Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital heart disease (CHD) is the most common congenital defect, affecting approximately 1% of live births ![]() ( - ,
( - , ![]() ) ( ; ). The population of children and adults with repaired, palliated, or unrepaired CHD continues to increase. With declining mortality from CHD, survival to adulthood (≥18 years) in well-developed medical systems is approximately 98%, 90%, and 56% for children with mild, moderate, and severe disease, respectively ( ; ; ). The prevalence and distribution of patients with CHD continues to evolve, with a rapidly growing number of adolescents and adults, especially with severe disease ( ). In Quebec, overall CHD prevalence from 2000 to 2010 increased 11% in children and 57% in adults, with the greatest increase (55%) seen in adults with severe CHD ( ). It is estimated that the number of patients with CHD in the US grew from ≈850,000 in 2000 to ≈2.4 million (1 million children) in 2010 ( ). Although synonymous with childhood, the majority of patients with CHD are still adults, and because children of parents with CHD have an increased incidence of CHD, the total incidence and prevalence are likely to increase generation by generation ( ).
) ( ; ). The population of children and adults with repaired, palliated, or unrepaired CHD continues to increase. With declining mortality from CHD, survival to adulthood (≥18 years) in well-developed medical systems is approximately 98%, 90%, and 56% for children with mild, moderate, and severe disease, respectively ( ; ; ). The prevalence and distribution of patients with CHD continues to evolve, with a rapidly growing number of adolescents and adults, especially with severe disease ( ). In Quebec, overall CHD prevalence from 2000 to 2010 increased 11% in children and 57% in adults, with the greatest increase (55%) seen in adults with severe CHD ( ). It is estimated that the number of patients with CHD in the US grew from ≈850,000 in 2000 to ≈2.4 million (1 million children) in 2010 ( ). Although synonymous with childhood, the majority of patients with CHD are still adults, and because children of parents with CHD have an increased incidence of CHD, the total incidence and prevalence are likely to increase generation by generation ( ).
The heterogeneous nature of the CHD population is made more so by the use of different treatment strategies for the same or similar lesions in conjunction with advances in pediatric cardiac surgery, interventional cardiac catheterization, and electrophysiologic techniques. For example, in dextro-transposition of the great arteries (D-TGA), the majority of adults have had the atrial switch operation (the Mustard or Senning procedure), whereas most children have had the arterial switch operation (the Jatene) ( ). These two operations for the same underlying condition carry vastly different intermediate and long-term outcomes ( ).
Shunts (both physiologic and anatomic), obstructive lesions, intercirculatory mixing, and single-ventricle physiology are hallmarks of CHD. Cardiac dysrhythmias are not usually a prominent presenting feature but become more common as patients age and the pathophysiologic sequelae of abnormal cardiac structure, function, and surgery accrue. Table 30.1 summarizes the pathophysiology and clinical picture associated with a wide variety of congenital heart defects.
| Lesion Type | Pathophysiology | Clinical Signs and Symptoms |
|---|---|---|
| Shunt Lesion Without Outflow Tract Obstruction | ||
|
|
|
| Shunt Lesions With Right Ventricular Outflow Tract Obstruction | ||
|
|
|
| Transposition Physiology (Intercirculatory Mixing) | ||
|
|
|
| Single-Ventricle Physiology | ||
|
|
|
| Left Ventricular Obstructive Lesions | ||
|
Left ventricular pressure overload from aortic lesions Increased left atrial pressure from left ventricular systolic and diastolic dysfunction OR obstruction to left atrial emptying |
|
| Mixing of Systemic and Pulmonary Venous Blood With Series Circulation | ||
|
|
|
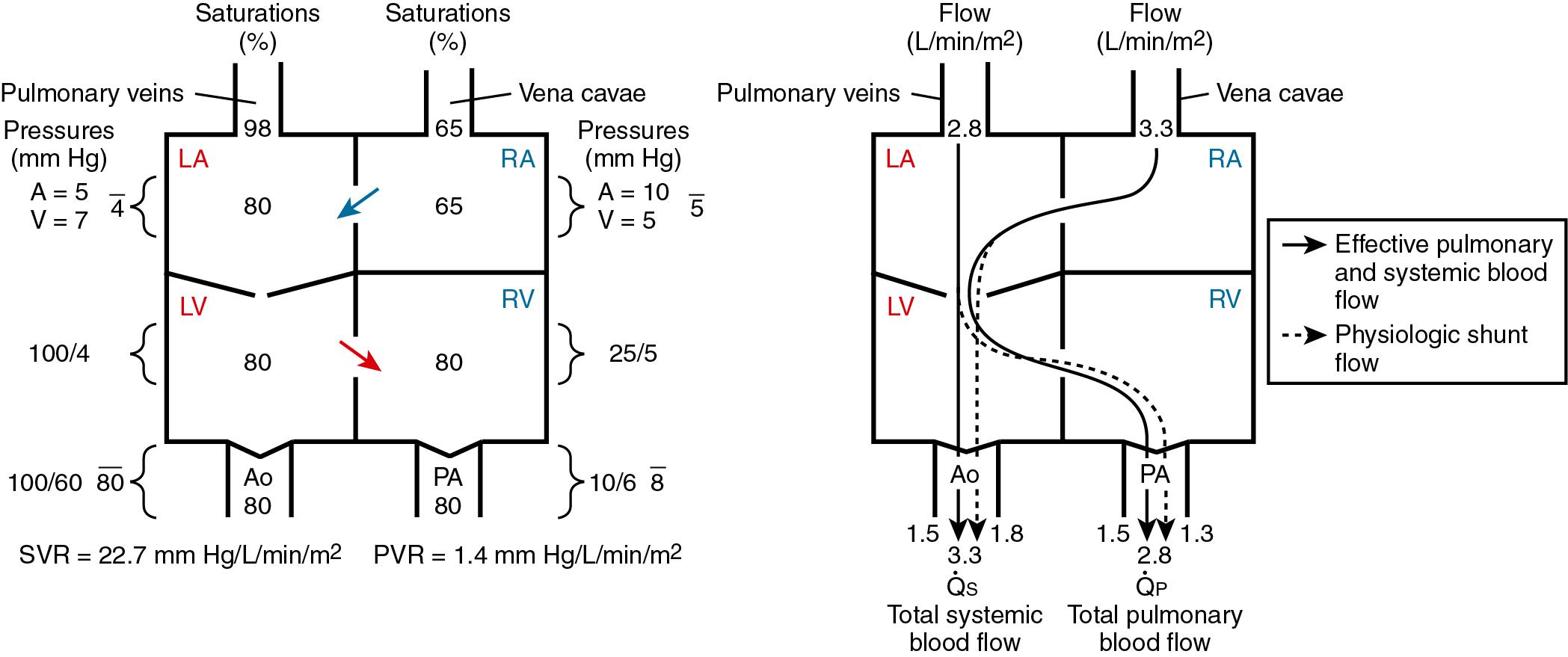
A shunt is an abnormal communication between the systemic and pulmonary circulations, allowing blood to flow directly from one circulatory system to the other. Shunting is the process whereby venous return into one circulatory system (systemic or pulmonary) is recirculated through the arterial outflow of the same circulatory system. Flow of blood from the systemic venous atrium or right atrium (RA) to the aorta produces recirculation of systemic venous blood. Flow of blood from the pulmonary venous atrium or left atrium (LA) to the pulmonary artery (PA) produces recirculation of pulmonary venous blood. Recirculation of blood produces a physiologic shunt. Recirculation of pulmonary venous blood produces a physiologic left to right (L-R) shunt, whereas recirculation of systemic venous blood produces a physiologic right to left (R-L) shunt.
Effective blood flow is the quantity of venous blood from one circulatory system reaching the arterial system of the other circulatory system. Effective pulmonary blood flow is the volume of systemic venous blood reaching the pulmonary circulation, whereas effective systemic blood flow is the volume of pulmonary venous blood reaching the systemic circulation. Effective pulmonary blood flow and effective systemic blood flow are the flows necessary to maintain life, and they are always equal, no matter how complex the lesions. Effective blood flow is usually the result of the normal pathway through the heart, but it may occur as the result of an anatomic R-L or L-R shunt, as in transposition physiology.
Total pulmonary blood flow (
p) is the sum of effective pulmonary blood flow and recirculated pulmonary blood flow. Total systemic blood flow (
s) is the sum of effective systemic blood flow and recirculated systemic blood flow. Total pulmonary blood flow and total systemic blood flow do not have to be equal. Therefore it is best to think of recirculated flow (physiologic shunt flow) as the extra, noneffective flow superimposed on the nutritive effective blood flow. These concepts are illustrated in Figs. 30.1 to 30.3 .
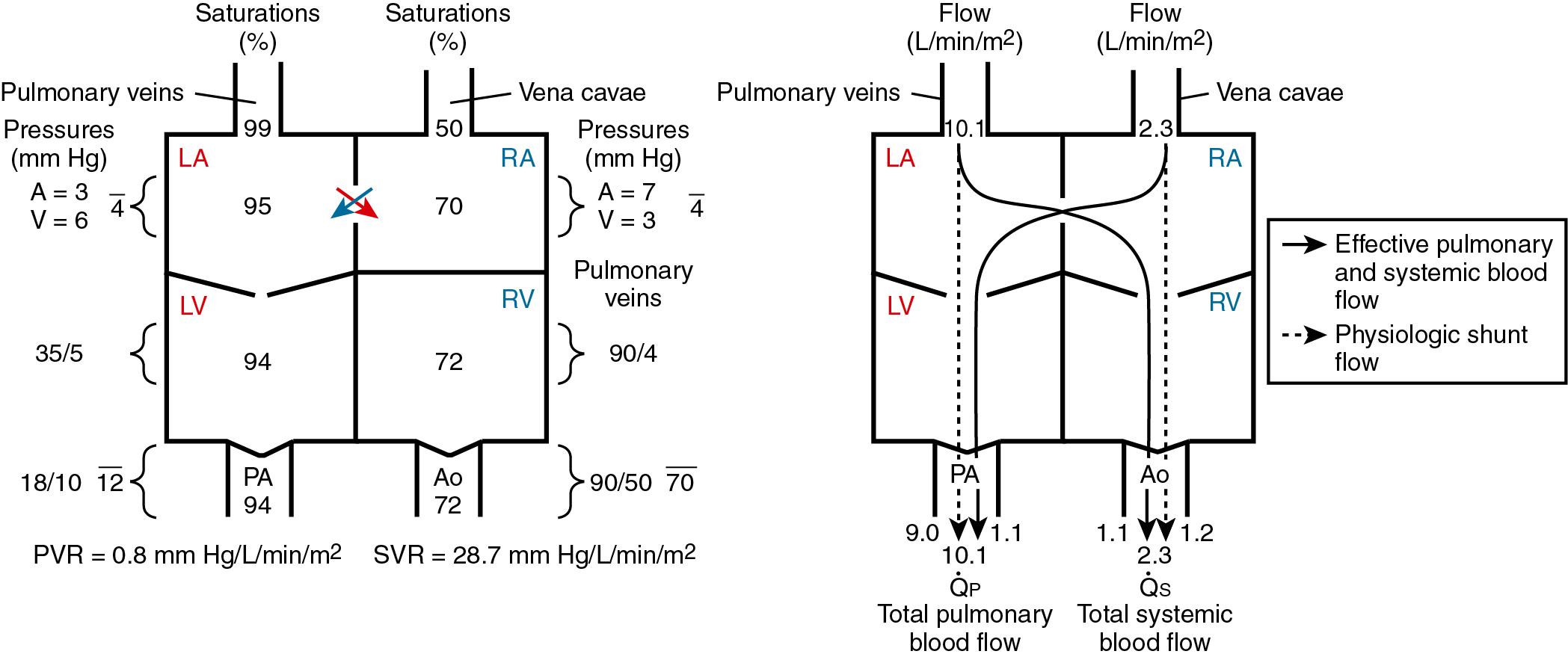
Clinically, shunting makes the circulation less efficient and places an increased demand on the ventricles. The severity of symptoms in most patients is determined by the degree (volume) of shunting ( ). Factors influencing the direction and degree of shunting include the size of the shunt orifice, the pressure gradient between the chambers or arteries involved in the shunt (sometimes counterintuitive; e.g., a large ventricular septal defect [VSD] will have a low gradient between the left ventricle [LV] and right ventricle [RV] but a high shunt flow), the relative compliance of the right and left ventricles, the ratio of pulmonary vascular resistance (PVR) to systemic vascular resistance (SVR), and the blood viscosity (hematocrit). L-R shunting results in increased pulmonary blood flow (PBF), increased pulmonary artery pressures (PAP), increased PVR, pulmonary edema, increased left atrial volume and/or pressure, and volume overload of both the right and left ventricles, leading to biventricular failure. Low aortic diastolic pressure accompanying large systemic–to–pulmonary artery shunts can lead to myocardial ischemia as well as organ hypoperfusion (e.g., bowel ischemia). Pulmonary overcirculation is associated with decreased lung compliance, increased airway resistance, and airway compression ( ; ; ). R-L shunting results in decreased oxygen content of the systemic arterial blood, with the decrease in proportion to the volume of deoxygenated systemic venous blood mixing with the oxygenated pulmonary venous blood. Even with normal cardiac output, the decrease in tissue oxygen delivery limits exercise tolerance ( ).
Anesthetic considerations: Important considerations are avoidance of air bubbles in intravenous (IV) catheters to prevent systemic embolization and attention to pulmonary vascular tone and its influence on the PVR-to-SVR ratio. Factors influencing PVR are discussed later in the Pulmonary Hypertension section. In patients with L-R shunts, the major perioperative concerns are threats to already limited systemic blood flow. Routine clinical monitoring tools are unlikely to indicate an evolving problem in this regard until severe hypotension and evidence of myocardial ischemia ensue; with L-R shunt physiology, it is important both to maintain myocardial pump function (i.e., avoid myocardial depression) and to try to limit decreases in PVR. Such decreases (e.g., those that can be produced by hyperventilation or hyperoxia) can lead to a steal phenomenon with increased PBF. The major acute consequence will be decreased
s with increased risk for significant systemic hypotension and hypoperfusion; in the longer term, the increased
p that results can increase pulmonary congestion, lung water, and cardiac volume overload.
In patients who have or who are at risk for significant R-L shunting or who are dependent on a narrowed systemic–to–pulmonary artery shunt for pulmonary blood flow (e.g., after stage I repair of hypoplastic left heart syndrome [HLHS] or as the initial palliation for reduced blood flow lesions such as tricuspid atresia), primary management considerations are similarly the maintenance of pump (cardiac) function and blood pressure plus the avoidance of factors that further increase PVR or decrease SVR (or both). It is important to note that the resultant arterial oxygen saturation (Sao 2 ) in such patients will be affected by both the magnitude of the R-L shunting and the saturation of the shunted (essentially systemic venous return) blood.
Congenital narrowing of the ventricular outflow tracts, semilunar valves, and great arteries may occur as isolated lesions or part of more complex malformations ( ). Ventricular outflow obstruction may be subvalvar, valvar, supravalvar, or a combination thereof, and fixed or dynamic. Residual or recurrent obstruction can occur following repair. Outflow obstruction produces increased afterload on the ventricle (pressure overload), leading to ventricular hypertrophy, a less compliant or “stiff” ventricle (diastolic dysfunction), higher filling pressures, and ultimately systolic and diastolic ventricular dysfunction ( ; ).
Left ventricular outflow obstruction may occur with aortic stenosis, coarctation of the aorta (CoA), interrupted aortic arch (IAA), and variants of HLHS and Shone’s anomaly ( Table 30.1 ) ( ). Right ventricular outflow obstruction is seen with pulmonary stenosis (PS), tetralogy of Fallot (TOF), hypoplastic pulmonary arteries, some forms of double-outlet right ventricle (DORV), and pulmonary hypertension. Right ventricle–to–pulmonary artery (RV-PA) conduits are used in the repair of pulmonary atresia, truncus arteriosus, TGA with left ventricular outflow obstruction (Rastelli procedure), and some DORV defects. Conduits calcify and narrow, and together with the increasing stroke volume that occurs with growth, significant obstruction can develop. The septal shift associated with severe RV pressure overload can compromise LV function via a reduction in LV filling and LV outflow obstruction.
Congenital mitral stenosis (MS) is rare, and in many parts of the world mitral stenosis is the result of rheumatic heart disease ( ). The degree of obstruction depends on the valve area, heart rate, and cardiac output. Pulmonary edema develops when high LA pressure leads to pulmonary venous pressures above 30 to 40 mm Hg.
Anesthetic considerations: Pressure-overloaded ventricles are at significant risk for myocardial ischemia during anesthesia, particularly in association with systemic hypotension and tachycardia. Together with hypertrophy-induced increases in myocardial O 2 consumption and elevated end-diastolic pressure (EDP), subendocardial ischemia can occur with systemic or suprasystemic RV pressures because systolic coronary blood flow may be markedly diminished or absent. The overall goal of anesthetic induction and maintenance is to optimize and maintain the major determinants of ventricular function, coronary blood flow, and cardiac output in the face of outflow obstruction and often some degree of both diastolic and systolic dysfunction. Hemodynamic goals are sinus rhythm, a normal to slower heart rate, and normal to increased preload to maintain cardiac output (CO). Adequate preoperative hydration in accordance with fasting guidelines or a bolus of intravenous fluid prior to or during induction is recommended. Volume administration in the presence of a stiff LV or MS must be done judiciously because of the potential to cause an excessive increase in left atrial pressure with consequent pulmonary edema. With severe ventricular dysfunction, an intravenous induction with agents that maintain contractility and systemic blood pressure without significant alterations in heart rate is usually indicated. Although the majority of patients can tolerate at least modest concentrations of inhalational agent, for the patient with significant ventricular dysfunction a balanced technique employing a potent opioid such as fentanyl in combination with a lower concentration of inhaled agent may offer greater hemodynamic stability. Relief of severe outflow tract obstruction, which can frequently be performed in the cardiac catheterization laboratory, should be considered and discussed with the patient’s cardiologist prior to elective surgery.
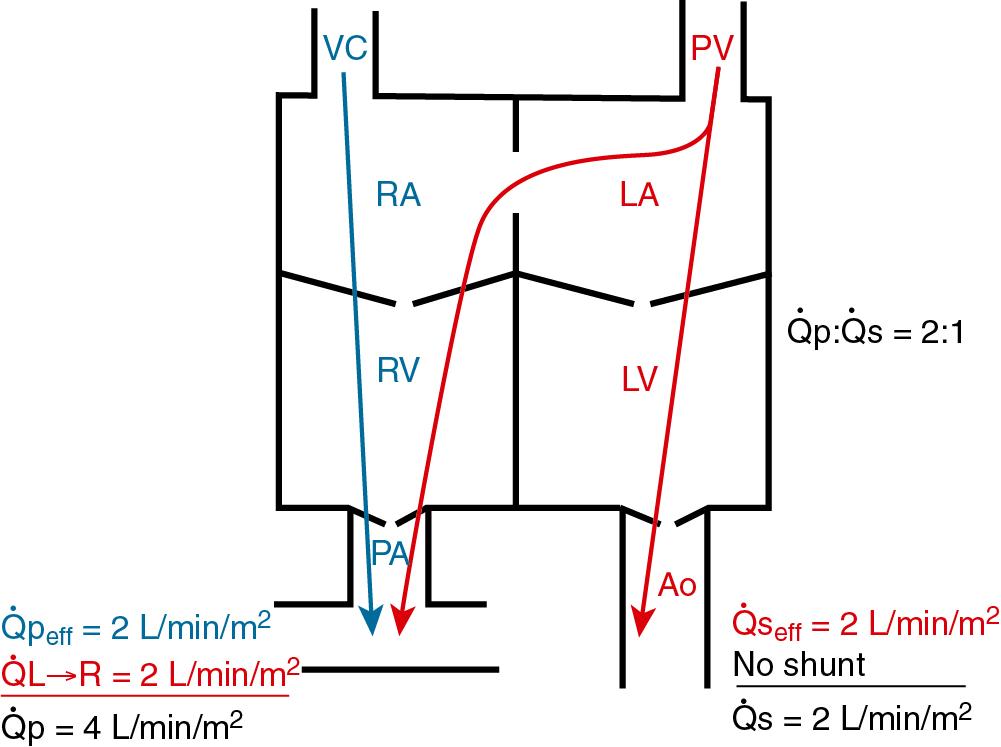
Intercirculatory mixing is the unique situation that exists in transposition of the great arteries. In TGA, there are two parallel circulations because of the existence of atrioventricular concordance (right atrium to right ventricle, and left atrium to left ventricle) and ventriculoarterial discordance right ventricle to aorta, and left ventricle to pulmonary artery. This produces a parallel rather than the normal series circulation. In this arrangement, parallel recirculation of pulmonary venous blood in the pulmonary circuit and systemic venous blood in the systemic circuit occurs. Therefore the physiologic shunt or the percentage of venous blood from one system that recirculates in the arterial outflow of the same system is 100% for both circuits. This lesion is incompatible with life unless there are one or more communications (atrial septal defect [ASD], patent foramen ovale [PFO], VSD, patent ductus arteriosus [PDA]) between the parallel circuits to allow intercirculatory mixing. In the presence of mixing, arterial oxygen saturation (Sao 2 ) is determined by the relative volumes and saturations of the recirculated systemic and effective systemic venous blood flows reaching the aorta (see Fig. 30.2 ).
