy (SMA), cerebral palsy (CP), Friedreich’s ataxia, syringomyelia, Duchenne muscular dystrophy (DMD), Rett syndrome, spinal cord injury, and myelomeningocele, is far less common but poses significantly greater challenges to the anesthesiologist ( ; ). The most common types of scoliosis are listed in Box 36.1 .
Congenital scoliosis
Vertebral anomalies
Rib anomalies
Spinal dysraphism
Idiopathic scoliosis
Infantile (<3 years of age)
Juvenile (3–10 years of age)
Adolescent (>10 years of age)
Scoliosis associated with neuromuscular disease
Cerebral palsy
Poliomyelitis
Myopathies
Muscular dystrophies
Syringomyelia
Friedreich ataxia
Traumatic scoliosis
Fractures
Irradiation
Burns
Surgery
Syndromes associated with scoliosis
Neurofibromatosis (von Recklinghausen disease)
Marfan syndrome
Osteogenesis imperfecta
Mucopolysaccharidosis
Rheumatoid arthritis
Neoplastic disease
Congenital scoliosis is a curvature of the spine that is the result of a rib or vertebral anomaly. Idiopathic scoliosis is the most common of the spinal deformities and has three periods of onset, all coincident with periods of rapid growth spurts: infantile (<3 years old), juvenile (3 to 10 years old), and adolescent (>10 years old). Progression of the deformity depends on the age of onset. Infantile idiopathic scoliosis is associated with an increased incidence of intellectual disability, inguinal hernias, congenital dislocation of the hip, and congenital heart disease. Juvenile idiopathic scoliosis can usually be managed conservatively ( ). Adolescent idiopathic scoliosis is the most common form of scoliosis and occurs most commonly in girls ( ). The curve may resolve, remain stable, or progress in severity. The most significant prognosticators of curve progression in girls are age at onset, premenarchal status, and bone age ( Table 36.4 ) ( ). Postulated mechanisms for the progression of adolescent idiopathic scoliosis include abnormal RNA expressions, upregulation of TGF-ß, melatonin deficiency, leptin-induced increased sympathetic nervous system activity, and increases in platelet-derived calmodulin. Despite strong familial patterns, no specific polymorphism or single gene has been exclusively related to scoliosis ( ). Children with congenital syndromes and early-onset scoliosis, defined as the diagnosis of spinal deformity before the age of 5 years, can be divided into idiopathic, neuromuscular/syndromic, and congenital etiologies. These include thoracic insufficiency syndromes, Marfan syndrome, and arthrogryposis, and all pose extreme challenges to the perioperative team ( ; ).
| Age | Menarchal Status | Bone Maturity |
|---|---|---|
| <11 years (88%) | Premenarche (53%) | Immature (68%) |
| >15 years (29%) | Postmenarchal (11%) | Mature (18%) |
The natural history of scoliosis varies according to the cause and the pattern of vertebral involvement. Uncorrected, scoliosis can result in curve progression, cosmetic deformity, back pain, and physiologic compromise ( ; ; ). In most cases of idiopathic scoliosis, the spinal curvature remains small and conservative nonoperative management is appropriate ( ; ; ). In 0.3% to 0.5% of cases, the curve increases, necessitating surgical intervention ( ). In patients with idiopathic scoliosis, a Cobb angle of >50 degrees at skeletal maturity is a predictor of decreased pulmonary function, and those with thoracic apices and curves of >100 degrees are at increased risk of death from cor pulmonale and right ventricular failure ( ; ). The timing of surgery for this condition is controversial. The worse the curve and the more compromised the cardiorespiratory function, the greater the risk of perioperative morbidity and mortality. The pulmonary hypertension of progressive uncorrected idiopathic scoliosis often results in life-compromising respiratory failure in the fourth or fifth decade ( ).
Even asymptomatic scoliotic patients have demonstrable abnormalities in pulmonary function. As the curvature progresses, vertebral rotation leads to thoracic cage asymmetry and deformation; lung volumes and pulmonary compliance are often but not always inversely related to the degree of this curve ( ). When the scoliotic curve is >60 degrees, oxygen saturations are lower at maximal exercise. When the scoliotic curve is >65 degrees, pulmonary function tests demonstrate the characteristic pattern of restrictive lung disease ( ). The first manifestation of this restrictive lung disease is a reduction in vital capacity; in many cases, the vital capacity (normal, 60 mL/kg) is severely reduced, often to <60% of predicted and is often predictive of a need for postoperative mechanical ventilation ( ). Of the subdivisions of vital capacity, inspiratory capacity is affected to a greater extent than is expiratory reserve volume. Functional residual capacity and residual volume are not as severely affected. These alterations in lung volumes are caused by the scoliotic changes in chest wall compliance and the resting position of the thoracic cage rather than to parenchymal changes. The thoracic deformity of early-onset scoliosis damages pulmonary vascular development and inhibits physiologic alveolar growth ( ). In addition to restrictive lung disease, idiopathic scoliosis can also produce obstructive symptoms. Specifically, bronchus intermedius obstruction can occur when the natural kyphoscolioric curve is lost in patients with dextroscoliosis ( ).