Single-ventricle (SV) physiology describes the situation in which there is complete mixing of pulmonary venous and systemic venous blood at the atrial and/or ventricular level, and the ventricle (or one normal and one hypoplastic ventricle) then distributes output to both the systemic and pulmonary circulations. As a result of this physiology, (1) ventricular output is the sum of
p and
s, (2) distribution of systemic and pulmonary blood flow is dependent on the relative resistances to flow (PVR and SVR) into the two parallel circuits, and (3) oxygen saturations are the same in the aorta and the pulmonary artery. In patients with SV physiology, the Sao 2 is determined by the relative volumes and saturations of pulmonary venous and systemic venous blood flows that have mixed and reach the aorta. Complete obstruction necessitates the presence of two shunt pathways.
In the case of a single anatomic ventricle, there is always obstruction to either pulmonary or systemic blood flow as the result of complete or near-complete obstruction to inflow or outflow (or both) from the hypoplastic ventricle. In this circumstance there must be a source of both systemic and pulmonary blood flow to ensure postnatal survival. In some instances of a single anatomic ventricle, a direct connection between the aorta and the pulmonary artery via a PDA is the sole source of systemic blood flow (e.g., HLHS) or of pulmonary blood flow (e.g., pulmonary atresia with intact ventricular septum [PA/IVS]). This is known as a ductal dependent circulation. In other instances of a single anatomic ventricle, intracardiac pathways provide both systemic and pulmonary blood flow without survival dependent on a PDA. This is the case when tricuspid atresia occurs along with normally related great vessels, a nonrestrictive VSD, and minimal or absent pulmonary stenosis. Patients with SV physiology will ultimately undergo the staged surgeries that comprise the single-ventricle pathway and result in Fontan physiology (described later).
Single-ventricle physiology can also exist in the presence of two well-formed anatomic ventricles: (1) TOF with pulmonary atresia (in which pulmonary blood flow is supplied via a PDA or multiple aortopulmonary collateral arteries), (2) unrepaired truncus arteriosus, and (3) severe neonatal aortic stenosis and interrupted aortic arch (in which a substantial portion of systemic blood flow is supplied via a PDA). Table 30.2 lists a number of single-ventricle physiology lesions. Patients with two well-formed ventricles are usually able to undergo a two-ventricle repair. In some cases, the two-ventricle repair will be complete. In others, significant residual lesions (VSD, aortopulmonary collaterals) will remain.
| Aortic Blood Flow From | Pulmonary Artery Blood Flow From | |
|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fontan physiology is a series circulation in which one ventricle has sufficient systolic, diastolic, and atrioventricular valve function to support systemic circulation. This ventricle must in turn be in unobstructed continuity with the aorta and pulmonary venous blood return; there must also be unobstructed delivery of systemic venous blood to the pulmonary circulation (total cavopulmonary continuity). The physiology of the Fontan circulation is described in detail in the section Single-Ventricle Lesions and Hypoplastic Left Heart Syndrome. See Figs. 30.4 and 30.5 .
The factors that determine the natural history and pathophysiologic consequences of congenital cardiovascular malformations also influence perioperative risk. Although the majority of anesthesiologists are not familiar with the natural history of each and every lesion prior to and following surgical and/or catheterization intervention, it is possible to develop a rational approach to the anesthetic management of this group of patients by focusing on the factors listed in Box 30.1 . Identification of patients at increased risk and development of an appropriate strategy to prevent adverse events are the cornerstones of anesthetic management. As cardiac arrest is more frequent in children with heart disease, it is essential to understand the unique anatomy and physiology in order to provide effective cardiopulmonary resuscitation (CPR). The American Heart Association (AHA) has issued a scientific statement discussing high-risk cardiac lesions that impact prearrest stabilization and resuscitation ( ).
Anatomic vs. physiologic repair
Ventricular dysfunction
Rhythm and conduction abnormalities
Ventricular outflow obstruction
Hypoxemia and cyanosis
Pulmonary hypertension
Myocardial ischemia
Infective endocarditis
End organ dysfunction and injury
Extracardiac anomalies
Heart transplant recipients
Although it is common to differentiate between corrective and palliative surgery, total correction or cure is not the rule for the vast majority of children with CHD ( ). Cure or definitive repair, in the strictest sense, means that normal cardiovascular structure and function are achieved and maintained; life expectancy is normal; further medical, surgical, and catheter-based treatments for the CHD are unnecessary; and noncardiovascular (e.g., renal, neurologic) consequences are absent. With cure, there are therefore no cardiac or vascular residua (abnormalities that were part of the original defect and are still present after repair), sequelae (disorders intentionally incurred at the time of reparative surgery and deemed unavoidable), or complications (unintentional aftermath) after surgery ( ). Lesions that lend themselves to cure are uncomplicated closure at an early age of an uncomplicated, nonpulmonary hypertensive PDA, ASD, VSD, and in some instances, catheter ablation of tachyarrhythmias ( ; ).
Palliative repair implies that future procedures are anticipated or necessary to maintain or restore the patient to a state of normal (or at least compensated) physiology and to improve life span. Even if a complete repair is performed for many lesions, virtually all forms of CHD require long-term surveillance. Complete repair is not synonymous with cure. Many lesions carry substantial risk for residual and potentially progressive structural, contractile, hemodynamic, electrophysiologic, and end organ abnormalities. Table 30.3 summarizes the surgical repair options for each type of lesion.
| Lesion Type | Repair |
|---|---|
| Shunt Lesions | |
| Left-to-Right | |
|
|
|
|
|
|
|
|
|
|
| Right-to-Left | |
|
|
|
|
|
|
|
|
| Transposition Physiology | |
|
|
| Single-Ventricle Physiology | |
| One-Ventricle Lesions | |
|
|
|
|
|
|
| Two-Ventricle Lesions | |
|
|
|
|
|
|
| Left Ventricular Obstructive Lesions | |
| Mitral Stenosis | |
|
|
|
|
| Aortic Stenosis | |
|
|
| Subvalvar (subaortic membrane) | |
|
|
| Coarctation of Aorta | |
|
|
| Mixing of Systemic and Pulmonary Venous Blood With Series Circulation | |
|
|
|
|
In an anatomic repair, there is both atrioventricular and ventriculoarterial concordance (i.e., normal 4-chamber anatomy), the circulation is in series, and cyanosis is corrected. An anatomic repair may be categorized as either a simple or complex reconstruction. With a simple anatomic repair, the heart is structurally normal, and correction for the most part is “curative” without long-term sequelae. Repair of uncomplicated, nonpulmonary hypertensive PDA, ASD, or VSD, as well as vascular rings or successful balloon dilation of pulmonary valve stenosis, would fall into this category. In a complex anatomic repair, anatomic correction is achieved, but because of the complex nature of the surgical repair there may be significant long-term sequelae. Complex repairs comprise extensive ventricular outflow tract reconstruction (right and/or left), atrioventricular (AV) valve repair, and placement of conduits or baffles. Lesions necessitating a complex anatomic repair include D-TGA, TOF with or without pulmonary atresia, aortic stenosis (AS), some PS, atrioventricular canal (AVC) defects, MS, truncus arteriosus, coarctation of the aorta, interrupted aortic arch, and Ebstein anomaly.
In a physiologic repair, the circulation is in series and the cyanosis is relieved, but the heart is either univentricular (single ventricle) or biventricular, with the morphologic RV connected to the aorta and functioning as the systemic ventricle and the morphologic LV connected to the PA and functioning as the pulmonary ventricle. Single-ventricle repairs result in connection of the systemic venous return directly to the pulmonary artery, thereby excluding the pulmonary (pumping) ventricle. By relieving hypoxemia, single-ventricle repairs are “functionally corrective.” A physiologic biventricular repair is seen with the atrial switch operation (Mustard or Senning) for TGA, effectively resulting in a switch at the atrial level and the RV functioning as the systemic ventricle. Physiologic repairs are characterized over time by progressive dysfunction of the single or systemic right ventricle, progressive insufficiency of the systemic atrioventricular valve, dysrhythmias and conduction defects, and baffle leaks or obstruction.
Anesthetic considerations: Patients with simple anatomic repairs who are asymptomatic and have normal to near-normal hemodynamics and exercise tolerance can generally be anesthetized in the same manner as patients with a structurally normal heart. Patients with complex anatomic repairs are at increased perioperative risk, and although parents and older patients may report few symptoms or limitations to activities of daily living, significant limitations (e.g., reduced exercise or aerobic capacity, decreased heart rate and blood pressure response to exercise, ventilation/perfusion [V/Q] mismatch) may be evident on objective testing. Physiologic repairs are always palliative, and because of potentially significant pathophysiology, the anesthetic management is complex and can be associated with considerable intraoperative difficulties if the underlying physiology is not well understood ( ; ; ).
Congenital heart disease with progressive ventricular dysfunction is the commonest cause of heart failure in children and the leading cause of death in adults ( ; ). Beyond infancy, children in general have greater cardiac reserve than adults and can compensate for a longer period of time. However, the transition to acute heart failure can be rapid ( ). Risk factors include type of malformation, advancing age, older age at surgery, and frequency of reoperation. Patients with single ventricles, systemic right ventricles, and TOF are particularly at risk ( ; ; ). The etiology is multifactorial and includes genomic variation and modification, epigenetic modification, environmental triggers, many years of abnormal volume and pressure loading (which cause pathologic remodeling of numerous cardiomyocyte and nonmyocyte functions and processes), chronic hypoxemia, damage during surgical repair (inadequate myocardial preservation, scarring, poor repair, damage to coronary arteries), arrhythmias, and acquired disease ( ; ; ). Ventricular volume overload occurs with intracardiac or extracardiac L-R shunts, valvar regurgitation, and single-ventricle lesions. The time course over which irreversible ventricular dysfunction develops is variable, but if surgical intervention to correct the volume overload is undertaken within the first 2 years of life, residual ventricular dilation and dysfunction are uncommon (except for single ventricle lesions) ( ). Ventricular pressure overload results from residual or recurrent ventricular outflow obstruction or pulmonary hypertension. The time to develop significant ventricular dysfunction is longer compared with a chronic volume load, so symptoms are uncommon unless the obstruction is severe and prolonged or it is combined with a volume load ( ). Chronic hypoxemia and cyanosis decrease ventricular oxygen supply and increase oxygen demand through increased work related to increases in pulmonary and systemic vascular resistance associated with polycythemia. Myocardial ischemia resulting from coronary artery anomalies or kinking or torsion after reimplantation may also cause ventricular dysfunction.
Anesthetic considerations: The chronic presence or potential to develop or exacerbate low systemic cardiac output is the single most important consideration for anesthetizing patients with CHD. Sometimes, the correlation between a patient’s or parent’s report of clinical status and functional myocardial reserve can be unreliable. No single parameter or test is best to assist with this assessment. Rather, one is advised to carefully review historical, physical, and diagnostic test information pertaining to myocardial performance to arrive at an integrated assessment of the degree of ventricular dysfunction, as well as the potential for further decompensation that can occur as a result of anesthetic choices and procedural factors. Although there is no single recipe, key aspects of successful management include selection of anesthetic induction and maintenance techniques most likely to maintain contractile function and hemodynamic stability and appropriate fluid administration, most commonly with maintenance of a normal to modestly increased preload. In patients with significant myocardial dysfunction, inotrope administration before, during, or soon after induction and/or during the procedure may be beneficial. Airway management is crucial, as airway obstruction and/or hypoventilation will increase PVR and RV afterload, and in the presence of shunts will also increase R-L shunting. According to Laplace’s law, positive pressure ventilation is likely to improve the function of a dysfunctional systemic ventricle by decreasing the transmural myocardial pressure and thus ventricular afterload; additional fluid administration may be necessary to counteract the associated decrease in venous return and preload.
Arrhythmias and conduction defects have a major impact on the prognosis and management of patients who have undergone repair or palliation of CHD and are a leading cause of impaired quality of life, morbidity, and mortality ( ; ). Rhythm disturbances that may be well tolerated in a structurally normal heart may be life-threatening in a structurally or functionally abnormal heart. The etiology is multifactorial, and as reviewed by includes abnormal anatomy or congenitally displaced or malformed sinus nodes or AV conduction systems, abnormal hemodynamics, primary myocardial disease, hypoxic tissue injury, residual or postoperative sequelae (damage to the arterial supply or direct injury to the SA and AV nodes and conduction system, atrial or ventricular scarring, suture lines, patches, myocardial ischemia, fibrosis), and genetic influences ( ).
Arrhythmias may occur in the perioperative period or many years after surgery, and tend to vary with age, type of underlying heart disease, and surgical procedures performed ( ; ). Supraventricular tachycardias (atrial flutter/intraatrial reentrant tachycardia, AV node reentrant tachycardia, atrial fibrillation) and sinoatrial node dysfunction (bradycardia, tachy-brady syndrome, exit block, sinus arrest) are more common in lesions that required extensive intraatrial surgery or have residual elevations in right atrial pressure, such as the atrial switch (Mustard or Senning) procedure for TGA, the Fontan procedure, and TOF repair. Although the 20-year risk of developing atrial arrhythmias is 7% for a 20-year-old, the prevalence increases to 50% by age 65 years ( ). Isolated right bundle branch block is frequent after right ventriculotomy ( ). The QRS duration may be an independent predictor of arrhythmia, right ventricular dysfunction, and sudden death in patients after TOF repairs ( ). Electromechanical dyssynchrony can lead to pathologic ventricular remodeling and heart failure ( ). Efficacy of cardiac resynchronization therapy (CRT) can vary with the underlying structural and functional substrate, with the best response observed in patients with a systemic LV who were upgraded from RV pacing to CRT ( ; ). Ventricular arrhythmias include mono- or polymorphic VT and ventricular fibrillation (VF) and are more common in lesions with residual ventricular pressure or volume load, such as aortic stenosis, hypertrophic cardiomyopathy, TOF, following the atrial switch and Fontan procedures, and Eisenmenger syndrome. Tachyarrhythmias and ventricular dysfunction are a dangerous combination and a cause of sudden cardiac death ( ).
Anesthetic considerations: Any new onset of palpitations, dizziness, or syncope should be investigated before elective surgery. Consultation with a pediatric cardiologist (electrophysiologist) can be very helpful in understanding the risk factors and causes for various rhythm disturbances in the CHD population, the need for additional diagnostic or therapeutic procedures, and the preferred pharmacologic and electrical approaches to the rhythm disturbances likely to arise. The electrophysiologist may also be helpful for patient optimization. Examples include whether temporary intravenous pacing is placed in select unpaced patients with bradycardia, or whether catheter ablation of amenable tachyarrhythmias should be performed prior to major procedures. Such discussions help ensure availability and appropriate use of antiarrhythmic agents and cardioversion/defibrillation devices. The availability of similar expert consultation during procedures is also advisable, particularly in situations that fail to resolve with usual therapy. In patients with tachyarrhythmias, there is at least a theoretical reason to avoid agents with vagolytic or sympathetic-stimulating properties. The electrophysiologic impact of most inhaled or intravenous agents, with the possible exception of dexmedetomidine, is probably modest ( ; ; ; ; ).
The American Society of Anesthesiologists (ASA) has published an update to the Practice Advisory for the Perioperative Management of Patients with Cardiac Implantable Electronic Devices (CIED) ( ). It is essential to know the reason for pacemaker placement and hence the likely response should the pacemaker fail; for example, whether there is an escape rate in the setting of AV block (this rate is likely to decrease or become absent under general anesthesia). Existing pacemakers should be checked preoperatively, may require reprogramming to an asynchronous mode if electromagnetic interference will interfere with pacemaker function, and may require antitachyarrhythmia and/or defibrillation functions to be suspended. Temporary (transcutaneous) pacing in the event of pacemaker malfunction and defibrillation equipment should be immediately available. For high-risk patients, the external pacing and defibrillator unit should be in the operating room and pads applied around the time of induction of anesthesia. The need for and use of electrocautery (monopolar vs. bipolar) in pacemaker-dependent patients should be discussed with both the surgeon and cardiologist. Postoperatively, it is essential that implanted pacemakers be interrogated and reprogrammed if there has been electromagnetic interference, and that antitachyarrhythmia and/or defibrillation functions are restored. reviewed the perioperative interrogation and management of CIEDs. The same authors have provided detailed guides for specific devices: Medtronic (Minneapolis, MN), Boston Scientific (Marlborough, MA), Biotronik (Lake Oswego, OR), and St. Jude Medical (St. Paul, MN) ( ; ; ; ).
Although MRI has traditionally been contraindicated in patients with CIEDs, in recent years manufacturers have developed MRI-conditional CIEDs. These devices are not MRI safe, and the Heart Rhythm Society released a consensus statement providing detailed instructions for all healthcare providers on the management of children and adults with these devices undergoing MRI (as well as CT scanning and radiation therapy) ( ).
The introduction of leadless transcatheter-deployed intracardiac pacemakers, subcutaneous implantable cardioverter defibrillators, and wireless endocardial pacing introduce new challenges for perioperative management ( ). Although societal perioperative practice guidelines still have to be developed for these newer devices, many of the principles for the management of transvenous systems can be applied.
Ventricular outflow obstruction may be subvalvar, valvar, supravalvar, or a combination thereof, isolated or part of more complex malformations, residual or recurrent, and fixed or dynamic. Outflow obstruction results in pressure overload on the ventricle, ventricular hypertrophy, a “stiff” ventricle (noncompliant), and ultimately systolic and diastolic ventricular dysfunction ( ). Left ventricular outflow obstruction may occur with aortic stenosis, CoA, IAA, and variants of HLHS and Shone’s anomaly ( ). Right ventricular outflow obstruction is seen with PS, TOF, hypoplastic pulmonary arteries, RV-PA conduits (performed in repair of pulmonary atresia, truncus arteriosus, D-TGA with pulmonary stenosis (Rastelli procedure), some forms of double-outlet right ventricle [DORV]), and pulmonary hypertension. Conduits calcify and narrow, and together with the increasing stroke volume that occurs with growth, significant obstruction can develop. The septal shift associated with severe RV pressure overload can compromise LV function via a reduction in left ventricular filling and systemic outflow obstruction.
Anesthetic considerations: Pressure-overloaded ventricles are at significant risk for myocardial ischemia during anesthesia, particularly in association with systemic hypotension and tachycardia. Right ventricular subendocardial ischemia is a potential risk in patients with systemic or suprasystemic RV pressures because systolic coronary flow may be markedly diminished or absent. The overall goal of anesthetic induction and management is to optimize and maintain the major determinants of ventricular function in the face of a fixed (sometimes dynamic) outflow obstruction and often some degree of both systolic and diastolic dysfunction. Hemodynamic goals are maintenance of sinus rhythm, normal to slower heart rate, normal to modestly increased preload, and inotropic support if significant ventricular dysfunction is present. Adequate preoperative hydration in accordance with fasting guidelines or a bolus of intravenous fluid prior to or during induction is recommended. Volume infusion or replacement in the presence of a noncompliant LV or MS must be done judiciously, because it has the potential to cause an excessive increase in LA pressure and consequent pulmonary edema. As severity of ventricular dysfunction increases, an intravenous induction with agents that maintain contractility and systemic blood pressure without significant alterations in heart rate is probably indicated. Although the majority of patients can tolerate at least modest concentrations of inhalational agent, a balanced technique employing a potent opioid such as fentanyl in combination with low-concentration inhaled agent and muscle relaxant may offer greater hemodynamic stability. When feasible, relief of severe outflow tract obstruction, which can frequently be performed in the catheterization laboratory, should precede all but emergency surgery.
Hypoxemia and cyanosis usually result from decreased PBF and/or intracardiac mixing lesions but can also occur in lesions with increased PBF causing pulmonary edema with V/Q mismatch and shunt. Prior to repair, cyanotic lesions include tetralogy of Fallot, tricuspid atresia, pulmonary atresia, HLHS and other single ventricles, transposition of the great arteries, truncus arteriosus, and heterotaxy. Unrepaired TOF, tricuspid atresia, and pulmonary atresia are all lesions that typically have reduced PBF, while truncus arteriosus is associated with increased PBF. In lesions with intracardiac mixing (e.g., TGA, HLHS, SV, heterotaxy), hypoxemia can occur with decreased or increased PBF, depending on whether there is obstruction to PBF. Cyanosis may also be found in the setting of very low cardiac output, increased systemic oxygen consumption, and respiratory pathology. With the advent of early infant repair, chronic hypoxemia is now most frequently encountered in the young child undergoing staged repair, children from resource poor settings, and in the adult with unrepaired or palliated CHD. Indeed, one stimulus for early, definitive repair is to eliminate hypoxemia and the compensatory polycythemia with its rheologic, hemostatic, neurologic, renal, hepatic, and metabolic consequences. Secondary erythrocytosis is one adaptive physiologic response to compensate for the low blood oxygen tension, but does result in increased blood viscosity ( ). As blood viscosity increases, systemic (including coronary) and pulmonary vascular resistances increase markedly, resulting in decreased tissue perfusion and somewhat offsetting the benefits of increased O 2 carrying capacity. The duration and degree of hypoxemia and polycythemia are important historical factors in the evaluation of possible long-term residual cardiac muscle blood flow abnormalities. Hemostatic abnormalities associated with cyanotic CHD include thrombocytopenia; platelet dysfunction; shortened platelet survival; decreased production of coagulation factors II, V, VII, IX, and X; fibrinogen dysfunction; and accelerated fibrinolysis ( ; ). Although low-grade disseminated intravascular coagulation (DIC) has been proposed as a mechanism, other data do not substantiate this ( ; ). Although sludging of red blood cells increases the risk for thromboembolism and stroke, phlebotomy regimens are used less frequently because of the paradoxical risk of stroke, iron depletion, and reduced oxygen-carrying capacity ( ). Guidelines from the American Heart Association/American College of Cardiology (AHA/ACC) recommend therapeutic phlebotomy for hemoglobin greater than 20 g/dL and hematocrit greater than 65% associated with headache, increasing fatigue, or other symptoms of hyperviscosity in the absence of dehydration or anemia ( ; ). Prenatal hypoxemia, pre- and postoperative hypoxemia, and chronic hypoxemia during infancy and early childhood are significant risk factors for reduced cognitive performance ( ; ; ; ).
Anesthetic considerations: It is important to maintain adequate preoperative hydration by encouraging liberal clear fluid intake in accordance with fasting guidelines or placement of an intravenous catheter and administration of maintenance fluids. Although the data are scant, preoperative hydration may be especially important as the hemoglobin concentration approaches 20 g/dL. There is an increased risk of bleeding in association with the increased tissue vascularity, hemostatic abnormalities, and anticoagulant medications. The risks of regional anesthesia in the presence of a hemostatic abnormality should be carefully considered. The effect of anemia on oxygen-carrying capacity is exaggerated because hemoglobin values “within the normal range” in cyanotic patients may represent a significant deficit. reviewed the general approach to cyanotic CHD and hypoxemia, and other aspects of anesthetic management should be based on the underlying pathophysiology.
Pediatric pulmonary arterial hypertension (PAH) is a vascular disease characterized by remodeling of the pulmonary vasculature resulting in elevated PAP; RV dysfunction (systolic and diastolic); compression of the LV resulting in decreased LV diastolic and systolic function, which causes further decrements in RV function; heart failure; and ultimately death ( ). The definition of pulmonary arterial hypertension (precapillary pulmonary hypertension) for biventricular circulations has been redefined as a mean pulmonary artery pressure >20 mm Hg and a pulmonary vascular resistance index (PVRI) ≥3.0 Wood units with a pulmonary artery wedge pressure ≤15 mm Hg ( ; ). Following a cavopulmonary anastomosis, PAH is defined as a PVRI >3.0 Wood units/m 2 or a transpulmonary gradient >6 mm Hg (mean PAP minus mean left/common atrial pressure). The etiology of pediatric pulmonary hypertension is multifactorial, but the most frequent causes are hereditable, idiopathic, or associated with CHD ( ). Survival is closely related to RV function ( ). Treatment for PAH includes supportive therapy (diuretics, O 2 , digoxin, anticoagulants), targeted drug therapy (Ca-channel blockers, endothelin receptor antagonists, phosphodiesterase-5 inhibitors, prostacyclins (epoprostenol, treprostinil, iloprost), and surgical interventions (atrial septostomy, “reverse” Potts shunt, and lung ± heart transplantation) ( ; ). Guidelines on the diagnosis and treatment of pediatric PAH have been published by the American Heart Association/American Thoracic Society (AHA/ATS) and the European Pediatric Pulmonary Vascular Disease Network (EPPVDN) ( ; ).
In the child with unrepaired CHD, unrestricted L-R shunting with increased PBF produces a volume load on the heart (
p:
s >1) and structural changes in the pulmonary vascular bed (muscularization of peripheral arteries, medial hypertrophy of large pulmonary arteries, loss of small precapillary arteries, progressive intimal hyperplasia, and progression to necrotizing arteritis), leading to vessel occlusion and PAH ( ; ). The time course for developing pulmonary vasoocclusive disease (PVOD) depends on the size and site of the shunt and the age at corrective surgery. Progression is more rapid when both the volume and the pressure load on the pulmonary circulation are increased, such as with a large VSD. For the majority of infants with an unrestrictive shunt, repair of the defect in the first year of life is usually associated with regression of the pulmonary vascular changes. Pulmonary arterial hypertension develops more slowly with increased pulmonary blood flow in the absence of elevated pulmonary artery pressures, as with an ASD, where the absence of PAH into the third decade or beyond is not uncommon. Eisenmenger syndrome is characterized by irreversible PVOD and cyanosis related to reversal of the L-R shunt ( ).
The increased PVR can be reactive, fixed, or a combination thereof. The reactivity of the pulmonary vascular bed is determined during cardiac catheterization by the changes in PAP and PVR in response to vasodilators, most commonly oxygen and nitric oxide (NO) ( ; ). A positive response to acute vasoreactivity testing is a ≥20% decrease in both the PVRi and PVRi/SVR ratios, with final values <6 and <0.3 Wood units/m 2 , respectively ( ). The major pathophysiologic consequences of a pulmonary hypertensive crisis are acute RV dysfunction with resultant low systemic cardiac output. The cycle is increased RV afterload, causing RV dilation with increased wall stress/RV EDP, decreased RV stroke volume, decreased left heart return, decreased cardiac output with decreased coronary perfusion pressure, and myocardial ischemia. Additionally, RV dilation can cause a leftward shift of the interventricular septum, impairing LV filling and function, and further decreasing cardiac output. The compensatory increase in heart rate can aggravate myocardial ischemia, potentially leading to bradycardia and cardiac arrest. If an intracardiac communication (PFO, ASD, VSD) is present, increases in PAP lead to R-L shunting and desaturation, albeit with better maintenance of LV filling and systemic cardiac output. If the patient has a Potts shunt (side-to-side anastomosis of the left pulmonary artery to the descending artery), the systemic circulation may be maintained but with desaturation.
Anesthetic considerations: reviewed the evaluation and perioperative management of pediatric PAH. Severe PAH imparts major anesthetic risk for both children and adults, even for minor procedures and in the current era with disease-modifying treatments ( ; ; ; ). Important risk factors for complications are younger age (<1 year), severity of PAH (systemic or suprasystemic PAP), and RV dysfunction ( ; ). Of note, many perioperative deaths occur in the postoperative period because of hypovolemia (bleeding, fluid shifts, vomiting), excessive pain with a stress response, atelectasis and/or pneumonia worsening hypoxemia, or other end organ dysfunction ( ; ). In older patients with Eisenmenger syndrome, most deaths appear to occur as a result of the nature of the surgical procedure rather than from anesthesia ( ).
The goals of anesthesia are to prevent increases in PVR and to support the RV. Preoperative knowledge of the degree of PAH, pulmonary vascular reactivity, RV function, and the presence of an intra- or extracardiac communication is imperative ( ). Factors that increase PVR include hypoxemia, hypercarbia, acidosis, extremes of lung volume, sympathetic stimulation associated with stress or light anesthesia, and hypothermia. It is not possible to recommend a specific anesthetic technique because all anesthetic techniques have been used successfully. Excellent airway management is crucial, as is the provision of adequate sedation/depth of anesthesia and analgesia without compromising ventricular function. Hemodynamic goals are avoidance of excessive tachycardia, maintenance of sinus rhythm, normal to increased preload, avoidance of systemic hypotension (risk of RV ischemia), and support of hemodynamics with early use of inotrope and/or vasoactive agent. Although a loading dose of dexmedetomidine over 10 minutes did not produce significant pulmonary vasoconstriction in children without (or with treated) PAH, it has been reported to cause a severe increase in PVR in patients with brittle or severe PAH ( ; ). Ketamine has been used safely, provided that airway control and ventilation is satisfactory ( ; ). The use of invasive monitoring (i.e., arterial catheter, central venous catheter) is usually determined by the nature of the surgical procedure. Endotracheal intubation as a potential mechanical trigger of pulmonary vasoreactivity should be recognized; this also applies to extubation on emergence from anesthesia. In addition to adequate anesthetic depth, lidocaine spray to the vocal cords and trachea may offer some degree of protection during intubation. Noninvasive ventilation may be an attractive alternative to support adequate gas exchange during anesthesia and surgery under some conditions. Although allowing better control of oxygenation and ventilation, positive pressure ventilation increases RV afterload and decreases RV filling, so that excessive inspiratory pressures and volumes, high positive end-expiratory pressure (PEEP), and short expiratory times (reduces venous return) should be avoided.
It is critical that pulmonary vasodilator therapy not be interrupted perioperatively, particularly epoprostenol or treprostinil infusions, the discontinuation of which can result in severe rebound PAH. Epoprostenol has an elimination half-life of 6 minutes, so rebound PAH can occur in as little as 10 to 15 minutes; the elimination half-life of treprostinil is approximately 4 hours. Higher Fio 2 is generally recommended. With severe pulmonary hypertension and known responsiveness of the pulmonary vasculature to NO, it is advisable to have NO available for immediate or even prophylactic administration. Because of the significant morbidity and mortality of surgery and anesthesia for the patient with severe PAH, a risk-benefit analysis involving the cardiologist, surgeon, and anesthesiologist is essential before performing elective procedures.
Coronary artery anomalies (e.g., intramural coronary, anomalous origin from the other sinus or pulmonary artery), and early and late coronary artery obstruction associated with the arterial switch operation (ASO) or supravalvar aortic stenosis (SVAS), can result in myocardial ischemia ( ; ; ; ; ; ). In many patients with congenital defects and normal coronary arteries, ischemia is more commonly secondary to imbalances in myocardial oxygen supply and demand. There is some evidence that in lesions associated with abnormal load, including those where the RV is the systemic ventricle, coronary angiogenesis and capillary supply may not keep pace with increased muscle mass. Subendocardial perfusion is largely determined by coronary perfusion pressure (aortic diastolic pressure minus the ventricular end-diastolic pressure) and the time interval available for perfusion (predominately diastole). As a result, the relationship between diastolic blood pressure, ventricular end-diastolic pressure, and heart rate determines whether subendocardial ischemia occurs. These three factors place patients with CHD at risk for ischemia in the following situations: (1) the systolic pressure in the ventricles is abnormally elevated; a frequently forgotten condition is systemic or suprasystemic pressures in the RV; (2) the aortic diastolic pressure is compromised by diastolic runoff of aortic blood into the lower-resistance pulmonary circuit in ductal-dependent circulations and systemic-to-pulmonary artery shunts (coronary perfusion is further compromised if the coronary ostia are perfused with desaturated blood, as in patients with HLHS or unrepaired D-TGA); (3) elevated ventricular end-diastolic pressure or volume, which may be the result of impaired systolic and/or diastolic function (reduced ventricular compliance and relaxation); and (4) increases in heart rate, which geometrically reduce the duration of diastole (the duration of systole stays relatively constant) so the time available for coronary perfusion falls and consequently a higher diastolic pressure is necessary to maintain the same degree of subendocardial perfusion. The combination of a high heart rate and low diastolic blood pressure can, in some situations, produce significant ischemia.
Early studies in adults with cyanotic CHD reported a lower burden of atherosclerosis with dilated epicardial coronary arteries and mural attenuation caused by medial abnormalities ( ; ; ). The paucity of atheroma was believed to be the result of hypocholesterolemia, hypoxemia, upregulated nitric oxide, thrombocytopenia, and hyperbilirubinemia. However, this concept was later challenged. In a study comparing young adults (median age 50 years) with cyanotic CHD to age-, sex-, smoking status-, and body mass index matched controls, no significant differences in lipoprotein profile, cardiovascular risk score, and prevalence of carotid and coronary subclinical atherosclerosis were found ( ).
Anesthetic considerations: Standard principles are followed to ensure that myocardial oxygen supply exceeds demand. In particular, maintaining aortic perfusion pressures, in combination with avoiding excessive tachycardia, frequently appears to be critical. With cyanotic lesions, a hemoglobin level above the normal range may be necessary.
Infective endocarditis (IE) is a devastating disease with a high incidence of morbidity (heart failure, stroke, cardiac surgery) and overall mortality (pediatric 6.6%, adult 24% to 28.6%) ( ; ). Recommendations for IE prophylaxis are based on expert consensus rather than randomized controlled trials (RCTs), and prevention remains an empirical practice with uncertainty and controversy ( ). Guidelines have been published by the AHA, European Society of Cardiology (ESC), and UK National Institute for Healthcare and Excellence (NICE) ( www.nice.org.uk/CG064 ) ( ; ; ). In 2007 the American Heart Association concluded that only an extremely small number of cases of IE could be prevented by antibiotic prophylaxis for dental procedures ( ). Accordingly, prophylaxis for dental procedures was recommended only for patients with underlying cardiac conditions ( Box 30.2 ) associated with the highest occurrence and risk for adverse outcome from IE. Prophylaxis in this group was recommended for all dental procedures that involve manipulation of gingival tissue or the periapical region of teeth or perforation of the oral mucosa. The antibiotic is administered in a single dose before the procedure ( Table 30.4 ). Administration of antibiotics solely to prevent endocarditis was not recommended for patients with the same conditions listed in Box 30.2 who undergo a genitourinary or gastrointestinal tract procedure. Although there is some overlap between the various guidelines, they are not identical. The AHA and ESC favor antibiotic administration in select cardiac patients, while the NICE recommended that antibiotic prophylaxis be abandoned in any patient for any procedure ( ). Furthermore, clinicians frequently vary in their interpretation of the same guidelines.
Prosthetic cardiac valve or prosthetic material used for cardiac valve repair
Previous infective endocarditis
Congenital heart disease (CHD) *
Unrepaired cyanotic CHD, including palliative shunts and conduits
Completely repaired congenital heart defect repaired with prosthetic material or device, whether placed by surgery or by catheter intervention, during the first 6 months after the procedure †
Repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device (both of which inhibit endothelialization)
Cardiac transplant recipients with valve regurgitation resulting from a structurally abnormal valve
* Except for the conditions listed above, antibiotic prophylaxis is no longer recommended for any form of CHD.
Prophylaxis is recommended because endothelialization of prosthetic material occurs within 6 months after the procedure.
| REGIMEN: SINGLE DOSE 30–60 MIN BEFORE PROCEDURE | |||
|---|---|---|---|
| Situation | Agent | Adults | Children |
| Oral | Amoxicillin | 2 g | 50 mg/kg |
| Unable to take oral medication | Ampicillin OR cefazolin or ceftriaxone |
2 g IM or IV 1 g IM or IV |
50 mg/kg IM or IV 50 mg/kg IM or IV |
| Allergic to penicillins or ampicillin—oral | Cephalexin * † OR clindamycin OR azithromycin or clarithromycin |
2 g 600 mg 500 mg |
50 mg/kg 20 mg/kg 15 mg/kg |
| Allergic to penicillins or ampicillin and unable to take oral medication | Cefazolin or ceftriaxone † OR clindamycin |
1 g IM or IV 600 mg IM or IV |
50 mg/kg IM or IV 20 mg/kg IM or IV |
* Or other first- or second-generation oral cephalosporin in equivalent adult or pediatric dosage.
† Cephalosporins should not be used for an individual with a history of anaphylaxis, angioedema, or urticaria with penicillins or ampicillin.
Congenital heart disease is a significant risk factor for IE and its complications. A pediatric population-based study in Quebec found that cyanotic CHD, left-sided lesions, endocardial cushion defects, the 6-month postoperative period of cardiac surgery, and age <3 years were associated with an elevated risk of developing IE ( ). An accompanying editorial commented that although cyanotic lesions and cardiac surgery in the previous 6 months are in line with current guidelines for IE prophylaxis, further research is necessary before extending prophylaxis to other congenital lesions ( ). A more recent retrospective cross-sectional study of the Kids’ Inpatient Database (KID) found that the incidence of IE was stable in the 2000 to 2012 period, but that mortality following implementation of the 2007 AHA IE prophylaxis guidelines was higher for CHD patients than non-CHD patients (11.1% vs. 2.4%, respectively; p <0.001) ( ).
In contrast to high-income countries, rheumatic heart disease accounts for up to 50% of IE cases in low- and middle-income countries, and the rate of access to surgery is dismal ( ).
Rarely, patients scheduled for sedation or anesthesia have IE, in which case the clinical status and results of blood cultures should guide antibiotic therapy and perioperative management.