The pulmonary impairment resulting from the scoliosis of neuromuscular disease is exacerbated by coexisting abnormalities in central respiratory drive, swallowing function, and innervation of the upper airway and respiratory musculature. Pulmonary dysfunction in these patients is exacerbated by their increased frequency of respiratory infections, predilection to aspiration, and impaired ability to clear pulmonary secretions. Patients who have abnormal pulmonary function tests, particularly a forced vital capacity (FVC) of <30%, or those who have hypercapnia preoperatively are likely to require postoperative (or chronic) ventilation ( ). Children with neuromuscular scoliosis should be enrolled in a preoperative respiratory training program of noninvasive positive pressure ventilation and mechanical insufflation-exsufflation for 30 minutes per day for at least 1 to 4 weeks before surgery, and it should be continued in the postoperative period to minimize the duration of postoperative mechanical ventilation and pulmonary complications ( ).
Echocardiographic evidence for increased pulmonary artery pressures has been demonstrated in individuals with only modest degrees of scoliosis in the absence of abnormal pulmonary function. Patients with curves >70 degrees develop pulmonary hypertension on exercise; those with curves >110 degrees have pulmonary artery hypertension at rest. Kafer proposed that this increase in pulmonary vascular resistance is not just the result of lung compression from thoracic cage abnormalities but also from an increased incidence of hypoxic pulmonary vasoconstriction ( ). Impaired development of the pulmonary vascular bed results in a reduction in the number of functional vascular units per lung ( ). Chronic hypoxia induces pulmonary vascular remodeling, which contributes significantly to these patients’ pulmonary hypertension ( ).
Myopathies affect both cardiac as well as skeletal muscle. Any child with a myopathy or muscular dystrophy should have an electrocardiogram and an echocardiogram performed to assess the presence of cor pulmonale, ventricular wall motion, ejection fraction, and ventricular wall thickness ( ). Duchenne muscular dystrophy (DMD) is the most common muscular dystrophy occurring in children who present for surgery (see Chapter 51 : Genetic and Muscular Disorders) ( ; ; ). An X-linked recessive disorder, DMD is a progressive, debilitating disease that affects skeletal, cardiac, and smooth muscle. Typically, afflicted boys become wheelchair dependent by the age of 10 years, and in the past, death from respiratory or cardiac failure occurred in this population before the age of 20. Glucocorticoid therapy may prolong ambulation by 2 to 3 years, decrease the incidence and severity of scoliosis, and modulate the course of their pulmonary and cardiac decline in the second decade of life ( ; ). Recent data demonstrate that boys with DMD have impaired sympatholysis, which produces muscle ischemia, injury, and fatigue. Phosphodiesterase type 5 inhibitors alleviate this ischemia in an immediate dose-dependent manner ( ). With the continued introduction of these pharmacologic advances, in concert with noninvasive home ventilation, these men are living longer ( ; ; ; ; ). Scoliosis surgery for patients with debilitating diseases is often performed to help with daily care and improve the patient’s quality of life.
Numerous anesthetic challenges are presented by patients with DMD ( ; ). Clinically significant cardiomyopathies and rhythm disturbances manifest by 10 years of age (see Chapter 51 : Genetic and Muscular Disorders). Many of these children are obese because of muscle weakness, fatty degeneration of muscle fibers, and lack of exercise. Succinylcholine can cause a fatal hyperkalemia in these patients, who may present for surgery before the diagnosis has been definitively made; therefore the routine use of this muscle relaxant is no longer recommended in all children ( ; ; ). In addition, prolonged administration of inhaled anesthetic agents has been associated with rhabdomyolysis and hyperkalemia.
The most important aspects of the preoperative evaluation include determination of the location and degree of spinal curvature, the cause of the scoliosis, the patient’s history of exercise tolerance, respiratory symptoms, and the presence of coexisting diseases. A directed physical examination of the cardiorespiratory system should evaluate the presence of tachypnea; crackles; wheezing; and signs of right heart failure, such as hepatomegaly, jugular venous distention, and peripheral edema. Any preoperative neurologic deficits should be recorded. Based on the severity of the curve and the degree of respiratory impairment, the preoperative laboratory studies listed in Box 36.2 should be requested. Preoperative electrocardiogram should be performed in all patients and echocardiography should be performed in patients who have signs or symptoms of heart failure or pulmonary hypertension. This is particularly important in patients with severe neuromuscular scoliosis and patients with muscular dystrophy ( ; ; ; ). Historically, pulmonary function tests are useful in establishing the risk of pulmonary complications in the immediate postoperative period ( ; ). Peak inspiratory and expiratory forces with the airway occluded of at least –30 cm H 2 O and +40 cm H 2 O, respectively, are necessary for effective sighs and postoperative coughing and expulsion of secretions. Although some studies demonstrate an increased likelihood of postoperative intubation with an FVC <30% predicted, other studies suggest that postoperative intubation might be increased with an FVC of <30 mL/kg (or <50% of predicted) or a forced expiratory volume at 1 second (FEV 1 ) that is <50% predicted. However, , in a retrospective observational study noted only a small percentage of patients whose PFTs suggested a high risk (FVC <40% predicted or maximal inspiratory pressure <30 cm/H 2 O) for postoperative intubation actually needed to remain intubated postoperatively.