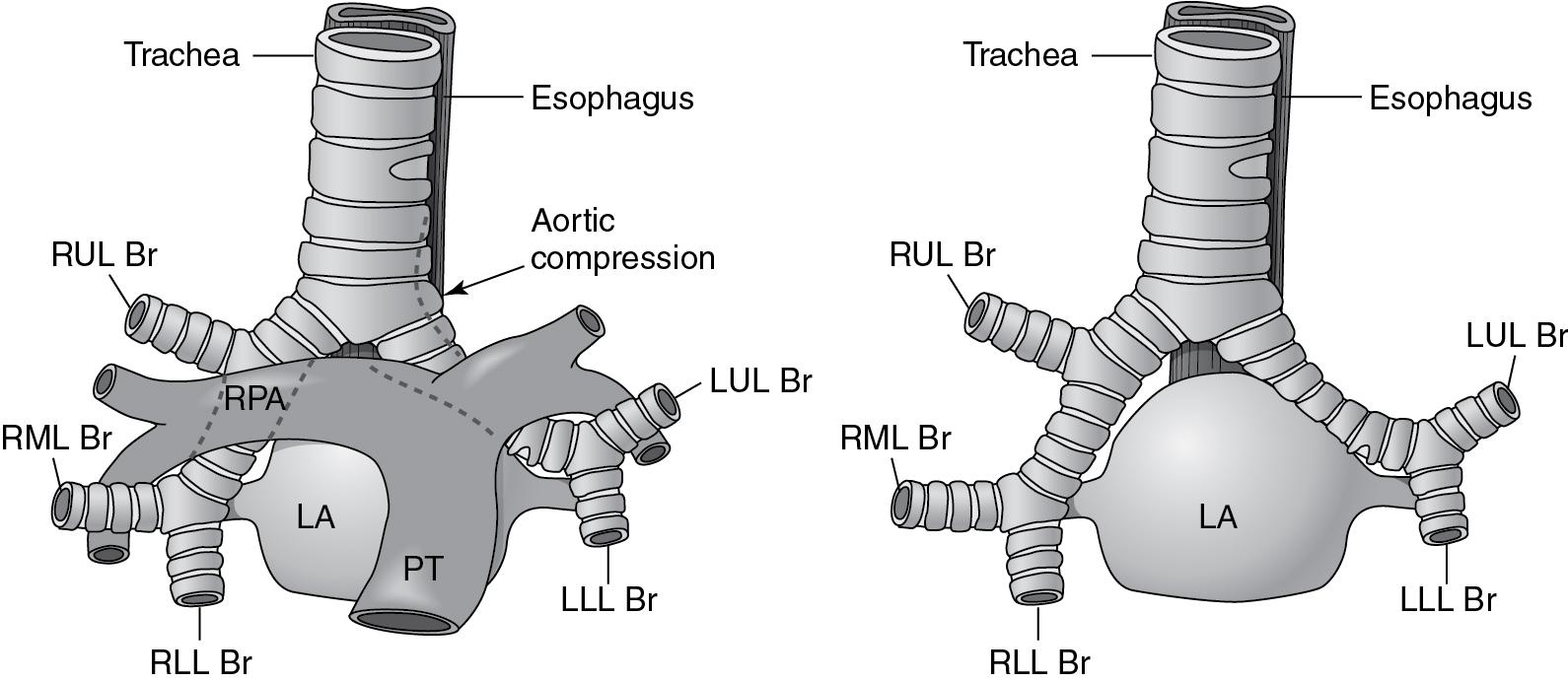
Unrestricted L-R shunting, in addition to increasing PAP and PVR, produces alterations in lung mechanics and airway compression. The primary effects are a decrease in lung compliance and an increase in airway resistance ( ; ). Decreased compliance will necessitate higher-than-expected airway pressures, with care being taken not to insufflate the stomach during mask ventilation. Even patients with decreased PBF, such as TOF, can have pathologic lung function parameters (functional residual capacity [FRC], ventilation homogeneity) because of the effects of pulmonary hemodynamics on the alveolar architecture ( ; ). Children and adults with reduced lung flow prior to repair of cyanotic CHD have decreased lung volumes as a result of the lower density of intrapulmonary vessels and fewer alveoli ( ). Airway compression can result from dilated pulmonary arteries, left (or right) atrial dilation, massive cardiomegaly, or intraluminal bronchial obstruction ( ) (see Fig. 30.e1 ). The pulmonary lymphatics are also compressed in these circumstances, perhaps explaining an increased incidence of pulmonary infectious symptoms in patients with large L-R shunts.
Neurologic injury and adverse neurodevelopmental outcome in patients with CHD is very common, particularly with moderate and severe disease ( ; ). Children undergoing surgery in infancy have been found to have impairment in executive function, cognition, language skills, learning, fine and gross motor skills, visual-spatial skills, attention, memory, academic achievement, and social skills ( ; ). Less commonly seen in the current era are clinical seizures, major stroke, and choreoathetosis. The etiology is multifactorial and includes innate patient factors (genetic, epigenetic, maternal education and IQ, socioeconomic status); abnormal brain growth and development; and acquired intrauterine, pre-, intra-, and postoperative brain injury ( ; ; ; ; ; ; ; ; ). Smaller total brain volume, delayed maturation, brain injury, and structural and metabolic abnormalities have been found with MRI both antenatally and before any intervention, as well as after surgery or balloon atrial septostomy (BAS). Acquired cardiovascular comorbidities in adulthood make the risk factors for brain injury in the CHD population cumulative and synergistic ( ). Complex CHD may have anywhere from a mild to a profound impact on a child’s psychosocial development ( ; ; ; ). These issues necessitate additional sensitivity with the patient and family, altering the amount of detail discussed in front of the child when obtaining informed consent, and can preclude certain sedation or regional anesthetic techniques.
Renal and hepatic dysfunction frequently develop in response to chronic hypoxemia and cyanosis, low systemic cardiac output, and/or congestion with high central venous pressures ( ; ; ). This may not be evident on routine biochemical testing (e.g., serum creatinine or liver enzymes) but may predispose to perioperative dysfunction in response to relatively minor changes in organ perfusion and oxygen delivery or to otherwise relatively mild toxic stresses (e.g., NSAIDs). Renal dysfunction is associated with higher mortality, and may be present in 50% of young adults (36.0 ± 14.2 years) with CHD ( ). Severe renal dysfunction develops in 3% to 10% of heart transplant recipients within the first 10 postoperative years ( ). Contrast-induced nephropathy (CIN) associated with cardiac angiography increases the risk for perioperative renal dysfunction. Although preprocedural hydration with sodium bicarbonate reduced the incidence of CIN in adults with preexisting renal insufficiency, sodium bicarbonate did not lower the risk for dialysis and mortality ( ). Apart from good hydration, the best strategy to prevent CIN has yet to be defined ( ).
Liver dysfunction associated with single-ventricle physiology and the Fontan circulation appears to begin with chronic congestion, inflammation, and sinusoidal fibrosis, which progresses to centrilobular fibrosis, bridging fibrosis, and portal hypertension ( ; ; ). Fibrosis may begin before the Fontan operation and is associated with pre-Fontan morbidity ( ). Other factors contributing to Fontan-associated liver disease include perioperative liver injury (hypotension, hypoxia), genetic/metabolic factors, and hepatotoxins ( ).
Extracardiac anomalies are very common in patients with CHD and, depending on the case series, may be present in up to 50% of patients ( ; ). The anomalies are often multiple and may result from chromosomal, genetic, teratogenic, or unknown causes. Abnormalities are most frequently found in the central nervous system, gastrointestinal tract, kidneys and urinary tract, lung, craniofacial structures, musculoskeletal system, and spleen. In patients with TOF, the risk of 1-year mortality was associated with the number of extracardiac birth defects ( ).
Anesthetic considerations: A focused airway examination and thorough assessment of all organ systems is essential.
The worldwide annual transplant rate in children as reported to the International Society for Heart and Lung Transplantation (ISHLT) for the period July 1, 2017, to June 30, 2018, was 620, comprising 14.2% of cardiac transplants ( ). The primary indication varies by age, with CHD being the commonest indication in infants (age <1 year at transplant) and cardiomyopathy in older children (1 to 17 years). Factors to consider in this population are cardiac physiology and functional status, cardiac allograft vasculopathy, rejection, the side effects of immunosuppressive agents, and the development of renal dysfunction, hypertension, and malignancy. The physiology of the transplanted heart involves efferent denervation resulting in resting tachycardia (withdrawal of vagal tone), impaired chronotropic response to stress (slower increase in heart rate, lower peak heart rate, and delayed return to resting rate), and greater dependence on preload and endogenous circulating catecholamines to maintain cardiac output ( ). Cardiac physiology is restrictive, with mildly elevated filling pressures, a low-normal ejection fraction, and increased afterload. There can be a shift from β 1 to β 2 receptors, and sinus node dysfunction can occur in the early postoperative period. Afferent denervation results in silent ischemia and alterations in cardiac baro- and mechanoreceptors (less stress-induced increase in systemic vascular resistance). Cardiac reinnervation is complex and has been reviewed in detail ( ). Reinnervation may occur months to years following heart transplant, is partial and regionally heterogeneous, and does not appear in all recipients.
Anesthetic considerations: Anesthesia for the patient with a transplanted heart has been reviewed ( ). Briefly, it is important to maintain adequate preload as the heart rate response is limited. The restrictive physiology, particularly with rejection, increases the risk for pulmonary edema when fluid administration is excessive. Hypotension and/or decreased cardiac output should be treated with direct-acting sympathomimetic agents. Sensitivity is increased to direct-acting catecholamines, β-blockers, adenosine, and verapamil, and it is decreased to digoxin and indirect-acting sympathomimetic agents. Myocardial ischemia is an ever-present threat from coronary artery vasculopathy. A new onset of dysrhythmias or heart block is ominous, suggesting rejection and/or myocardial ischemia. Immunosuppression requires strict aseptic technique, and the hypertension and nephrotoxicity associated with some agents and possible need for stress-dose corticosteroids need to be addressed.
The preoperative evaluation should be complete enough to provide a clear understanding of the anatomy and pathophysiology of the “original” cardiac defect, and if applicable that following any surgical or catheterization procedures, current medications, involvement of other organ systems, and the likely acute physiologic consequences of the planned surgical procedure.
Specific symptoms that should be sought are feeding difficulties and sweating in infants, poor growth, cyanotic spells, decreased activity level such as inability to keep up with healthy peers, fatigue, dyspnea, palpitations, chest pain, and syncope. New or worsening symptoms require cardiology consultation. Recent respiratory tract infections can cause changes in pulmonary vascular resistance and airway reactivity, increasing anesthetic risk, particularly in the setting of decreased pulmonary compliance, pulmonary hypertension, systemic-to-pulmonary artery shunts, and cavopulmonary anastomosis.
The physical examination should include general appearance, level of activity, vital signs, and presence of cardiac and respiratory dysfunction. Arterial oxygen saturation (Spo 2 ) varies with clinical status, but it is expected to be above 94% after definitive procedures and in the range of 75% to 85% after palliative interventions that create shunted or intracardiac mixing circulations. Evidence of cyanosis, tachycardia, tachypnea, labored breathing, congestive heart failure, and poor peripheral perfusion should be sought. Airway assessment is important, because craniofacial anomalies may be present in up to 20% of patients with CHD ( ) (Fig. 30.e1). Peripheral pulses and four extremity blood pressures should be assessed in the setting of known or suspected aortic arch obstruction, present or previous Blalock-Taussig shunts, or after multiple cardiac catheterizations.
The extent of laboratory and diagnostic testing depends on the child’s clinical status and the complexity of the planned procedure. For cardiac surgery, a hemoglobin/hematocrit, white blood cell count, and platelet count are routine, as is blood typing and crossmatching. Some centers routinely perform coagulation studies (prothrombin time, partial thromboplastin time, fibrinogen), even for patients not receiving anticoagulants. Serum electrolytes, glucose, urea and creatinine are usually performed for complex procedures, renal dysfunction, and certain medications (diuretics, angiotensin-converting enzyme [ACE] inhibitors, and digoxin). There is very wide variability in preoperative laboratory testing across institutions performing pediatric cardiac surgery ( ). Diagnostic studies for most patients include an electrocardiogram, chest x-ray, and echocardiogram (transthoracic and/or transesophageal). Some patients will have had a cardiac catheterization, cardiac MRI (CMR), or cardiac computed tomography (CT). Much of the testing is driven by institutional practice and the preferences of the cardiologists and cardiac surgeons.
The considerations common to all pediatric anesthesia also apply for cardiac surgery, including appropriately sized airway and monitoring equipment, adequate vascular access equipment and supplies (including pressure transducers and flush systems), temperature control (capability for warming and cooling), and weight-appropriate sets for fluid and blood administration. An extra blood pressure cuff and pulse oximeter should be available and used in some situations (repair of CoA or IAA, PDA ligation). The capability for cardioversion, defibrillation, and external pacing (including external pads or paddles and internal paddles of appropriate sizes) is essential. Ultrasound devices for vascular placement is the norm in many centers for arterial (peripheral or femoral) and central venous (usually internal jugular) cannulation and is helpful for the child with difficult IV access. In addition to standard drugs and heparin, one should have predrawn and therefore immediately available syringes containing weight-appropriate concentrations for bolus administration of emergency drugs, including epinephrine, calcium (gluconate or chloride salt), phenylephrine, atropine, and ephedrine. Other agents, such as sodium bicarbonate, dextrose, potassium chloride, antiarrhythmics (e.g., adenosine, procainamide, lidocaine, β-blockers), and inotropes and other vasoactive drugs for infusion (e.g., dopamine, epinephrine, phenylephrine, milrinone, vasopressin, nitroglycerin, nitroprusside, esmolol) should be immediately available. Many providers find it helpful before starting the case to complete a patient-specific emergency card for each patient that contains weight-based concentrations, dosages, and bolus volumes or infusion rates for the most frequently used agents.
The younger child will usually benefit from oral premedication before placement of an intravenous catheter. Premedication is especially beneficial for children with cyanotic CHD, especially those with hypercyanotic spells, catecholamine-induced arrhythmias, and preexcitation syndromes. Although the potential effects of hypoventilation and hypoxemia on PVR need to be considered in the setting of pulmonary hypertension, sympathetic stimulation in a distressed patient may have a more deleterious effect.
Midazolam, 0.5 to 0.75 mg/kg orally, is usually sufficient. Patients who have had multiple surgical procedures with long ICU stays are likely to be more anxious and are frequently quite tolerant to sedative medications; for these patients it is often necessary to increase the dose to 1 mg/kg ( ); sometimes oral ketamine, 3 to 10 mg/kg, is added as well ( ; ; ; ). Troublesome ketamine-stimulated secretions can usually be controlled with glycopyrrolate (0.005 mg/kg) once intravenous access is established. If the patient has intravenous access in situ or after placement, a small bolus of midazolam, 0.05 mg/kg, repeated as necessary, will provide anxiolysis and facilitate separation from the parents. Intramuscular sedation (generally induction of general anesthesia) with ketamine, 3 to 5 mg/kg, with or without midazolam, 0.05 to 0.1 mg/kg, may be necessary for the uncooperative or combative child who will not accept oral premedication and for whom an intravenous induction is most desirable. After any heavy premedication, the anesthesiologist should remain with the patient, and, particularly for patients with cyanotic CHD, oxygen saturation should be monitored and oxygen administered as needed ( ).
No one anesthetic induction technique is suitable for all patients with CHD. The patient’s age, cardiopulmonary function, degree of cyanosis, and emotional state all are factors in the selection of an anesthetic technique. The paramount consideration for all patients is ensuring adequacy of the airway and gas exchange. In some CHD patients, loss of the airway can lead to hypoxemia and rapidly downhill to cardiac arrest. Intravenous administration of induction agents clearly affords the greatest flexibility in terms of drug selection and drug titration and allows for prompt control of the airway. Although an inhalation induction is commonly feasible, we believe that intravenous induction is the preferred technique in the majority of patients, particularly those with significantly impaired ventricular systolic function and limited cardiac reserve, significant obstruction to blood flow (e.g., severe aortic stenosis), systemic or suprasystemic RV/PA pressures, and a shunt-dependent circulation. Cardiac medications, apart from diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor antagonists (which are typically held), are generally administered on the morning of surgery.
Mask induction of anesthesia can be accomplished safely in the subset of children without severe cardiorespiratory compromise. However, reduced pulmonary blood flow in cyanotic patients will prolong the length of induction and the interval during which the airway is only partially controlled. In addition, in these patients, even short intervals of airway obstruction or hypoventilation may result in hypoxemia. Sevoflurane has become the inhalation induction agent of choice (halothane, even if desired, has become unobtainable in many places). Sevoflurane causes less myocardial depression, hypotension, and bradycardia than halothane. Isoflurane and particularly desflurane are unsuitable agents, because their pungency causes copious secretions, airway irritation, and laryngospasm.
With respect to intravenous induction, many of these patients come to the operating room with functioning IV access. For those without it, effective oral premedication may facilitate IV placement and allow the attendant risks of mask induction in this population to be avoided. A high-dosage synthetic narcotic in combination with a muscle relaxant is commonly used for IV induction in neonates and young infants. While the vagolytic and sympathomimetic effects of pancuronium counteract the vagotonic effect of synthetic opioids, the drug is not available in many countries; for this technique, rocuronium is a suitable alternative. In patients with a low aortic diastolic blood pressure and a high baseline heart rate (at risk for myocardial ischemia), vecuronium or cisatracurium may be used without affecting heart rate. In older children with moderately depressed systolic function, lower dosages of a synthetic opioid can be used in conjunction with etomidate (0.1 to 0.3 mg/kg) ( ). Ketamine (1 to 3 mg/kg) is a very useful induction agent for patients with moderate to severe ventricular dysfunction. Ketamine provides hemodynamic stability through sympathetically mediated increases in heart rate and systemic vascular resistance, albeit with direct myocardial depressant effects ( ). Although reports of the effect of ketamine on pulmonary vascular resistance are conflicting, it can be used without risk of major increases in PVR in patients with CHD as long as the airway is supported and elevations in arterial CO 2 are prevented ( ; ; ; ). The tachycardia and increase in SVR induced by ketamine may make it unfavorable for use in patients with systemic outflow tract obstructive lesions. The vasodilatory and myocardial depressive effects of propofol and thiopental can make them unsuitable as induction agents in patients without adequate cardiovascular reserve to tolerate these adverse effects ( ).
Intramuscular induction is an alternative to IV induction in patients with difficult peripheral IV access or when initial IV access will have to be obtained via a central vein (internal or femoral). One technique, provided a difficult airway is not suspected, is ketamine (3 to 5 mg/kg), succinylcholine (3 to 4 mg/kg), and atropine (0.02 mg/kg). Atropine is used to prevent the bradycardia that may accompany succinylcholine administration and to reduce airway secretions. The required dosage of succinylcholine per kilogram body weight is highest in infants. This technique provides prompt induction, hemodynamic stability, and almost immediate (within 1 min) control of the airway and tracheal intubation. One potential problem is that the short duration of action of succinylcholine limits the period of patient immobility. Some providers use rocuronium (1 to 2 mg/kg) instead of succinylcholine, but this technique is limited by the longer time interval until attainment of adequate intubating conditions.
Anesthesia is generally maintained using a synthetic opioid–based technique. These opioids may be used in high dosages (25 to 100 mcg/kg fentanyl or 2.5 to 10 mcg/kg sufentanil) or in low to moderate dosages (5 to 25 mcg/kg fentanyl or 0.5 to 2.5 mcg/kg sufentanil). In both instances, opioids are typically used in combination with an inhalation agent (generally isoflurane 0.5% to 1.0% or sevoflurane 1.0% to 2.0%) or a benzodiazepine (generally midazolam 0.05 to 0.1 mg/kg), or both. Caution must be exercised because the combination of narcotics and benzodiazepines is synergistic in reducing SVR. High-dosage opioid techniques are particularly useful for neonates and very young infants. Patients in this age group often have significant ventricular pressure or volume overload, and many have tenuous subendocardial and systemic perfusion secondary to elevated end-diastolic pressures in combination with low aortic diastolic blood pressure from runoff into the pulmonary circulation (via PDA). Given the limited contractile reserve available in the immature myocardium, it is not surprising that the myocardial depressive and systemic vasodilatory effects of inhalation agents and the synergistic vasodilatory effects of benzodiazepines and opioids may be poorly tolerated in this patient group. Many patients will require inotropic support prior to cardiopulmonary bypass (CPB).
Sevoflurane and isoflurane have similar effects on cardiac index and are associated with less myocardial depression and better hemodynamic stability than halothane, particularly in infants ( ; ; ; ). The inhalational anesthetic agents (sevoflurane, isoflurane, halothane) or fentanyl-midazolam do not change the ratio of pulmonary-to-systemic blood flow (
p:
s) in children with atrial and ventricular septal defects when cautiously administered with 100% oxygen ( ). In patients with SV physiology, sevoflurane (1 minimum alveolar concentration [MAC]) or fentanyl-midazolam has no significant effect on myocardial function in patients with a single ventricle ( ). Halothane (1 and 1.5 MAC) depresses cardiac index and contractility more than comparable levels of sevoflurane, isoflurane, or fentanyl-midazolam anesthesia ( ). Notwithstanding the foregoing, inhalational agents require more careful titration in the presence of CHD.
Exposing neonates to large amounts of opioids and benzodiazepines can lead to tolerance that may persist, whereas inadequate analgesia for infants may lead to specific centrally mediated pain sensitization and thus increased sensitivity to pain and greater fear of painful procedures ( ; ). This has implications for premedication (as discussed above), opioid-benzodiazepine anesthesia, and postoperative pain control.
Dexmedetomidine is widely used in children, including those with cardiac disease and pulmonary hypertension ( ). In children without cardiac disease, the decreases in heart rate and systolic blood pressure are dose related, and the initial transient hypertension seen in adults is not consistent in children ( ; ; ). In children with pulmonary hypertension receiving chronic vasodilator therapy, a dexmedetomidine loading dose of 0.5, 0.75, and 1 mcg/kg over 10 minutes was associated with a decrease in heart rate and increase in mean arterial pressure and systemic vascular resistance index but no significant changes in pulmonary artery pressure and pulmonary vascular resistance index ( ). However, limitations of this study included the degree of pulmonary hypertension in the majority of subjects being not very severe (PAP <70% systemic arterial pressure), the exclusion of patients with newly diagnosed untreated pulmonary hypertension, and that 81% of the patients were on chronic vasodilator therapy. Because the high risk of dexmedetomidine in some patients with pulmonary hypertension was subsequently reported, further research is necessary, particularly in children with severe or brittle pulmonary hypertension ( ).
Congenital heart disease and cardiomyopathy add significant risk for morbidity and mortality in patients undergoing sedation and anesthesia for both cardiac and noncardiac procedures ( ; ; ; ; ; ; ; ; ; ; ; ). Many of the principles of anesthesia for congenital heart surgery also apply to the anesthetic management of CHD patients for noncardiac procedures. This is true whether the patient is a child or an adult, and whether or not they have had a procedure (surgical or catheterization).
Broadly speaking, there are three categories of patients with CHD: those who have undergone a reparative (corrective) procedure, those who have undergone a palliative procedure, and those who have not undergone any procedure. The goal of the preoperative evaluation is to understand the current anatomy, physiology, and functional status and how this will interact with the planned procedure. As a general rule, patients with CHD who are doing well clinically (i.e., have good functional status, few or no medications, and only routine cardiology visits) tend to do well with anesthesia and surgery. Not surprisingly, the unrepaired or palliated patient presents a greater risk, as does the more complex and stressful surgical procedure.
This is the same as for patients presenting for congenital heart surgery. Because the history may be incomplete or misleading with complex CHD, close collaboration with the patient’s cardiologist is valuable. The cardiologist can help identify patients at higher risk, clarify pathophysiologic issues, establish if the current clinical status is the best possible, and provide the findings of recent cardiologic studies. The focus of the history should be on the type of lesion and factors listed in Box 30.1 , prior surgical and catheterization procedures and complications thereof, anesthetic experience, medications, allergies, and current functional status. It is worth noting that lack of cardiology follow-up begins in childhood, and between the ages of 18 to 22 years approximately 61% of patients have failed to receive cardiac follow-up ( ). The message is that the adolescent and young adult with CHD may not be in their best state of health.
The extent of laboratory testing depends on the child’s clinical status and the complexity of the planned procedure. General recommendations for blood testing include (1) hematocrit or hemoglobin if the child is pale, cyanotic, or undergoing a procedure with the potential for significant blood loss; (2) serum electrolytes for patients with renal dysfunction or those receiving diuretics, angiotensin-converting enzyme inhibitors, or digoxin (although preoperative electrolyte disturbances in children and young adults presenting for cardiac surgery are uncommon ( ); (3) platelet count and coagulation studies for cyanotic children or those on anticoagulants or antiplatelet agents; and (4) blood typing and cross-matching if significant blood loss is anticipated. A chest radiograph should be obtained with new cardiorespiratory symptoms or abnormal findings on clinical examination, or if dictated by the surgical procedure. Cardiologic studies should be coordinated with the child’s cardiologist, because some tests will have been recently completed, and investigations such as cardiac catheterization, Holter monitoring, exercise stress tests, and cardiac MRI mandate the cardiologist’s input. The electrocardiogram is reviewed for rhythm abnormalities, impaired conduction, chamber enlargement, and ischemia. Changes from prior studies need to be explained before proceeding. Preoperative consultation with the cardiologist is essential for patients with pacemakers, with evaluation of the pacemaker and a clear plan made for appropriate adjustment on the day of surgery (see earlier) ( ). In all but the simplest lesions, recent (within 3 to 6 months) echocardiography is probably useful to document the current status of anatomy and ventricular function.
Institutional practices vary in the provision of anesthetic care for the highest-risk children and adults with CHD. The aging of the CHD population has also resulted in adults with CHD undergoing cardiac and noncardiac procedures at pediatric institutions ( ; ; ; ). In some children’s hospitals, anesthesia is provided by those who routinely practice pediatric cardiac anesthesia, while in others, pediatric anesthesiologists who do not practice cardiac anesthesia provide the care ( ; ). In one academic anesthesia department, providers who did not practice cardiac anesthesia were found to have low levels of knowledge and comfort providing perioperative or obstetric care to adults with CHD ( ). The important point is that the case be assigned to an anesthesiologist who will understand the cardiac pathophysiology and know how to prevent and promptly deal with cardiac-related complications.
Ideally, patients with significant CHD should be scheduled for surgery early in the day. Clinical advantages include minimizing the effects of dehydration on hemodynamic function (particularly in infants and in cases of obstructive lesions, shunt-dependent circulations, cyanotic disease, single-ventricle physiology), possible reduction in the increased myocardial oxygen consumption associated with prolonged anxiety, provision of additional time to monitor patients in the postanesthesia care unit if discharge to home is being considered, greater availability of additional support if necessary, and avoidance of multiple care teams.
Standard principles of anesthetic management apply to patients with CHD who present for noncardiac procedures. The choice of anesthetic technique (local, regional, or general anesthesia), selection of pharmacologic agents, and requirement for invasive monitoring are determined by the physical (functional) status of the patient and the nature of the planned procedure. As for cardiac surgery, there is no one-size-fits-all approach. The perioperative administration and holding of cardiac medications are discussed in the preceding section. The patient’s cardiologist should discuss with the surgeon the withholding of aspirin, other antiplatelet agents, and Coumadin. Depending on the circumstances, institution of an appropriate perioperative anticoagulation strategy may be necessary. In general, patients with prosthetic valves or a history of stroke and other thromboembolic events mandate the greatest degree of concern. Approaches to anticoagulation vary and depend to some extent on both the specific indication and the degree of concern regarding the relative risks. Management strategies vary from temporarily discontinuing Coumadin (and usually following the prothrombin time and international normalized ratio [INR] and performing the surgery in the ensuing 24 to 48 hours) or discontinuing Coumadin and instituting low-molecular-weight heparin or IV heparin prior to surgery.
An intravenous induction for general anesthesia is recommended for patients with limited cardiac reserve, particularly those at risk for a marked decrease in cardiac output or circulatory instability with induction. Such high-risk cases include the more severe presentations of ventricular dysfunction, left-sided obstruction, pulmonary hypertension, and Williams syndrome ( ). Dependency on a systemic-to-pulmonary artery shunt (and thus maintenance of adequate cardiac output and peripheral resistance, as well as avoidance of increased PVR) may also be better served by a hemodynamically stable intravenous induction, particularly if there is evidence (e.g., echocardiographic, history of decreasing arterial oxygen saturation, need for supplemental O 2 , or rising hemoglobin) of shunt narrowing or outgrowth over time. Although less of an issue with sevoflurane, patients with R-L shunts have a slower inhalation induction because of the effects of reduced
p ( ). There is no technique that guarantees preservation of myocardial function and hemodynamic stability during anesthetic induction at the extremes of ventricular dysfunction (e.g., severe end-stage dilated cardiomyopathy), valvar obstruction, or pulmonary hypertension; reasons are multiple and include decreased sympathetic tone (resulting in vasodilation) and the deleterious effects of institution of positive pressure ventilation. Clearly, only absolutely necessary or emergent procedures should be undertaken for such patients. Preoperative treatment with inotropes or inodilators (e.g., milrinone) or both for patients with severe ventricular dysfunction, and balloon dilation of severe valvar stenosis, are examples of preoperative optimization techniques that should be considered. In addition to including inotropic support, the ability to rapidly convert to support with extracorporeal membrane oxygenation should be available in some situations ( ; ; ).
Standard monitoring according to the guidelines of the American Society of Anesthesiologists is recommended, with the need for invasive monitoring determined by the physical status of the patient and the complexity of the planned procedure. Clinical assessment is important and includes skin color (pallor, mottling, duskiness) and temperature (cool peripheries), quality of the pulse, capillary refill time, breathing pattern, and core temperature (one cause of increasing temperature is low cardiac output). As discussed earlier, the site of previous Blalock-Taussig (BT) shunts, the presence or history of aortic arch obstruction or interruption, and a history of multiple cardiac catheterizations need to be considered when choosing a site for the blood pressure cuff or arterial catheter. Classic BT shunts (subclavian artery–to–pulmonary artery anastomosis with ligation of distal subclavian artery) result in decreased or absent pulses on the side of the shunt. A modified BT shunt (synthetic graft between subclavian or innominate artery and pulmonary artery) usually does not but can result in stenosis of the subclavian artery with decreased pressure on the side of the shunt. The left subclavian artery is sometimes used as part of a flap in the repair of CoA. Lower limb blood pressures may be lower after narrowing or occlusion of the femoral arteries as a result of cardiac catheterization or as a result of a technically inadequate repair (e.g., residual or recurrent coarctation, arch obstruction in patients with HLHS). Arterial access may be limited after radial artery cutdowns or femoral artery occlusion from multiple cardiac catheterizations.
Venous access may be limited as a result of multiple surgical procedures, cardiac catheterizations, and intensive care admissions. Multiple cardiac catheterizations can also lead to femoral vein occlusion. It is imperative to avoid the introduction of air into the venous system, leading to systemic air embolization, in patients with intracardiac or intravascular communications and the potential for R-L shunting. For the more complex surgical procedures, central venous catheters allow monitoring of trends in central venous pressure and oxygen saturation, as well as fluid and inotrope administration ( ). Internal jugular or subclavian vein catheters can be quite useful but also carry a risk for thrombosis in the superior vena cava, as well as postoperative catheter-associated infection. The thrombotic complication can be disastrous in SV patients with a cavopulmonary anastomosis; consideration should be given to placing a femoral venous catheter if central access is necessary in such patients. Central venous catheters should be used for the shortest duration possible, access frequency should be limited, and special access ports and disinfection techniques should be employed to reduce the risk for catheter-associated infection. A low-dosage postoperative heparin infusion (10 IU/kg per hour) through the catheter can reduce the risk for thrombosis. Urinary catheters are placed as a guide to the adequacy of cardiac output and renal perfusion if major fluid shifts or blood losses are anticipated or if the procedure will be prolonged.
Practice guidelines for perioperative transesophageal echocardiography (TEE) have been published ( ). TEE has been shown in adults to be useful for intraoperative assessment of ventricular preload and function, but the often complex anatomy in patients with CHD mandates additional expertise ( ; ; ). Although TEE, and sometimes intermittent transthoracic echocardiography, is widely used in patients with and without CHD undergoing noncardiac procedures, point-of-care ultrasound (POCUS) is becoming an integral part of anesthesia practice and is now being taught in medical schools ( ; ; ).
Pulmonary artery catheters (PAC) are used much more sparingly in the current era, and in many instances ultrasound provides superior and more accurate information. Evidence generally supporting the use of PAC is mostly level IV (nonrandomized, historical controls, expert opinion) ( ). The risks are increased in the setting of pulmonary hypertension, and correct placement may be more difficult in some patients with CHD (absence of pulsatile flow, need to traverse atrial baffles). If desired for monitoring, PACs can be placed under fluoroscopic guidance in the cardiac catheterization laboratory. Although usually not essential, it may be helpful in patients with very severe ventricular dysfunction or poor Fontan physiology undergoing major noncardiac surgery (e.g., spinal fusion) in the prone position; in this situation TEE carries the risk of accidental extubation.
The preoperative clinical status, nature of the surgical procedure, and perioperative course determine whether postoperative admission will be to a general ward, a cardiology ward, an intensive care unit, or the patient discharged to home. Hemorrhage, hypoxia, hypoventilation, fever, uncontrolled pain, or myocardial ischemia may convert a well-tolerated surgical procedure into a crisis. Day surgery is possible for those patients whose cardiac status is well controlled, who have undergone a minor surgical procedure, and who have attained their baseline status prior to discharge. Higher-risk patients, particularly those undergoing major surgical procedures, are usually best managed in an intensive care setting or on a cardiology ward rather than a general (surgical) ward if changes in cardiac physiology pose a greater risk than complications from the surgical procedure. The healthier cardiac patient with a more complex surgical procedure is usually admitted to the appropriate surgical ward with cardiology consultation and follow-up. Standard fluid replacement guidelines are employed, and postoperative laboratory testing is guided by intraoperative blood loss, fluid shifts, and the child’s preoperative status. Effective pain management is important and can be especially difficult if the patient is tolerant to sedatives and narcotics or has recently been discharged after prolonged hospitalization and is still on a withdrawal regimen. Involvement of colleagues with pediatric pain management expertise is recommended. Despite the best possible anesthetic and procedure, complications could still develop in the postoperative period. Patients with PAH, Eisenmenger syndrome, or preexisting end organ dysfunction are especially prone to complications ( ).
An atrial septal defect is a communication between the left and right atria, and it may be single or multiple, vary widely in size, be located within or external to the atrial septum, and be isolated or associated with other congenital heart defects (50%). ASDs are classified by their location ( Fig. 30.6 ). The four morphologic types are the secundum ASD ( ![]() ), primum ASD ( Fig. 30.7 ,
), primum ASD ( Fig. 30.7 , ![]() – ), sinus venosus ASD, and coronary sinus ASD. Strictly speaking, only secundum and primum ASDs are defects in the interatrial septum. A secundum ASD lies within the fossa ovalis and is due to single or multiple defects in the septum primum ( ). Isolated ostium primum ASDs, also known as partial atrioventricular canal defects, extend from the anterior-inferior margin of the fossa ovalis to the atrioventricular valves, and are discussed later (see Atrioventricular Canal Defects). In a sinus venosus ASD, there is a communication between the right pulmonary vein(s) and the cardiac end of the superior vena cava (over 80%) or the posterior-inferior atrial wall just above the inferior vena cava-right atrial junction; there is no direct communication between the RA and LA ( ). A coronary sinus ASD comprises partial or complete unroofing of the tissue between the coronary sinus and the left atrium. Patent foramen ovale (PFO) is a normal interatrial communication during fetal life and is seen in almost all newborns. A probe-patent foramen ovale, present in up to 25% of adults, is not a true defect in the atrial septum but results from incomplete fusion of the septum primum and the superior limbic band of the fossa ovalis (septum secundum). Paradoxical embolism is a major anesthetic consideration in these patients.
– ), sinus venosus ASD, and coronary sinus ASD. Strictly speaking, only secundum and primum ASDs are defects in the interatrial septum. A secundum ASD lies within the fossa ovalis and is due to single or multiple defects in the septum primum ( ). Isolated ostium primum ASDs, also known as partial atrioventricular canal defects, extend from the anterior-inferior margin of the fossa ovalis to the atrioventricular valves, and are discussed later (see Atrioventricular Canal Defects). In a sinus venosus ASD, there is a communication between the right pulmonary vein(s) and the cardiac end of the superior vena cava (over 80%) or the posterior-inferior atrial wall just above the inferior vena cava-right atrial junction; there is no direct communication between the RA and LA ( ). A coronary sinus ASD comprises partial or complete unroofing of the tissue between the coronary sinus and the left atrium. Patent foramen ovale (PFO) is a normal interatrial communication during fetal life and is seen in almost all newborns. A probe-patent foramen ovale, present in up to 25% of adults, is not a true defect in the atrial septum but results from incomplete fusion of the septum primum and the superior limbic band of the fossa ovalis (septum secundum). Paradoxical embolism is a major anesthetic consideration in these patients.
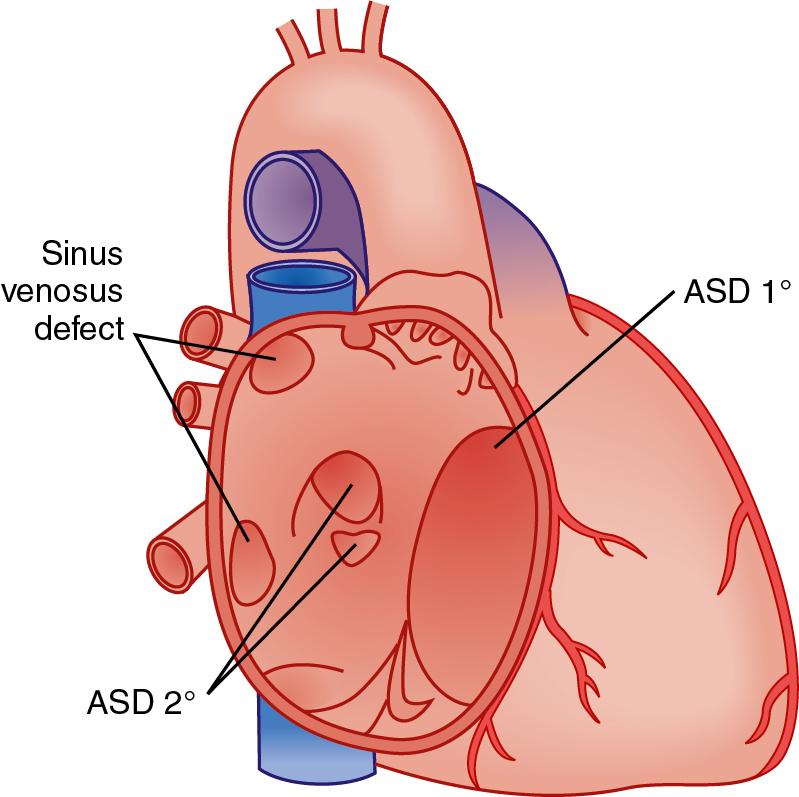
ASDs are shunts, and the amount of blood that passes through the defect is determined by the size of the defect and the relative compliance of the right and left ventricles. Early in infancy, because the right ventricle is less compliant, L-R shunting is minimal. With declines in pulmonary artery pressure and remodeling (increased compliance) of the right ventricle, shunting increases as the right atrial pressure becomes lower than the left atrial pressure. The normally decreasing left ventricular compliance with age further increases L-R shunting ( ). ASDs result in increased pulmonary blood flow and place a volume load on the right atrium, right ventricle, and left atrium. Isolated ASDs are generally asymptomatic in infancy and childhood. Although CHF is more common after 40 years of age, a few infants develop CHF as a result of pathophysiology that is incompletely understood ( ). Symptoms and the incidence of atrial fibrillation (and risk of thromboembolism) increases with advancing age ( ). The murmur of an ASD is related to increased flow through the tricuspid and/or pulmonic valve. The second heart sound is widely split and does not change with respiration.
Spontaneous closure of secundum defects is related to defect diameter. The closure rate is 100% for defects <3 mm in diameter, 87% if 3 to 5 mm, and 80% if 5 to 8 mm; defects >8 mm are unlikely to close ( ). Indications for closure are a hemodynamically significant shunt (
p:
s ≥1.5:1) with enlargement of the right heart structures ( ; ). Closure is generally done before starting school; closure beyond 25 years is associated with reduced survival ( ; ). Normal ventricular function after early repair is the rule, but ventricular dysfunction and atrial arrhythmias can occur with late repair. Pulmonary hypertension is uncommon in children with an isolated ASD ( ; ). Pulmonary vascular occlusive disease will develop in approximately 5% to 10% of unrepaired ASDs, most commonly in the second decade or thereafter, and can result in shunt reversal and Eisenmenger syndrome ( ; ). Secundum ASDs can be closed surgically or percutaneously with a device in the cardiac catheterization laboratory ( ). Primum, sinus venosus, and coronary sinus ASDs require surgical closure. Residua and sequelae are more commonly seen after repair of sinus venosus defects (loss of sinus node function, atrial fibrillation, pulmonary venous obstruction, and residual shunt) and ostium primum defects (see Atrioventricular Canal Defects) ( ).
The anesthetic goals for pre- and post-CPB management are presented in Boxes 30.3 and 30.4 , respectively. The anesthetic management for patients with an unrepaired ASD for noncardiac procedures is the same as that before CPB ( Box 30.1 ).
Maintain heart rate, contractility, and preload to maintain cardiac output.
A reduction in cardiac output compromises systemic perfusion, despite a relatively high pulmonary blood flow.
Avoid maneuvers that decrease the PVR-to-SVR ratio.
The increase in pulmonary blood flow that accompanies a reduced PVR-to-SVR ratio necessitates an increase in cardiac output to maintain systemic blood flow, thereby compromising systemic (e.g., coronary, cerebral, GI, renal) perfusion.
Maintain normoxia and normocarbia or mild hypercarbia.
Some patients overcirculate (excessive pulmonary blood flow) despite these measures, and it may be necessary to support systemic perfusion prior to CPB, with additional volume expansion or inotropes (or both).
The right pulmonary artery can be partially or completely occluded to mechanically control the magnitude of a left-to-right shunt and thus support systemic perfusion once adequate surgical exposure is attained.
Avoid large increases in the PVR-to-SVR ratio.