Chest radiograph
Electrocardiogram
Echocardiogram
Pulmonary function tests
Arterial blood gas
Spirometry
Forced vital capacity (FVC)
Forced expiratory volume at 1 second (FEV 1 , FEV 1 /FVC)
Peak expiratory flow rate (PEFR)
Peak inspiratory pressure (Pi max )
Peak expiratory pressure (Pe max )
Coagulation studies
Platelet count
Prothrombin time, partial thromboplastin time
Electrolyte panel
Liver function tests
The treatment of spinal curvature is dictated by the type of scoliosis and by the surgeon’s expertise and preferences ( ). For curves in the range of 20 to 40 degrees bracing can be an effective means of treating early scoliosis by preventing its progression. Risser casts have been used in patients with early onset scoliosis. Risser casts remain on the patient continuously for 2 to 3 months at a time.
Because most cases of congenital scoliosis will progress rapidly, potentially culminating in neurologic deficits or cor pulmonale, conservative management is rarely appropriate. The mainstay of therapy is posterior spinal fusion. Current experience suggests that instrumented and uninstrumented fusions present comparably low neurologic risk and similar reoperation rates. Instrumentation has a better initial correction, and present practice has shifted to posterior fusion with instrumentation for these children ( ; ). Continued improvements in spinal surgery technology have changed the treatment pathway for these young children with early-onset scoliosis. Implantable magnetically controlled growing instrumentation rods and vertical expandable prosthetic titanium prostheses (VEPTR) allow noninvasive lengthening without the need for recurring extensive surgical replacment of rods in the skeletally immature patients with rapidly progressive curves ( ; ). VEPTR devices work to straighten the spine and separate the ribs.
The goal of scoliosis surgery is to achieve a spinal fusion and stabilization of the curve. Since Harrington first established the standard treatment of posterior fusion with instrumentation in 1962, systems have been continuously modified to optimize correction and minimize postoperative neurologic complications and implant failures. The Harrington system used a stainless steel rod, connected to the inferior facets and pedicles, that applied distraction forces along the concave aspect of the curve adjusted using the ratchet principle ( ). The next generation of devices, the Luque and Cotrel Dubousset systems, are three-dimensional segmental spinal techniques that applied rod and rotational forces to affect a correction ( ). Segmental pedicle screws and hybrid systems using pedicle screws, hooks, and wires are the instrumental trend of today ( ). Initially used for lumbar curves, they are now used for total curve correction. The limitation of posterior spinal fusion with or without instrumentation is that the anterior growth plates, which play a major role in the development of the deformity, are not affected. Late torsional deformities can result.
Advances in imaging and intraoperative navigation have improved the safety of posterior spinal fusion and the ability of the surgery team to place pedicle screws with much higher accuracy. In the 1970s, with free-hand pedicle screw placement it was estimated that misplacement may have been as high as 40%; accuracy has improved more than two-fold when employing 2D fluoroscopy. Now with intraoperative 3D imaging and navigation systems becoming more prevalent in these surgeries, the operative workflow has become more streamlined and the accuracy of pedicle screw placement has improved dramatically ( ; ).
The anterior approach to spinal deformities has been advocated for several specific deformities, including severe kyphosis and lordotic paralytic curves in patients with cerebral palsy. Surgery consists of discectomies with or without instrumentation, performed alone or in combination with a posterior spinal fusion. Video-assisted thoracoscopic surgery has evolved to be the standard approach to the anterior spine in children with spinal deformities, avoiding thoracic muscle disruption and pulmonary complications. The surgical approach used to expose the anterior portion of the spine depends on the exact spinal deformity. Thoracic curves are usually approached through a left thoracotomy, and the procedure is facilitated by insertion of a double-lumen endotracheal tube and one-lung ventilation. Alternatively, single-lung ventilation in young children is performed by advancing a tracheal tube into the mainstem bronchus opposite the side of surgery or by positioning a bronchial blocker into the mainstem bronchus on the operative side. Multiple techniques for placing a variety of bronchial blockers outside the tracheal tube have been described for use in children (see Chapter 32 : Anesthesia Thoracic Surgery).
Dramatic hemodynamic instability, substantial blood and heat loss, potential for catastrophic spinal cord injury, blindness, hair loss, and postoperative infections are the major concerns with scoliosis surgery. In addition to the monitors routinely used in conducting a pediatric general anesthetic, large-bore IV access, intraarterial catheterization, and bladder catheterization are standard. In high-risk patients, such as patients with Cobb angles greater than 70 degrees or with a history of coagulopathies or neuromuscular scoliosis, a central venous catheter should be considered. These invasive catheters allow monitoring of beat-beat changes in blood pressure, adequacy of oxygenation, ventilation, organ perfusion, and access sites for intravascular volume and cardiotonic drug administration. Hypothermia is common, and continuous temperature monitoring along with thermoprotective strategies are an essential element of care.
The organization of the spinal cord blood supply is segmental in a cross-sectional and rostral-caudal fashion ( Fig. 36.4 ). The intrinsic spinal cord vasculature consists of the anterior median and the paired posterior spinal arteries. The vasculature supplying these vessels arises from the segmental arteries of the aorta and branches of the subclavian—the vertebral arteries—and the internal iliac arteries. The solitary anterior median spinal artery runs along the entire length of the cord in the anterior sulcus, giving off penetrating branches that supply the ventral two-thirds of the spinal cord. Blood flow in the anterior spinal artery is not continuous throughout its span; instead, the anterior spinal artery functions as an anastomotic channel between the terminal branches of successive radicular arteries. Blood from the terminal aspects of these radicular arteries courses upward and downward in the anterior spinal artery. At points between adjacent radicular arteries, blood flows in either direction. The paired posterior spinal arteries, which supply the dorsal third of the cord, also have discontinuous segments and appear more like a plexus of pial vessels than paired arteries.
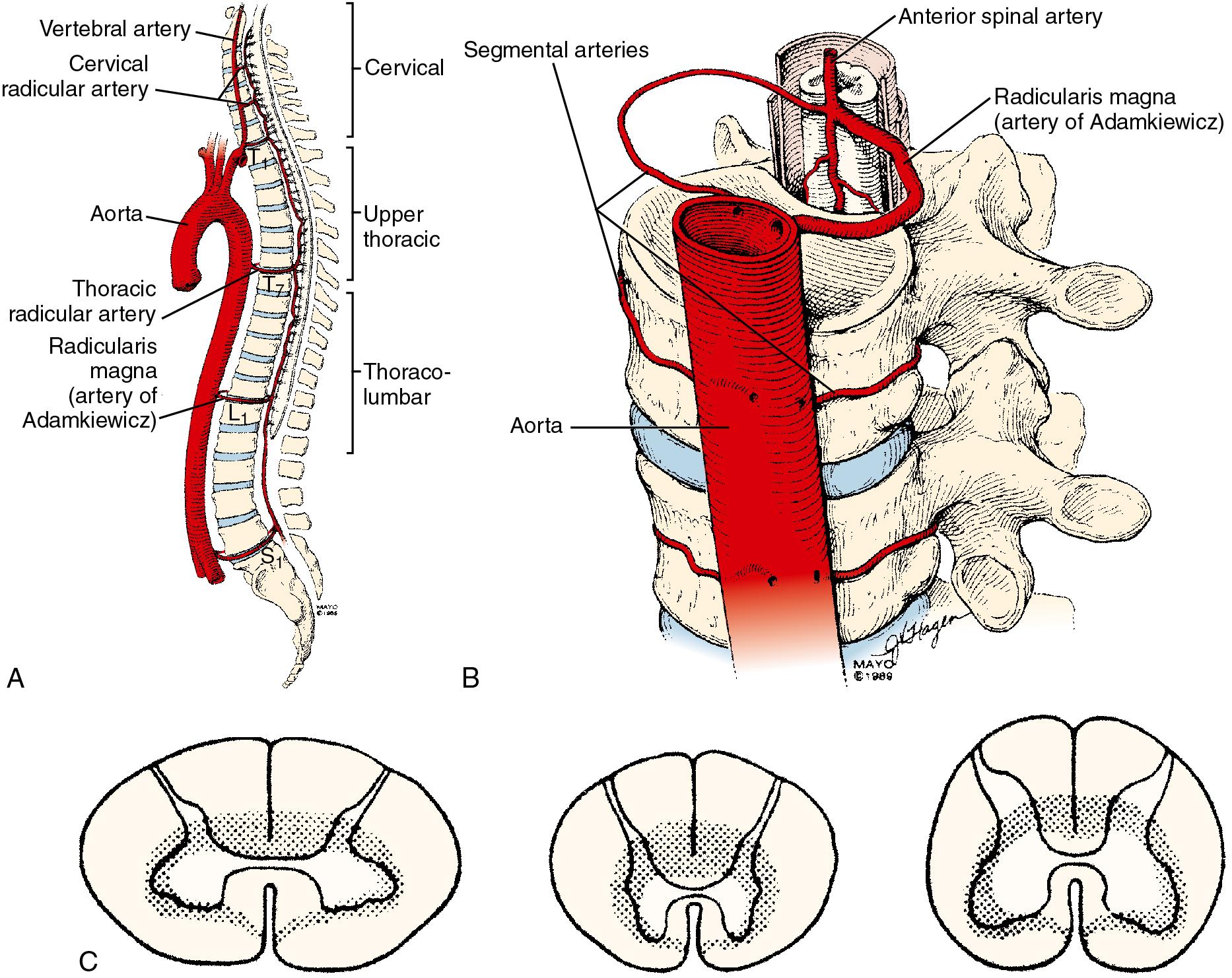
These three perimedullary vessels give rise to the intramedullary arterial system: the central arteries that supply the gray matter and the deep portions of the white matter and the radial arteries that supply most of the white matter. Nonfunctional anastomotic links exist between the central arterial supply and the radial arterial supply at a given spinal segment. This border zone and the radial circulation appear at highest risk for ischemic injury. The regional circulation of the spinal cord is divided into four segments. The cervical and lumbosacral regions each receive double the blood flow from the thoracic region ( Fig. 36.4 ). Although each vertebral level has paired segmental arteries, only six to eight important medullary arteries are formed. These medullary arteries join the spinal arteries. The segmental arteries at all other levels are functionally nonsuppliers of blood to the spinal cord itself. The vertebral arteries form the rostral origins of the anterior and posterior spinal arteries and represent the principal supply to the cervical cord. Branches of the thyrocervical and costovertebral arteries supply the lower cervical and upper thoracic cord. A radicular artery arising from T 7 provides perfusion for the middle thoracic cord. The most consistent and important of the anterior medullary arteries is the artery of Adamkiewicz—the arteria radicularis magna—which usually joins the anterior spinal artery between T 8 and L 3 . This artery is the predominant source of blood supply to the lower two-thirds of the spinal cord. The implications of this design dictate the clinical manifestations of impaired cord perfusion. Watershed areas, which