A substantial increase may result in production of a right-to-left shunt.
When a right-to-left shunt exists, ventilatory measures to decrease PVR should be entertained, and SVR must be maintained or increased.
These measures will reduce the magnitude of the right-to-left shunt.
A ventricular septal defect is the most common congenital cardiac defect (2.5 per 1000 live births) and is an opening in the ventricular septum permitting communication between the left and right ventricles ( ; ; ). VSDs may occur in isolation, but in nearly 50% will have an additional cardiac anomaly ( ). VSDs are classified by their location in the septum, with multiple classifications existing. The physiologic effects are determined by the degree of shunting, which is influenced by the size of the defect and the relative pulmonary and systemic vascular resistances. Smaller (restrictive) defects limit shunting and have a large pressure gradient across the defect. Large (nonrestrictive) defects have less pressure difference between the ventricles, so the magnitude and direction of shunting are more dependent on the relative pulmonary and systemic vascular resistances. The high PVR at birth limits L-R shunting, but the magnitude of the shunt increases over the first few months of life because of the normal postnatal decline in PVR; there is also a contribution from physiologic anemia of the infant (hematocrit has a disproportionately greater effect on PVR than SVR) ( ). Excessive L-R shunting with increased pulmonary blood flow leads to pulmonary vascular congestion, abnormal pulmonary mechanics (decreased compliance, increased airway resistance, airway compression), increased myocardial work, and pulmonary hypertension ( ; ). Congestive symptoms appear with a
p:
s ≥1.5. Pulmonary hypertension is reversible initially but becomes increasingly fixed as PVOD develops. Although the timing can be variable, irreversible pulmonary vascular disease is uncommon in infancy ( ).
Most VSDs are small, and many will decrease in size or close spontaneously ( ). Indications for surgical repair in infancy are heart failure refractory to medical management (poor growth), pulmonary artery pressure that fails to fall below 60% of LV pressure by 6 months of age, and aortic regurgitation associated with heart failure ( ). Although patients with irreversible or severe PVOD (PVR 6 to 9 Wood units/m 2 ) and pulmonary hypertension (Eisenmenger complex) are generally not candidates for VSD closure, these criteria are not rigid ( ). Over the long term, myocardial and pulmonary function are likely to be normal if complete repair is done within the first year or two of life ( ). Potential sequelae following repair include residual defects, heart block, subaortic obstruction, and aortic regurgitation ( ). Device closure of muscular VSDs, particularly difficult-to-reach apical and anterior septal defects, is possible in the catheterization laboratory provided the device will not impinge on the atrioventricular or semilunar valves or cause heart block ( ). Perventricular VSD device closure is a hybrid approach combining surgical and percutaneous techniques without CPB ( ). The hybrid technique is an option for very small patients, difficult-to-reach defects, or those with limited vascular access ( ). Although early studies of device closure for perimembranous defects found a significant risk of heart block and aortic regurgitation, results with new devices are more encouraging ( ; ; ; ).
Preoperative assessment should focus on the degree of systemic circulatory compromise and control of pulmonary vascular tone. Signs and symptoms of significant CHF in infants include (1) respiratory: tachypnea, grunting, flaring, retractions, pulmonary congestion on chest x-ray; (2) growth and nutrition: poor feeding, poor growth, failure to thrive; and (3) cardiovascular: tachycardia, diminished pulses, decreased peripheral perfusion, mottling, hepatic congestion (hepatomegaly). The anesthetic goals for pre- and post-CPB management are presented in Boxes 30.3 and 30.4 , respectively. Patients with relatively well-preserved ventricular function, smaller defects, and absence of severe pulmonary hypertension are suitable for an inhalational induction. Risks include large decreases in PVR with resultant decreased systemic flow and hypotension; conversely, loss of the airway will increase PVR that can lead to R-L shunting with desaturation.
The anesthetic management for patients with an unrepaired VSD for noncardiac procedures is the same as that before CPB ( Box 30.3 ).
Atrioventricular canal defects (AVCDs), also referred to as atrioventricular septal defects or endocardial cushion defects, result from abnormal endocardial cushion development. Embryologically, four endocardial cushions develop in the atrioventricular canal and contribute to the development of the lower atrial septum; the upper, posterior inlet portion of the ventricular septum; and the mitral and tricuspid valves. AVCDs may be complete (common AV valve with ostium primum ASD, moderate to large inlet VSD, and varying chordal attachments to the crest of the ventricular septum) ( Fig. 30.8 , ![]() – ); partial (two distinct AV valves with ostium primum ASD and usually a cleft in the anterior leaflet of the mitral valve; no VSD is present); or transitional (ostium primum ASD, restrictive VSD, common AV valve orifice with fused anterior and posterior bridging leaflets dividing the common AV valve into distinct mitral and tricuspid components). AVCDs are commonly associated with extracardiac defects and are present in approximately 45% of children with Down syndrome ( ; ). Abnormalities in alveolar and lung vascular development predispose children with Down syndrome to early pulmonary hypertensive arterial changes ( ; ).
– ); partial (two distinct AV valves with ostium primum ASD and usually a cleft in the anterior leaflet of the mitral valve; no VSD is present); or transitional (ostium primum ASD, restrictive VSD, common AV valve orifice with fused anterior and posterior bridging leaflets dividing the common AV valve into distinct mitral and tricuspid components). AVCDs are commonly associated with extracardiac defects and are present in approximately 45% of children with Down syndrome ( ; ). Abnormalities in alveolar and lung vascular development predispose children with Down syndrome to early pulmonary hypertensive arterial changes ( ; ).
The physiology resembles that of atrial and ventricular septal defects, with the possible added feature of AV valve regurgitation. There is an L-R shunt with increased pulmonary blood flow and volume overloading of the right and left ventricles, with the magnitude of shunting largely determined by the size of the VSD component. Large ventricular level shunts, although predominantly left to right, usually have a bidirectional component. Surgery for complete AV canal defects is usually undertaken in the first 2 to 3 months of life because of the risk for accelerated pulmonary vascular disease. Surgery is usually done later in infancy for partial and transitional AV canals because the pulmonary vasculature is protected and the normal AV valve tissue is thin and fragile ( ). The repair typically involves dividing the common AV valve and closing the ASD and VSD with either one or two patches, approximation and suspension of the valve apparatus, and suturing any cleft in the mitral valve.
The induction and maintenance of anesthesia is like that for VSDs, in that patients with relatively well-preserved ventricular function, partial or transitional defects, and absence of severe pulmonary hypertension are suitable for an inhalational induction ( Box 30.3 ). The young infant with a complete AVCD usually has a very compromised systemic circulation (myocardial dysfunction, intravascular volume depletion due to diuretics and fasting) such that hypotension can develop at low concentrations of sevoflurane. This is especially a problem with Down syndrome, whereby placement of an oropharyngeal airway can result in coughing and laryngospasm leading to increased PVR with shunt reversal and rapid desaturation. Consideration should be given to an IV (or sometimes intramuscular [IM]) induction. Post-CPB anesthetic goals are presented in Box 30.4 . Potential sequelae following repair include residual septal defects, AV valve regurgitation or stenosis (especially mitral) or both, AV block, subaortic obstruction, and occasionally pulmonary hypertension; the last is more likely in patients with Down syndrome.
Maintain heart rate (preferably sinus rhythm) at an age-appropriate rate.
Cardiac output is likely to be more heart-rate dependent in the post-CPB period.
Reduce PVR through ventilatory interventions.
This is particularly important if PVOD is present.
Inotropic support of the right ventricle may be necessary, particularly if PVR is high. In patients with VSD or AVC, the left ventricle will no longer contribute to ejection of blood via the pulmonary artery, and the right ventricle will face a high afterload.
Dobutamine (5–10 mcg/kg per min) or dopamine (5–10 mcg/kg per min) provides inotropic support without increasing PVR.
Milrinone (0.5–1.0 mcg/kg per min) after a loading dose of 50 mcg/kg has more direct PVR-reducing effects in addition to inotropic, lusitropic, and SVR-reducing effects.
Infants with large VSDs or AVC defects, particularly in association with trisomy 21, are candidates for development of severe pulmonary hypertension and hypertensive crises after CPB.
Consider inhaled nitric oxide (iNO) when pulmonary hypertension and resultant RV dysfunction and low cardiac output persist.
ASD, Atrial septal defect; AVC, atrioventricular canal; PVOD, pulmonary vascular occlusive disease; PVR, pulmonary vascular resistance; SVR, systemic vascular resistance; VSD, ventricular septal defect.
The anesthetic management for patients with unrepaired AVCDs for noncardiac procedures is the same as that before CPB ( Box 30.3 ).
The ductus arteriosus normally connects the origin of the left main pulmonary artery to the aorta and is a critical component of the fetal circulation ( Fig. 30.9 A). In utero, the high pulmonary vascular resistance results in most (∼90% to 95%) of the blood from the RV bypassing the lungs via flow through the ductus arteriosus to the descending aorta ( ). In most newborns, the ductus closes in two phases. Within 24 to 48 hours of birth, the smooth muscle contracts in response to an increase in Pao 2 and decreases in PGE 2 and PGI 2 as a result of placental removal and pulmonary metabolism. A zone of hypoxia in the media causes smooth muscle cell death and production of hypoxia-inducible growth factors, leading to endothelial proliferation, subintimal disruption, fibrosis, and permanent closure by 2 to 3 weeks of age. If not fully obliterated, a connection remains between the systemic and pulmonary circulations. The PDA may be isolated ( Fig. 30.9 ) or associated with almost any other congenital cardiac anomaly, in many instances of which it is lifesaving (ductal-dependent lesions). Isolated PDA is associated with increasing L-R shunting as the pulmonary vascular resistance falls.
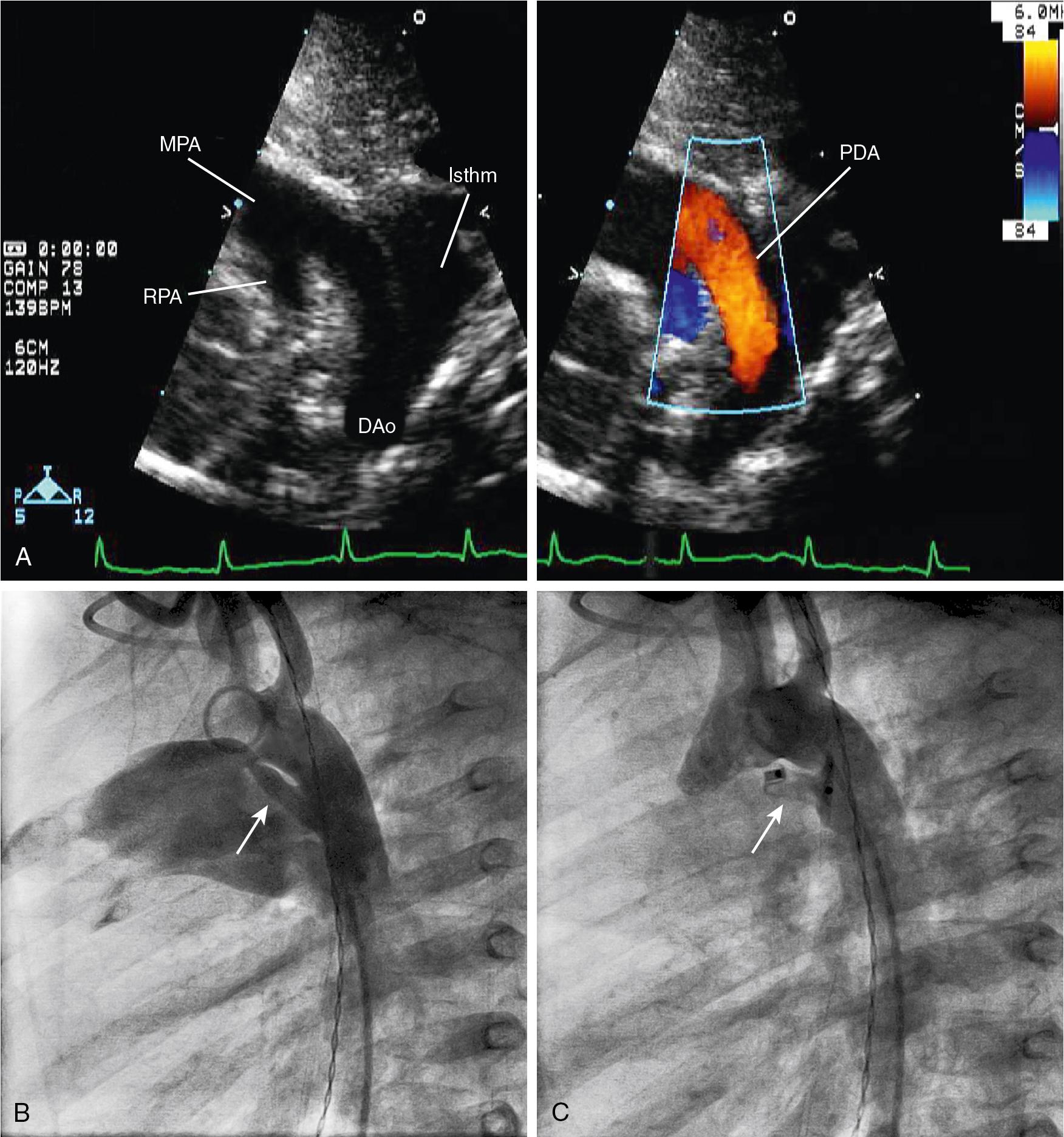
A tiny or small PDA is asymptomatic and associated with normal life expectancy, but in children and young adults closure is generally recommended because of the risk of infective endarteritis ( ; ). With a hemodynamically significant ductus, excessive blood flow to the lungs results in left heart and aortic enlargement, and CHF with very large shunts. Elevation of left atrial pressure may cause enlargement of a PFO with additional L-R shunting. In premature infants with respiratory distress, hypoxia promotes continued patency of the ductus, with the need for more aggressive ventilation. Runoff from the aorta into the pulmonary circulation during diastole reduces aortic diastolic pressure with decreased coronary, cerebral, and abdominal organ perfusion. Necrotizing enterocolitis and intracerebral and intraventricular hemorrhages are potential complications. See Chapter 27 : Neonatology for Anesthesiologists. Other cardiac anomalies determine the direction of shunting across a PDA. For example, with severe aortic coarctation or hypoplastic left heart syndrome, the shunting is predominantly right to left, and in fact is necessary to support systemic perfusion until more definitive interventions are undertaken.
In neonates and preterm infants, there is still no consensus on which PDAs to treat, as well as when and how to treat ( ; ). Closure of a PDA can be accomplished pharmacologically in preterm infants with indomethacin, ibuprofen, or acetaminophen ( ). Surgical management is typically via a small left posterolateral thoracotomy. For older infants and children with a small- to moderate-sized ductus, video-assisted thoracoscopic surgery (VATS) is an option ( ![]() – ). Percutaneous closure using interventional catheterization techniques is an established procedure with a 97% to 99% success rate, and is frequently employed as an alternative to surgical ligation ( ). Transcatheter techniques can also be used for preterm and term neonates ( Fig. 30.9 B–C;
– ). Percutaneous closure using interventional catheterization techniques is an established procedure with a 97% to 99% success rate, and is frequently employed as an alternative to surgical ligation ( ). Transcatheter techniques can also be used for preterm and term neonates ( Fig. 30.9 B–C; ![]() – ) ( ; ). Advantages include the ability to perform the procedure on an outpatient basis, reduced surgical scarring, less pain, faster recovery, and avoidance of late thoracotomy complications (cosmetic defects, rib fusion, scoliosis, chronic pain). Percutaneous closure, however, has its own set of risks beyond the usual risks of cardiac catheterization. Adverse events are more frequent in infants younger than 6 months and are mostly related to device or coil placement itself; these include embolization and malposition (more frequent with coils) ( ). Transcatheter techniques are best suited for a restrictive ductus with some length and an ampulla at the aortic end ( ). Understanding the genetics of development of the ductus arteriosus may lead to new strategies of management ( ).
– ) ( ; ). Advantages include the ability to perform the procedure on an outpatient basis, reduced surgical scarring, less pain, faster recovery, and avoidance of late thoracotomy complications (cosmetic defects, rib fusion, scoliosis, chronic pain). Percutaneous closure, however, has its own set of risks beyond the usual risks of cardiac catheterization. Adverse events are more frequent in infants younger than 6 months and are mostly related to device or coil placement itself; these include embolization and malposition (more frequent with coils) ( ). Transcatheter techniques are best suited for a restrictive ductus with some length and an ampulla at the aortic end ( ). Understanding the genetics of development of the ductus arteriosus may lead to new strategies of management ( ).
Anesthetic management for PDA ligation depends on patient age, clinical status, and interventional technique. Adequate vascular access and immediate availability of blood (at least one blood volume) are essential for all surgical procedures in the event of uncontrolled bleeding. Physiologic goals are similar to other patients with L-R shunts ( Box 30.3 ).
The highest risk and majority of patients are critically ill premature/very low birth weight neonates who are ventilator dependent because of respiratory distress syndrome. Systemic blood pressure and perfusion are frequently borderline. Transport of these neonates to the operating room can be hazardous, with the risks of accidental extubation, inadequate attention to ventilation, line disruption, and excessive cooling. Many centers perform PDA ligation in the neonatal ICU, either as routine practice or in cases where transport seems especially risky, but the unfamiliarity of the environment, available personnel, relative lack of surgical equipment should a complication arise, adequacy of lighting, and sterility need to be considered. Major anesthetic risks are hypoxemia during transfer of the baby from the incubator to the bed and accidental extubation during the case. Rapid placement of ECG leads is critical to identify hypoxia-induced bradycardia. Hemodynamic goals are to balance the PVR and SVR. Maneuvers that decrease PVR, in particular, as well as those that reduce myocardial contractility and SVR, should be avoided. Dopamine is often already in use or may be necessary to maintain arterial blood pressure. What constitutes a normal blood pressure or hypotension with decreased cerebral perfusion, particularly in the preterm neonate, remains to be determined ( ). Conversely, cerebral hyperperfusion increases the risk of cerebral hemorrhage. Pressure autoregulation monitoring may allow for optimization of cerebral perfusion for the individual patient ( ). A high-dose narcotic-based technique (e.g., fentanyl 25 to 50 mcg/kg, with a muscle relaxant) is usually well tolerated ( ). The desire to use less narcotic and supplement with low-dosage inhaled agent or sedative may be limited by the hemodynamics. Although invasive arterial pressure monitoring can be helpful in the sickest patients, it can be technically challenging and is not part of our routine practice. Blood pressure cuffs and pulse oximeters should be placed on the upper and lower extremities because clipping of the descending aorta is a known risk factor (the PDA can be larger than either the PA or the aorta). The right arm may be preferable in case the surgeon needs to place a clamp on the aorta if control of the aortic end of the ductus is lost during dissection or ligation. In neonates, retraction of the lung by the surgeon obviates the need for lung isolation. Not infrequently, the end-tidal CO 2 can drop into the single digits or disappear so that placement of a 9 Fr esophageal stethoscope can allow for prompt identification of accidental extubation. A murmur may not be heard if the PAP is very high. Vigilance for plugging of the small endotracheal tube is important. After ligation and/or clipping of the ductus, there is usually an acute increase in blood pressure, especially the diastolic, and disappearance of any murmur. As blood pressure can be labile following closure of the ductus and postoperatively, elevated blood pressure should be treated carefully. With an increase in LV afterload, increased inotropic support of the severely dysfunctional ventricle may be necessary. Mechanical ventilation is continued postoperatively. The most significant surgical risk is profound hemorrhage resulting from disruption or tearing of the ductus; other risk factors are inadvertent ligation of the left pulmonary artery or descending aorta, and damage to the recurrent laryngeal nerve (RLN).
Older infants and children with an isolated PDA undergoing surgical or catheterization procedures can usually be extubated at the end of the procedure, allowing for a variety of anesthetic techniques. Depending on the patient, an endobronchial blocker or double-lumen tube can be used. It is important to ensure adequate lung reexpansion, particularly if a thoracostomy tube is not left in situ, at the end of the procedure. Neuromonitoring using direct intraoperative stimulation of the RLN and evoked electromyogram monitoring to reduce the incidence of RLN injury has been described ( ). After uncomplicated repair and the absence of pulmonary hypertension, cardiovascular function should be normal so that future anesthetic management is straightforward.