are subject to ischemia during low-flow states, exist between the anterior and posterior circulations and between the four different spinal segments. The segments of T 4 –T 7 appear to be highly susceptible to injury during periods of hypoperfusion. The dependence of the lower two-thirds of the cord on the artery of Adamkiewicz puts this region at particular risk during surgical manipulation of the thoracolumbar aorta and spinal column, and this is referred to as the lumbar artery enlargement syndrome. Although the clinical picture of this syndrome is not constant, it is marked by the development of flaccid paraplegia or quadriplegia (depending on the level of the lesion) and dissociated sensory impairment in which heat and pain sensations are affected, whereas deep sensation is spared.
The same principles that regulate the cerebral blood flow apply to spinal cord blood flow. As such, cord blood flow depends on the perfusion pressure (i.e., mean arterial pressure [MAP] minus cerebrospinal fluid pressure), integrity of the circulation, microcirculatory autoregulation, and intrinsic regulation. If the perfusion pressure falls below 50 mm Hg, spinal cord blood flow may become compromised. Spinal cord blood flow autoregulates within the range of a MAP of 45 to 180 mm Hg. Spinal cord blood flow is also regulated on an intrinsic basis in response to arterial oxygen and carbon dioxide tensions, pH, and cord temperature in a fashion identical to that of the cerebral circulation. Hypercapnia increases flow, whereas a Pa o 2 below 60 mm Hg results in a vasodilatation that overrides the effects of hypocarbia and autoregulation ( ) (see Chapter 31 : Anesthesia for Neurosurgery).
Scoliosis surgery is high-risk surgery. Complications related to the surgery include surgical site infection, paralysis, and cardiovascular collapse from massive blood loss (and its therapy), air embolism, and anaphylaxis. Additional complications are related to the prone position, including air embolism, loss of the airway, postoperative blindness, and hair loss. Practiced crisis management is essential and the Society for Pediatric Anesthesia’s Pedi Crisis app can be an excellent resource ( ). Common intraoperative problems are listed in Table 36.5 .
| Problem | Monitoring Solution |
|---|---|
| Endotracheal tube malposition |
|
| Alteration in pulmonary compliance in the prone position |
|
| Alteration in cardiac function in the prone position |
|
| Hypotension |
|
| Coagulopathy |
|
| Electrolyte abnormalities (usually from blood transfusions) |
|
| Excessive heat loss |
|
| Neurologic injuries |
|
Hypotension and cardiovascular instability are a common perioperative occurrence. Physicians should always presume that hypotension is caused by hypovolemia until proven otherwise. Other causes are far less common and include an aphylaxis, anesthetic overdose, pneumothorax or hemothorax (particularly in a single-staged anterior posterior procedure), impaired venous return resulting from the prone position, neurovascular responses to surgical manipulation, and venous air embolism. Air embolism occurs during periods of relative hypovolemia when the pressure in the surgically exposed epidural veins that are above the level of the heart become subatmospheric. Venous air embolism should be considered in the setting of unexplained hypotension associated with a sudden decrease in end-tidal CO 2 levels. Transesophageal echocardiography can detect 0.02 mL/kg of air injected intravenously but is invasive and often requires extensive medical resources for continuous monitoring. Precordial Doppler is the most sensitive noninvasive modality, capable of detecting 0.05 mL/kg of intravascular air, well below the estimated lethal dose of intravascular air in the setting of a venous air embolism, which ranges from 0.4 mL/kg to 3 to 5 m/kg ( ).