Tetralogy of Fallot is the most common cyanotic lesion, accounting for approximately 10% of all CHD and with a birth prevalence of 0.34 per 1000 live births ( ; ). The defect is characterized by anterocephalad deviation of the outlet (conal) septum, resulting in right ventricular outflow tract obstruction (RVOTO), a nonrestrictive VSD, aortic override, and right ventricular hypertrophy ( Fig. 30.10 , ![]() – ). The RVOTO can be present at multiple levels: infundibular or subvalvar, valvar, supravalvar (hypoplasia of the main and branch pulmonary arteries), or a combination thereof. Infundibular obstruction may be fixed or dynamic (varying caliber of the RV infundibulum) and is invariably associated with some degree of pulmonary valve stenosis. Associated cardiac anomalies include a right-sided aortic arch with mirror image branching (25%), a second VSD (5%), and coronary artery anomalies (5%) such as anterior descending coronary artery arising from the right coronary artery and crossing the RV outflow tract, dual left anterior descending (LAD), and single right coronary artery ( ). At the extremes of the TOF spectrum are the “pink Tet” (large VSD with minimal RVOTO and pulmonary overcirculation) and TOF with pulmonary atresia or extreme pulmonary hypoplasia and aortopulmonary collaterals. Other less common variants of TOF include TOF with absent pulmonary valve, TOF with complete atrioventricular canal (CAVC) defect, and TOF with double-outlet RV. Chromosomal abnormalities are present in up to 25% of cases, with DiGeorge/velocardiofacial (22q11.2 microdeletions) and Trisomy 21 the most frequent. Other associations are Alagille, VACTERL, and CHARGE ( ).
– ). The RVOTO can be present at multiple levels: infundibular or subvalvar, valvar, supravalvar (hypoplasia of the main and branch pulmonary arteries), or a combination thereof. Infundibular obstruction may be fixed or dynamic (varying caliber of the RV infundibulum) and is invariably associated with some degree of pulmonary valve stenosis. Associated cardiac anomalies include a right-sided aortic arch with mirror image branching (25%), a second VSD (5%), and coronary artery anomalies (5%) such as anterior descending coronary artery arising from the right coronary artery and crossing the RV outflow tract, dual left anterior descending (LAD), and single right coronary artery ( ). At the extremes of the TOF spectrum are the “pink Tet” (large VSD with minimal RVOTO and pulmonary overcirculation) and TOF with pulmonary atresia or extreme pulmonary hypoplasia and aortopulmonary collaterals. Other less common variants of TOF include TOF with absent pulmonary valve, TOF with complete atrioventricular canal (CAVC) defect, and TOF with double-outlet RV. Chromosomal abnormalities are present in up to 25% of cases, with DiGeorge/velocardiofacial (22q11.2 microdeletions) and Trisomy 21 the most frequent. Other associations are Alagille, VACTERL, and CHARGE ( ).
Hypercyanotic episodes (“Tet spells”) are a hallmark of unrepaired TOF and can be life-threatening, with increased cyanosis, syncope, and convulsions. Spells occur more frequently as RVOTO gets more severe, with a peak frequency between 2 and 3 months of age. Although the mechanism of Tet spells is incompletely understood, infundibular spasm may play a role, and crying, defecation, feeding, fever, and awakening can be precipitating events. Spells are self-aggravating in that hypoxia induces a decrease in SVR, which further increases the R-L shunt. Paroxysmal hyperpnea increases oxygen consumption through increased work of breathing. Treatment of a Tet spell includes the following:
Administration of 100% oxygen.
Knee-chest position or pressure on the femoral arteries, as is commonly done in the pediatrician’s office.
Sedation with morphine sulfate (0.05 to 0.1 mg/kg IM or IV) to relieve distress and air hunger. Ketamine is a suitable alternative and can also be given IM or IV ( ).
Intravenous crystalloid (15 to 30 mL/kg) or colloid (5 to 10 mL/kg) is used to increase preload, which may increase the diameter of the RVOT.
Phenylephrine 0.5 to 1 mcg/kg IV as a bolus or 2 to 5 mcg/kg per minute as an infusion to increase the SVR and reduce R-L shunting. In the presence of severe RVOTO, phenylephrine-induced increases of PVR have little or no effect in increasing RV outflow resistance.
Sodium bicarbonate (1 to 2 mEq/kg) can be administered to correct the metabolic acidosis, increasing the SVR and lowering the PVR.
Judicious use of propranolol (0.1 mg/kg) or esmolol (0.5 mg/kg followed by an infusion of 50 to 300 mcg/kg per min) slows the heart rate and relaxes the infundibulum. β-Adrenergic agonists are contraindicated.
Failure to respond to the above or for a patient in the operating room or ICU, tracheal intubation and mechanical ventilation with 100% oxygen, low inspiratory pressures, and long expiratory times to promote venous return and antegrade flow across the RV outflow. An inhalation agent may be beneficial to reduce hyperdynamic right ventricular outflow obstruction. Manual compression of the abdominal aorta can be an effective means to temporarily increase SVR and decrease cyanosis in the anesthetized patient.
For the child undergoing cardiac repair, there may be the need to proceed very rapidly to cardiopulmonary bypass (CPB) if the spell is severe and not resolving.
Resuscitation by extracorporeal membrane oxygenation (ECMO) in refractory episodes, when immediate operative intervention is not possible ( ).
Primary complete repair in infancy has largely replaced the traditional two-stage repair sequence of a modified Blalock-Taussig shunt (mBTS) in early infancy followed by later repair ( ). Neonatal or young infant repair, however, carries significant perioperative risk so that in systems unable to provide the required postoperative care, early shunting may be necessary ( ). Because an mBTS also carries significant mortality, postoperative instability, and long-term morbidity, palliation with transcatheter techniques is an alternative approach, particularly with prematurity, low birth weight, and small pulmonary arteries ( ; ; ). Transcatheter techniques include balloon dilation of the RV outflow tract (RVOT) with/without stent placement, stenting of the PDA, and guidewire or radiofrequency perforation with/without stent placement for TOF with plate-like pulmonary valve atresia. In well-developed medical systems it is becoming fairly unusual to encounter an older infant or child with unrepaired or shunted TOF.
Surgical repair includes relief of RVOTO by resection of hypertrophied muscle bundles and enlargement of the outflow tract with a pericardial patch. Although pulmonary valve-sparing techniques are increasingly being employed and entail intraoperative balloon pulmonary valvuloplasty or placement of patches below and above the PV annulus, a very small pulmonic annulus and/or a very stenotic pulmonary valve usually dictates placement of the patch across the annulus (transannular patch), resulting in pulmonary regurgitation (PR) ( ; ). The VSD is closed with a Dacron patch; in neonates this is usually done through the right ventriculotomy created for resection of RVOT obstruction and with placement of the infundibular, pulmonary artery, or transannular patch. In infants and older children, the VSD can be closed via a trans–tricuspid valve approach, thereby avoiding the likely deleterious consequences of a right ventriculotomy. With TOF and long-segment pulmonary atresia, or when a major coronary artery crosses the RVOT, a right ventricle–to–pulmonary artery conduit is placed rather than an annular or periannular patch.
The majority of patients with repaired TOF will have residual hemodynamic and electrophysiologic abnormalities with increasing rates of morbidity and mortality beginning during the third decade of life ( ). In the first two decades after repair, many patients are asymptomatic with normal activity, although right ventricular dysfunction and dysrhythmias may only be evident on objective testing. Common indications for late reoperation or intervention are pulmonary regurgitation, residual or recurrent RVOT obstruction or RV-to-PA conduit stenosis, VSDs, arrhythmias, and ventricular dysfunction with electromechanical dyssynchrony (these problems are also shared by other lesions that require RVOT reconstruction or a RV-PA conduit such as truncus arteriosus, pulmonary atresia, and the Rastelli procedure for TGA) ( ; ). The presence of tricuspid regurgitation is a likely surrogate for substantial RV dysfunction. In adults, LV systolic and diastolic dysfunction may also be present ( ; ). Cardiac MRI is the best diagnostic modality for assessment of RV size and function and timing of pulmonary valve replacement ( ). Atrial reentrant tachycardia and high-grade ventricular arrhythmias develop in about 30% and 10% of patients, respectively ( ). The risk of sudden death is increased and estimated at 0.15%/year of follow-up ( ). Although a QRS duration of 180 milliseconds or greater is a highly sensitive marker for sustained ventricular tachycardia and sudden death, its positive predictive value is low ( ). Pulmonary valve replacement can be performed surgically or by a transcatheter technique (see section on cardiac catheterization). The ideal time is still controversial but should be performed before severe RV dilation and systolic ventricular dysfunction develop ( ; ). The presence of sustained ventricular tachycardia (VT) in a symptomatic patient (syncope, cardiac arrest) or electrophysiologic study is significant and necessitates treatment prior to elective surgery. Risk factors for VT are older age (>20 years), multiple cardiac surgeries, longer QRS duration, and left ventricular dysfunction ( ). Electrophysiologic methods are employed to assess and ablate atrial or ventricular arrhythmias and for deciding on the need for an implantable cardioverter-defibrillator (ICD) or resynchronization with biventricular pacing ( ; ).
The anesthetic and perioperative management for patients with TOF undergoing surgical repair has recently been reviewed ( ; ). The goals are similar to the management of a hypercyanotic spell as described above and are summarized in Box 30.5 .
Maintain heart rate, contractility, and preload to maintain cardiac output.
Euvolemia prevents exacerbation of dynamic RVOT obstruction from hypovolemia and reflex increases in HR and contractility.
Avoid increases in the PVR-to-SVR ratio.
Increases in PVR relative to SVR and decreases in SVR relative to PVR will increase R-L shunting, reduce pulmonary blood flow, and produce or worsen cyanosis.
Use ventilatory measures to reduce PVR.
Minimize mean airway pressure to avoid mechanical obstruction of pulmonary blood flow and effects to decrease preload.
Maintain or increase SVR.
This is particularly important when RV outflow obstruction is severe and changes in PVR have little or no effect on shunt.
Treat hypercyanosis promptly.
Avoid RV contractility depression with severe fixed RV outflow obstruction, except in patients with dynamic infundibular obstruction (Tet spell).
Reducing contractility during Tet spells may reduce RV outflow obstruction via relaxation of the infundibulum.
Precardiopulmonary Bypass: Preoperatively, it is important to avoid dehydration. β-Adrenergic antagonists (now used infrequently) should be continued until the induction of anesthesia in patients receiving these drugs for prophylaxis against hypercyanotic attacks.
Although an IV induction may be desirable, particularly in patients with significant outflow obstruction, the risk of precipitating a Tet spell with IV placement needs to be considered. Administration of adequate premedication (oral midazolam ± ketamine) can reduce this risk ( ). Mask induction of anesthesia with either sevoflurane or halothane (if still available) is usually effective, because contractility is typically preserved and there is a parallel decrease in PVR and SVR. Interestingly, halothane may better attenuate the dynamic component of RVOT obstruction than sevoflurane, because of halothane’s more potent negative inotropic effect. The increase in PVR associated with loss of the airway during a mask induction can precipitate rapid and profound desaturation. Ketamine is a useful induction agent in patients with TOF (and other cyanotic lesions) by increasing SVR and maintaining hemodynamic stability ( ). Fentanyl or sufentanil provides very stable induction and will blunt the tachycardia and stimulation-induced increases in PVR.
Regardless of induction technique, volume expansion with 10 to 15 mL/kg (or greater) of crystalloid or 5% albumin should be administered once intravenous access is obtained to avoid or treat systemic hypotension. This is particularly necessary in patients who have received nothing by mouth for a long interval before induction and is the most effective first-line therapy after airway and ventilatory management in preventing and treating dynamic RVOT obstruction. Systemic hypotension is particularly likely to cause or increase R-L shunting when RV outflow obstruction is severe, because anesthesia-induced decreases in PVR have little effect on decreasing RV outflow resistance. Fluid administration can be supplemented with phenylephrine (0.5- to 1.0-mcg/kg intravenous boluses). Maintenance of anesthesia with fentanyl or sufentanil, a muscle relaxant (perhaps avoiding pancuronium because of tachycardia and because of its effects on RV preload and contractility), and modest amounts of inhalation agent are appropriate. In addition to the periinduction period, pericardial incision and retraction and aortic and pulmonary artery manipulation are other frequent causes of Tet spell–like behavior with acute hypoxemia prior to CPB. These spells can occur in patients with no history of Tet spells. The management of a Tet spell has been discussed above. At times, rapid institution of CPB is the only effective therapy.
Postcardiopulmonary Bypass: After complete repair, the Sao 2 should be 100%, and most patients require only modest to moderate degrees of inotropic support with dopamine or dobutamine (3 to 10 mcg/kg per min). The need for substantially higher levels of support should raise the suspicion of a residual anatomic defect. Several factors may contribute to impaired RV systolic and diastolic function on weaning from CPB: (1) any preexisting dysfunction as a result of hypertrophy (decreased compliance) and cyanosis; (2) problems with myocardial preservation relating to the difficulties protecting hypertrophic muscle, increased tendency to free radical injury, and increased washout of cardioplegia due to collaterals; (3) a mechanical component of RV dysfunction because of a right ventriculotomy and RVOT patch; and (4) possible residual or new pressure and/or volume hemodynamic loads because of residual RVOT obstruction (infundibular), pulmonary regurgitation (transannular patch), or obstruction distal to the pulmonary valve (main or branch PA stenosis). RVOT obstruction should be nearly completely eliminated (gradient <20 mm Hg) after repair. A residual VSD is likely to be very poorly tolerated, with the most likely manifestation being low cardiac output syndrome associated with elevated central venous, left atrial pressure, and pulmonary arterial pressures. This results from a low (likely) PVR and compliant pulmonary vasculature, with the potential for a large L-R intracardiac shunt placing a large volume load on the LV and RV. The presence of pulmonary insufficiency will further exacerbate RV dysfunction by imposing an additional volume load. Any distal pulmonary artery stenoses, high mean airway pressures, or elevated PVR will increase the regurgitant volume and subsequent RV volume load. In neonates and young infants expected to have significant RV dysfunction, the surgeon may choose to leave a pop-off valve by leaving the PFO open or by creating a small (3- to 4-mm) atrial-level fenestration. This will allow physiologic intracardiac R-L shunting with the ability to augment systemic cardiac output at the expense of systemic oxygen saturation. In these patients, a Pao 2 of 40 to 50 mm Hg and a Sao 2 of 70% to 80% is acceptable until RV function improves over the course of days. A right bundle branch block is common. Rarely, heart block requiring temporary (or permanent) pacing can occur as a consequence of the VSD closure; a large VSD patch can also occasionally be associated with LV outflow tract obstruction.
The anesthetic management for patients with unrepaired TOF for noncardiac procedures is the same as that before CPB ( Box 30.5 ).
In older children and adults presenting for cardiac interventions or noncardiac procedures following surgical repair, the degree and etiology of RV dysfunction needs to be defined and the presence of any significant lesions and arrhythmias determined. Decreased functional status, including new onset palpitations or syncope, should prompt consultation with the pediatric cardiologist. In some cases, further medical, surgical, or catheterization management may be necessary to reduce anesthesia risk. In the presence of pulmonary regurgitation and/or RV hypertension, factors that increase PVR or are detrimental to the RV myocardial supply-demand relationship (tachycardia, hypotension, anemia, and acidosis) should be avoided. Right ventricular filling should be maintained at normal or slightly increased levels, understanding that excessive volume (or dysfunction) can lead to further RV dilation and resultant LV dysfunction (ventricular interdependence). As patients age, late development of LV dysfunction is another consideration ( ). Early administration of an inotrope to improve RV contractile performance should be considered in patients with significant RV dysfunction. (See Pulmonary Hypertension.) A means for external cardioversion or defibrillation should be readily available.
Bicuspid aortic valve occurs in 1% to 2% of the population and demonstrates a substantial male predominance ( ). It may be an isolated lesion or associated with other left-sided obstructive defects (e.g., aortic coarctation), and it may be asymptomatic or become progressively obstructive or regurgitant. The pathophysiology depends on the severity and duration of the obstruction and/or regurgitation ( ).
Critical neonatal aortic stenosis (AS) is a ductal-dependent lesion, with a clinical pattern similar to that of severe aortic coarctation. In its most severe forms, critical neonatal AS is poorly tolerated and produces left ventricular failure with poor systemic perfusion and pulmonary overcirculation (PFO, any associated ASD/VSD) with vascular congestion and edema; factors that increase myocardial O 2 demand even modestly (e.g., mild fever, anemia) can markedly exacerbate the low cardiac output state and potentially precipitate cardiovascular collapse. Supravalvar aortic stenosis (SVAS) is associated with variable-sized deletions in the elastin gene (chromosome 7q11.23) and adjacent segments and comprises a diffuse and variable arteriopathy ( ; ). SVAS is characterized by narrowing at the sinotubular junction, which in itself can cause obstruction of the coronary ostia. Associated lesions include coronary artery stenoses, main and branch pulmonary artery stenoses (37% to 75%), stenosis of the thoracic aorta that can extend into the descending aorta (middle aortic syndrome), renal artery stenosis and hypertension, and stenosis at other vascular sites ( Fig. 30.11 ) ( ). Subaortic stenosis may comprise a discrete fibromuscular ridge or membrane, a long fibromuscular tunnel, or hypertrophy of the interventricular septum (hypertrophic cardiomyopathy). Shone’s anomaly consists of aortic coarctation, subaortic stenosis, and mitral stenosis on the basis of both a parachute mitral valve and supravalvar mitral ring ( ). Although the relief of coarctation is typically straightforward, patients with Shone’s anomaly frequently have prolonged and recurrent left-sided obstruction arising from its other components, leading to reduced LV compliance and dysfunction despite one or more attempts at correction. The mitral stenosis (leading to pulmonary hypertension) and reduced ventricular function can make these patients particularly difficult to manage in the perioperative period, particularly with respect to fluid loads and shifts, anemia, fever, and stress response.
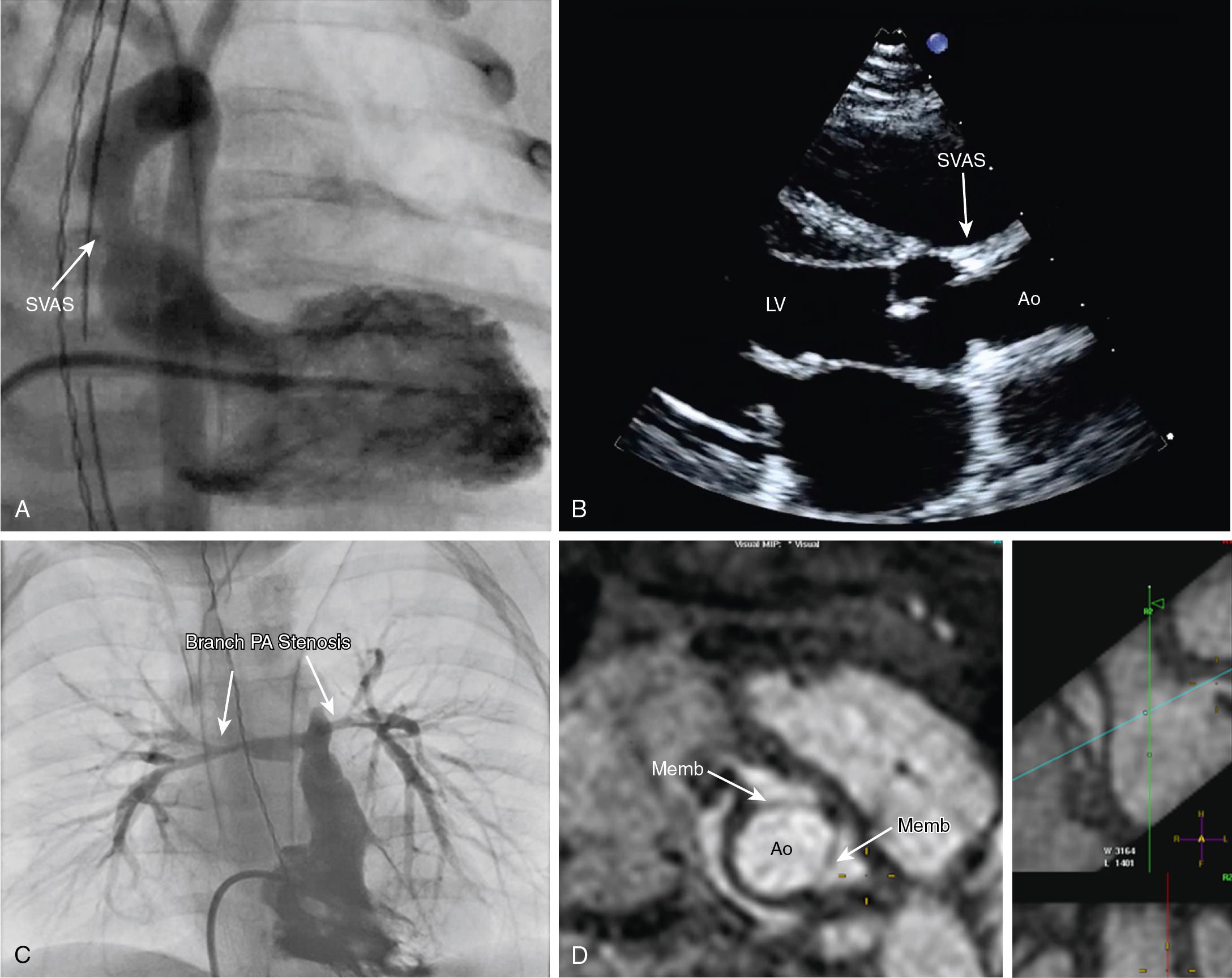
Congenital aortic valve stenosis can be relieved by surgical valvotomy or by percutaneous balloon valvuloplasty ( ; ). Although both strategies are effective in relieving the obstruction and promoting growth of the valve annulus, it is still unclear which is the better option ( ; ; ; ). The Ross operation is an alternative for unrepairable aortic valve disease, but in neonates it carries a high mortality ( ). In children and adolescents, surgical intervention becomes necessary when dilation of the stenosis is no longer effective or aortic regurgitation and consequent LV dilation becomes significant. Aortic valve repair rather than replacement is performed in many centers, obviating the risks of anticoagulation, allowing growth, and postponing aortic valve replacement (AVR).