Although case reports have indicated successful resuscitation in the prone position, a plan to turn the patient supine must be well established and rehearsed, beginning with the close proximity of a bed upon which the patient can be repositioned supine as quickly as possible ( ). As in any emergency, it is the anesthesiologist’s responsibility to declare the emergency and to call for help. Because the surgeon needs time to pack and cover the open wound with sterile towels and adhesive plastic, it is always better to begin the process early rather than waiting until the last possible moment (see Chapter 57 : Cardiopulmonary Resuscitation)
Postoperative paralysis or sensory loss is the most feared, devastating, and often unpredictable complication of scoliosis surgery ( ; ; ; ). Neurologic injury may result from direct injury to the spinal cord or nerves during instrumentation, from excessive traction during distraction, or from compromised perfusion of the spinal cord. Because the consequences of a motor deficit are significantly greater than those associated with a sensory deficit, surgically induced paraplegia has always been the major concern of scoliosis surgery.
Historically, the estimated risk of postoperative neurologic injury in patients undergoing spinal instrumentation is 0.4% to 1.4% ( ; ; ). Children with congenital scoliosis suffer neurologic complications disproportionately. To minimize the risk of these devastating neurologic injuries, a variety of intraoperative neurologic monitoring modalities have been used ( ; ; ; ; ). The goal of this monitoring is to identify and herald the onset of neurologic impairment and to provide the surgeon and anesthesiologist with the opportunity to implement appropriate interventions that may minimize permanent damage. These approaches include wake-up tests and neurophysiologic monitoring.
Vauzelle and colleagues first described the use of the wake-up test to assess the integrity of the spinal cord ( ). In this technique, patients are awakened intraoperatively to assess spinal cord motor function. The wake-up test requires an anesthetic that allows rapid recovery of consciousness and motor function. Ideally, the wake-up test should be rehearsed preoperatively. During rehearsal, the patient is informed that he or she will be momentarily awakened at the time of rod insertion to test the function of the spinal cord. Patients must be reassured that they will neither remember the event nor experience pain while they are “awake.” Preoperative preparation increases the speed and success of the test. The effectiveness of neurophysiologic monitoring during spinal deformity surgery is now so well established that the wake-up test is rarely used except in situations to confirm monitoring changes ( ).
When a wake-up test is performed, the OR must be quiet, the surgeon must stop operating, and an observer is positioned (usually under the drapes) to look for foot movement. After discontinuation of the anesthetic, the patient is first asked to move his/her hands (“squeeze my fingers”) to evaluate the level of consciousness and is then asked to move his/her feet (“wiggle your toes”). If the patient is unable to move his/her feet but can move his/her hands, spinal cord compromise is presumed and the spinal rod instrumentation is removed immediately. Spinal cord perfusion is maximized by raising the MAP, increasing the hemoglobin concentration, and normalizing arterial carbon dioxide and oxygen tensions. In one series of 166 patients in whom the wake-up test was used, three patients had demonstrable neurologic deficits when awakened ( ). These deficits disappeared immediately on release of the distracting force (i.e., rods) ( ).
Hazards associated with the wake-up test include dislodgment of spinal instrumentation rods and vascular catheters, accidental extubation, air embolization produced by deep inspirations, falls from the OR table, and patient recall with subsequent psychological trauma. This test has many other limitations: it tests only the anterior spinal cord (motor function) and not the dorsal column (sensory); it requires patient cooperation and has limited use in patients with baseline cognitive dysfunction; it provides a snapshot of a single moment of spinal cord function and can realistically be performed only once or twice during a procedure; and it can miss a spinal injury if the injury occurs after the wake-up test is performed. Also, depending on the anesthesiologist’s skill, the anesthetic technique employed, and the pharmacokinetic variability of the anesthetic agents, it may take 5 to 45 minutes after a wake-up test is requested before the patient’s wake-up status can be achieved.
Electrophysiologic (neurometric) monitoring provides real-time, continuous assessment of spinal cord function and does not require patient movement, arousal, or cooperation (see Chapter 18 : Monitoring) ( ; ). In order for neurometric monitoring to be effective, precise communication and coordination of efforts among the surgeon, anesthesiologist, and neurometric specialist are imperative when a change in neurometric potentials is observed. Normalization of the potentials may occur spontaneously with relaxation of the distraction instrumentation or by improving spinal cord perfusion (e.g., increasing blood pressure and Pa co 2 levels and/or hemoglobin levels).
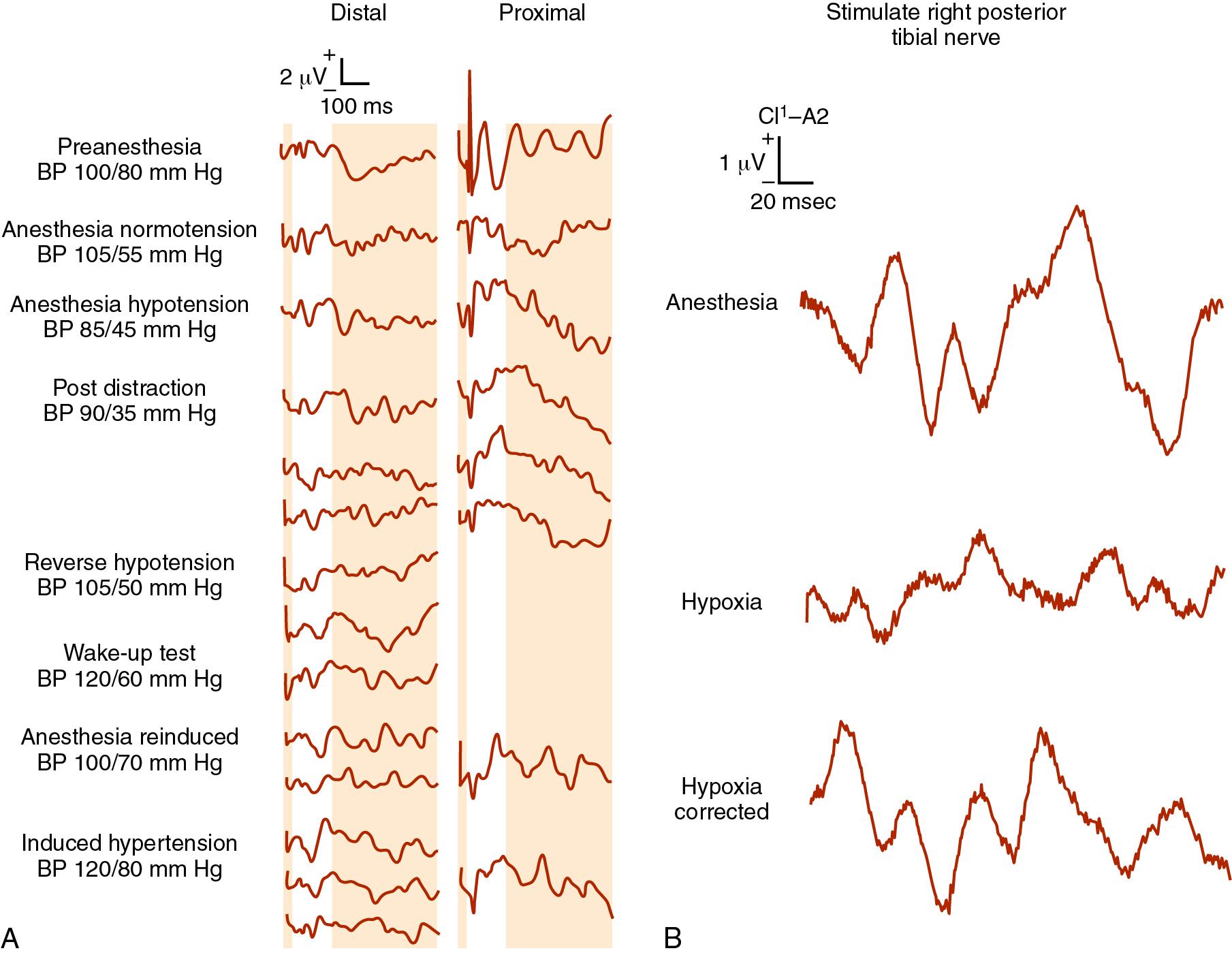
Somatosensory-evoked potentials (SSEPs) monitor the cortical and subcortical responses to peripheral nerve stimulation ( ; ; ; ). Typically, a peripheral mixed nerve (i.e., posterior tibial nerve, peroneal nerve, or median nerve) is stimulated at fixed intervals during a procedure. SSEPs are recorded repeatedly during surgery, and their amplitude (height) and latency (time of occurrence) are compared with baseline values ( ). Based on changes in these characteristics, it is possible to determine the functional status of the spinal cord, including the dorsal root ganglia and dorsal columns. A reduction in amplitude or an increase in latency may indicate neurologic dysfunction with a reduction in amplitude of >50% or an increase in latency of >10% relative to baseline values is generally considered significant ( ; ; ).
SSEPs monitor only the dorsal columns of the spinal cord and provide no direct evidence of motor function loss or anterior spinal cord injury. Motor deficits may occur in the absence of alterations in SSEPs, and numerous case reports have recorded the postoperative finding of paralysis despite unchanged intraoperative SSEPs (i.e., false-negative results). In the setting of spinal cord ischemia, the time to loss of SSEPs was almost three times longer than the time to motor-evoked potential loss ( ; ; ). The most comprehensive information regarding the false-negative rate of SSEPs comes from a survey of spine surgeons by the Scoliosis Research Society and the European Spinal Deformity Society, in which definite neurologic deficits despite stable SSEPs occurred during surgery in 0.063% of patients ( ). Children with neuromuscular scoliosis frequently have unreliable SSEP data. Despite these limitations, SSEP monitoring reduces postoperative paraplegia by more than 50% ( ; ). When SSEP monitoring is equivocal, we recommend an intraoperative wake-up test to assess motor function.
Many pharmacologic and physiologic variables affect the latency and amplitude of SSEPs and have been estimated to account for up to 44% of intraoperative SSEP changes ( ; ; ; ). The most important of these are the anesthetic agents, blood pressure, and body temperature, and these variables are summarized in Table 36.6 ( ; ; ; ). All of the potent inhaled anesthetic agents produce dose-dependent increases in latency and decreases in amplitude. These effects are less for sevoflurane and desflurane, permitting doses of 1.5 MAC (minimum alveolar concentration) with minimal SSEP changes. Nitrous oxide combined with volatile anesthetics results in a synergistic effect on cortical SSEPs ( ). Alone, nitrous oxide has no effect on SSEP latency but does decrease its amplitude by 50% ( ; ). Substantial recovery of latency and amplitude is achievable with discontinuance of nitrous oxide and the inhaled vapors. In general, the IV agents affect SSEP less than inhaled agents do; at high doses, they produce slight-to-moderate decreases in amplitude and increases in latency ( ). Barbiturates including thiopental at even high doses have no effect on amplitude or latency ( ). Benzodiazepines at clinical doses have no effect on latency ( ). On the other hand, ketamine and etomidate augment SSEP amplitude ( ; ). Propofol has minimal effect on amplitude or latency ( ; ). Opioids given in either analgesic or anesthetic doses produce minimal SSEP effects ( ). Although dexmedetomidine has been shown in some studies to have virtually no effect on SSEPs or MEPs ( ; ), there is conflicting data. Some studies demonstrate that patients receiving dexmedetomidine required significantly higher intensity and repetition rate of transcranial electric stimulation to evoke adequate transcranial MEPs (tcMEPs). Likewise, bolus administration of clonidine (1 to 2 mcg/kg) reduced tcMEP amplitude in upper and lower extremities in children under total intravenous anesthesia ( ; ). Based on previous studies that targeted blood concentrations, the effect of dexmedetomidine appears to have a clinically and statistically significant attenuation of amplitudes of transcranial electric motor-evoked potentials in a dose-dependent manner (intermediate and high plasma concentrations of 0.6 to 0.8 ng/mL plasma concentration) ( ).
| Agent | Amplitude | Latency |
|---|---|---|
| Desflurane | ↓ | ↑ |
| Isoflurane | ↓ | ↑ |
| Sevoflurane | ↓ | ↑ |
| Nitrous oxide | ↓ | ↔ |
| Barbiturates | ↓ | ↑ |
| Etomidate | ↑ | ↔ |
| Ketamine | ↑ | ↔ |
| Midazolam | ↓ | ↔ |
| Opioids | ↔ | ↔ |
| Propofol | ↔ | ↔ |
SSEP amplitude and latency are also affected by age, preexisting neurologic deficits, body temperature, Pa co 2 , hypoxia , hematocrit, and blood pressure ( Fig. 36.5 a and b) ( ). The reliability of spinal cord monitoring may be dramatically affected by the variability of the evoked responses. Muscle relaxants have no direct deleterious effects on the SSEP, and in fact may produce a more reliable recording by providing “quieter” conditions. An anesthetic milieu that is compatible with adequate neurometric monitoring and that allows rapid awakening can be created using a variety of approaches. Combining a half MAC of desflurane or sevoflurane with a remifentanil infusion produces ideal SSEPs and still allows for rapid wake-up if a wake-up test is required. Alternatively, the physician can substitute a continuous propofol infusion for the potent inhaled anesthetics in combination with an opioid. Because etomidate and ketamine augment SSEP amplitude, they may be useful in patients with abnormal preoperative SSEPs. However adding these drugs after SSEP signals change may produce false information and lead to catastrophe (see Box 36.3 ).
Monitors: standard anesthetic monitors
Arterial catheter, Foley catheter
Inhalational with sevoflurane and then IV access or intravenous induction 1–2 mg/kg of propofol
Two large-bore peripheral catheters
Rocuronium 0.5 mg/kg for intubation and maintenance
Dexamethasone 8 mg
Antimicrobial prophylaxis for surgical wound prophylaxis
Inhalation
O 2 /air/0.5–1.0 MAC inhaled anesthetic agent
Remifentanil: 0.2–0.3 mcg/kg/min
Sufentanil: 1.0 mcg/kg bolus, then 0.2 mcg/kg/hr
Fentanyl: 5–10 mcg/kg bolus, then 2 mcg/kg/hr
Dexmedetomidine 0.5–1.0 mcg/kg/hr
Propofol : 100–200 mcg/kg/min
Acetaminophen 15 mg/kg, max 1000 mg q6h
Tranexamic acid 10–30 mg/kg bolus, 5–10 mg/kg/hr
Ondansetron 0.1 mg/kg, max dose 4 mg
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here