Subaortic stenosis resulting from a discrete subaortic membrane is repaired by membrane resection. In addition to creating increased pressure within the LV, aortic valve damage and regurgitation caused by a jet arising from flow across the membrane is a common finding and an indication for surgery. Tunnel subaortic stenosis is managed by the modified Konno procedure, in which LV septal tissue is excised and a patch placed to enlarge the LV outflow tract.
Precardiopulmonary Bypass: The pathophysiology and anesthetic management of valvar aortic stenosis was discussed earlier in the section on ventricular outflow tract obstruction. Anesthetic management of the neonate with critical AS includes continuation of PGE 1 infusion (0.01 to 0.05 mcg/kg per min) pre-CPB to maintain ductal patency.
Postcardiopulmonary Bypass: Management will be influenced by the severity of preexisting ventricular dysfunction and the adequacy of myocardial protection. An inotropic agent is usually necessary to wean from CPB in the neonate and infant. Older children may only need an inotropic agent for a short period or not at all. Once the ventricle has recovered, the heart can be hyperdynamic so that infusions of a β-blocker (esmolol 100 to 300 mcg/kg per min) and/or a vasodilator (sodium nitroprusside 1 to 3 mcg/kg per min) are frequently necessary.
Anesthetic management of supravalvar aortic stenosis, whether associated with Williams (Williams-Beuren) syndrome or nonsyndromic, merits particular attention. These patients are at significantly increased risk for cardiac arrest and death during anesthesia, cardiac catheterization, and surgery ( ; ; ; ). The increased risk for sudden death appears to occur by at least two different but potentially interacting pathophysiologic features: (1) significant ventricular hypertrophy as a consequence of severe outflow obstruction; the presence of severe, bilateral (i.e., supravalvar aortic and pulmonic) obstruction and hypertrophy poses the greatest risk; (2) various forms of coronary artery obstruction. It is noteworthy that the degree of coronary involvement may not be accurately imaged by echocardiography or predicted by the degree of supravalvar aortic obstruction. Prolonged QTc, found in 13% of patients with Williams syndrome, has been proposed as a factor in sudden death ( ; ). Because torsade de pointes was not seen, the authors hypothesized that QTc prolongation was more likely the result of microvascular ischemia associated with the arteriopathy rather than a genetically determined channelopathy ( ). The lesion itself is usually repaired by resection of the stenotic area and patch placement, although more extensive aortic (or supravalvar pulmonary) reconstruction may be necessary ( ; ; ). The prebypass management is the same as anesthesia for noncardiac procedures, as discussed below. Post-CPB β-blockers and vasodilators are usually necessary if there has been good myocardial protection and repair of any coronary obstruction. Anesthetic management for noncardiac procedures, including imaging prior to repair, has been reviewed ( ; ; ). Key features include avoidance of prolonged fasting times and dehydration, hypotension, tachycardia, and other factors that compromise myocardial perfusion. Although uneventful inhalational inductions have been reported, our preference is for an IV induction with agents that maintain hemodynamic stability, as significant hypotension during induction can lead to cardiac arrest (especially when coronary artery obstruction is present) prior to establishing intravenous access ( ; ). Placement of an intravenous catheter (aided by premedication as necessary) and administration of a fluid bolus prior to or with induction is recommended. In the catheterization laboratory, both radiographic contrast injection (perhaps via its transient but significant ability to impair coronary oxygen delivery when injected into the aortic root or coronary arteries and/or occlusion of coronary blood flow by the catheter) and cardiac rhythm disturbances (as might be induced by a wire or catheter) are possible events leading to decompensation. Although not based on evidence, norepinephrine infusion can be administered should hypotension ensue or prophylactically administered during induction of anesthesia.
Coarctation of the aorta (CoA) is an arteriopathy with a focal narrowing of the upper thoracic aorta consistent with a discrete posterior shelf or invagination just opposite the insertion of the ductus arteriosus (juxtaductal) and distal to the left subclavian artery ( Fig. 30.12 , ![]() ). In some instances, this shelf may be circumferential or comprise a long segment. The precise etiology is unknown, but theories include extension of duct-like tissue into the wall of the aorta (ductal tissue theory), abnormal fetal blood flow patterns with lesions that reduce antegrade flow into the ascending aorta (hemodynamic theory), or abnormal cellular migration during arch development ( ). Not surprisingly, associations include aortic valve stenosis or bicuspid aortic valve (22% to 42%), ventricular septal defect (48%), aortic arch hypoplasia, hypoplasia of other left-sided structures (mitral valve, left ventricle, Shone syndrome), as well as intracranial aneurysms (usually of the circle of Willis; up to 10%) ( ). The pathophysiology is varied and depends on the severity of the coarctation, associated lesions, and age of the patient. A PDA is critical to provide systemic perfusion, so presentation is in infancy for all patients except those capable of tolerating ductal closure without overt hemodynamic compromise. In general, presentation beyond infancy is limited to patients with an isolated coarctation of mild to moderate severity. Critical neonatal coarctation with abrupt ductal closure results in profound circulatory collapse within the first days to a month of life, because perfusion to the lower body is dependent on patency of the ductus arteriosus and relatively high PVR. The presence of poor perfusion, acidosis, oliguria, and other signs and symptoms of acute systemic illness often results in these patients being seen emergently to rule out sepsis. Severe presentations typically involve some degree of acute myocardial dysfunction and can also include evidence of lung, liver, and renal injury. When a large VSD is also present, the aggravated degree of L-R shunting and heart failure (with elevated left atrial pressure and additional L-R shunting across the foramen ovale) results in presentation within days of birth. The initial treatment involves hemodynamic stabilization by reopening the ductus arteriosus with a PGE 1 infusion (0.01 to 0.05 mcg/kg per min). Other resuscitative measures may include intubation and mechanical ventilation, inotropic support, volume expansion, and sodium bicarbonate. Of note, once the duct is reopened, it is possible for blood pressures in the upper and lower limbs to be similar despite severe aortic narrowing. Patients who presented with severe decompensation and evidence of organ injury benefit from one or more days of restored systemic perfusion prior to surgical repair.
). In some instances, this shelf may be circumferential or comprise a long segment. The precise etiology is unknown, but theories include extension of duct-like tissue into the wall of the aorta (ductal tissue theory), abnormal fetal blood flow patterns with lesions that reduce antegrade flow into the ascending aorta (hemodynamic theory), or abnormal cellular migration during arch development ( ). Not surprisingly, associations include aortic valve stenosis or bicuspid aortic valve (22% to 42%), ventricular septal defect (48%), aortic arch hypoplasia, hypoplasia of other left-sided structures (mitral valve, left ventricle, Shone syndrome), as well as intracranial aneurysms (usually of the circle of Willis; up to 10%) ( ). The pathophysiology is varied and depends on the severity of the coarctation, associated lesions, and age of the patient. A PDA is critical to provide systemic perfusion, so presentation is in infancy for all patients except those capable of tolerating ductal closure without overt hemodynamic compromise. In general, presentation beyond infancy is limited to patients with an isolated coarctation of mild to moderate severity. Critical neonatal coarctation with abrupt ductal closure results in profound circulatory collapse within the first days to a month of life, because perfusion to the lower body is dependent on patency of the ductus arteriosus and relatively high PVR. The presence of poor perfusion, acidosis, oliguria, and other signs and symptoms of acute systemic illness often results in these patients being seen emergently to rule out sepsis. Severe presentations typically involve some degree of acute myocardial dysfunction and can also include evidence of lung, liver, and renal injury. When a large VSD is also present, the aggravated degree of L-R shunting and heart failure (with elevated left atrial pressure and additional L-R shunting across the foramen ovale) results in presentation within days of birth. The initial treatment involves hemodynamic stabilization by reopening the ductus arteriosus with a PGE 1 infusion (0.01 to 0.05 mcg/kg per min). Other resuscitative measures may include intubation and mechanical ventilation, inotropic support, volume expansion, and sodium bicarbonate. Of note, once the duct is reopened, it is possible for blood pressures in the upper and lower limbs to be similar despite severe aortic narrowing. Patients who presented with severe decompensation and evidence of organ injury benefit from one or more days of restored systemic perfusion prior to surgical repair.
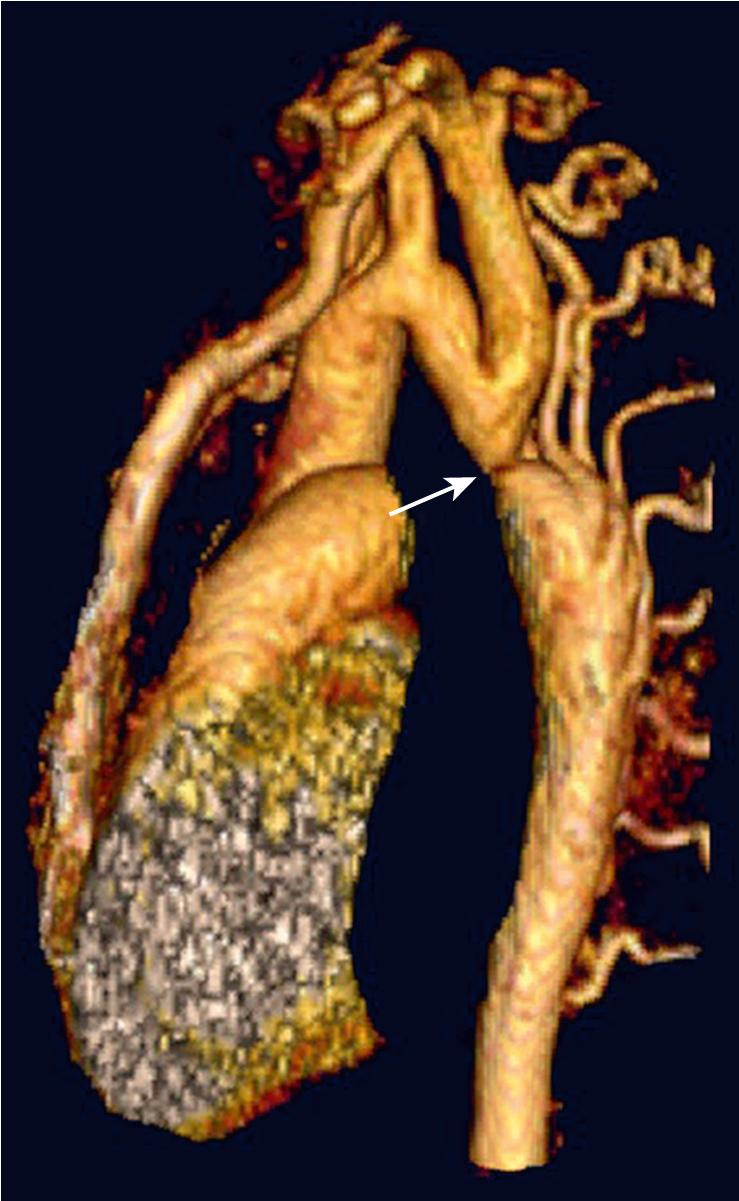
If the coarctation is not severe and closure of the ductus has occurred slowly, or if compensatory collaterals develop rapidly, presentation may be later in infancy, with tachypnea and failure to thrive. In such patients, the degree of coarctation commonly becomes more obstructive as the child grows. Blood pressure is elevated in the upper extremities, with an average gradient between upper and lower extremity sites of 30 to 40 mm Hg at rest ( ). Collaterals may originate from the internal thoracic, intercostal, subclavian, or scapular arteries, and with extensive collateral development, patients may be asymptomatic with little difference in blood pressure between the arms and legs. Large collaterals can be a source of an auscultatable bruit over the rib cage or elsewhere, as well as rib notching on a chest x-ray. Coarctation causes left ventricular hypertension and hypertrophy as well as systemic hypertension ( ). Symptoms (exercise intolerance, headache, chest pain, lower extremity claudication) are an absolute indication for surgical repair. For the asymptomatic patient, surgical indications include a blood pressure gradient between the upper and lower limbs of greater than 20 mm Hg, upper body blood pressure greater than two standard deviations above normal, or aortic diameter loss of 50% or greater.
The current strategies for CoA repair include surgical repair or catheter-based techniques (balloon aortic angioplasty, balloon-expandable stent implantation) ( ; ). Recoarctation can occur after both types of repair. Surgery comprises complete excision of the area of coarctation and surrounding ductal tissue with end-to-end anastomosis. With a hypoplastic aortic isthmus, complete excision in conjunction with an extended end-to-end or end-to-side anastomosis is performed ( ). Owing to insufficient studies and long-term follow-up of percutaneous treatment for primary repair, the superiority for the primary repair of a catheter technique over surgery has not been proven ( ; ; ). In some centers balloon angioplasty or stent angioplasty is reserved for children with recurrent coarctation after previous surgical repair or as the first-line treatment for older children and adults with newly diagnosed coarctation ( ). Paraplegia secondary to spinal cord ischemia is the most devastating complication associated with surgical repair. The incidence is relatively low (0.14% to 0.4%), and several factors are associated with an increased risk of developing paraplegia: hyperthermia, prolonged aortic cross-clamp time, elevated cerebrospinal fluid pressures, low proximal and distal aortic blood pressures, and poorly developed collaterals to the descending aorta.
In addition to recoarctation, long-term consequences of CoA increase risk of premature or sudden death resulting from persistent hypertension (over one-third of patients, even in the absence of a residual coarctation gradient), intracranial hemorrhage, heart failure (LV hypertrophy with diastolic dysfunction, premature coronary atherosclerosis), aortic aneurysm and fistula formation, aortic dissections and rupture, and endarteritis at the repair site and increased risk of premature or sudden death ( ; ; ; ; ). The etiology of persistent hypertension is multifactorial and includes reduced pulsatility of the postcoarctation descending aorta, reduced upper-body vascular responses to reactive hyperemia and glyceryl trinitrate (possibly abnormal smooth muscle relaxation or structural abnormalities of the arterial wall), aortic arch geometry, and abnormalities in baroreceptor reflexes and the renin-angiotensin-aldosterone system ( ; ; ; ). There is evidence to suggest that abnormalities in cardiovascular reflexes are already present in neonates with coarctation before repair ( ).
The newborn with critical coarctation requires medical stabilization, as outlined earlier, prior to repair. Anesthetic management includes continuation of PGE 1 infusion (0.01 to 0.05 mcg/kg per min) to maintain ductal patency, inotropic support of left ventricular function, adequate preload, and preservation of increased PVR as lower body perfusion is dependent on R-L flow across the ductus. Blood pressure above and below the coarctation should be monitored, with the arterial catheter preferentially in the right arm. The proximal aortic clamp may occlude or compromise the origin of the left subclavian artery, or the left subclavian artery may be sacrificed for the repair. A femoral arterial catheter and right arm cuff can be used if right radial artery cannulation is unsuccessful. Sick neonates and very young infants will benefit from a technique using high-dosage fentanyl in combination with a low inspired concentration of an inhalation agent and/or benzodiazepine and a period of postoperative ventilation (severe pulmonary edema). Ketamine may worsen hemodynamics in neonates with severe LV dysfunction by increasing SVR. It should be appreciated that proximal blood pressure response to stimulation will be exaggerated. In older children with only proximal systemic hypertension, intravenous or inhalational techniques are well tolerated. Lung isolation may be required for older children, who may also be candidates for early extubation.
The increase in proximal aortic pressure with application of the aortic cross-clamp in neonates and infants can usually be managed expectantly or with a volatile agent while continuing any inotropic support. In older children and adolescents, β-blockers and vasodilators may occasionally be necessary. We generally maintain the blood pressure in the upper limbs at or modestly higher than the preoperative level, with the hope that it encourages some degree of lower body perfusion via any existing collaterals. In general, hypertension during the cross-clamp period in neonates and infants is unlikely to need treatment unless it exceeds baseline arterial blood pressures by approximately 30% or more, or unless it approaches values that raise concern for cerebral hyperperfusion injury. Sodium nitroprusside has been shown to increase cerebrospinal fluid pressure and produce a greater decrease in distal aortic blood pressure, thereby reducing spinal cord perfusion pressure ( ). No such studies have examined these effects of sodium nitroprusside in pediatric patients. performed an RCT evaluating the effect of blood pressure regulation with sodium nitroprusside, nitroglycerine, or sevoflurane on tissue O 2 saturation during aortic cross-clamping. Although decreases in renal and muscle tissue O 2 saturation were larger and had a faster rate of decay with sodium nitroprusside, the clinical significance is still uncertain. Spinal cord protection is accomplished with a short cross-clamp time (preferably <20 min) and mild induced hypothermia (∼34°C). Two-site NIRS monitoring during coarctation repair found a greater decline in somatic rSO 2 in neonates and infants compared with children over 1 year of age, however interindividual variability was wide and a sensor over the flank is unlikely to reflect spinal cord perfusion ( ). Removal of the cross-clamp will result in vasodilation with reactive hyperemia in distal tissues leading to transient hypotension, and release of acid metabolites will increase Paco 2 . Anticipation by decreasing or discontinuing inhaled anesthetics or any vasodilators, volume expansion, and appropriate ventilation is usually sufficient to limit these effects. In some cases, judicious use of phenylephrine may be necessary. Postoperatively, rebound hypertension may occur and persist for some time. Esmolol and vasodilators may be necessary in the early postoperative period.
Anesthetic considerations are the need to address the possibility of residual or recurrent coarctation and the long-term consequences of CoA discussed above.
Transposition of the great arteries (D-TGA) accounts for 3% to 10% of all CHD and is rarely associated with congenital anomalies and syndromes ( ). Ventriculoarterial discordance results in the aorta arising from the anatomic right ventricle and the pulmonary artery from the left ventricle. In other words, a right-sided RA connects via a right-sided tricuspid valve and RV to a right-sided and anterior aorta, and a left-sided LA connects via a left-sided mitral valve and LV to a left-sided and posterior pulmonary artery ( Fig. 30.13 , ![]() ). TGA can occur with an intact ventricular septum (IVS) or a VSD (40% to 45%), with or without subpulmonic (LVOT) obstruction (25% of those with a VSD), and variability in coronary artery pattern ( ; ). A much less common form of TGA is congenitally (physiologically) “corrected” TGA (ccTGA), in which there is both ventriculoarterial and atrioventricular discordance (i.e., a series circulation in which the RA connects via the mitral valve to the LV and then the PA, and the LA connects via the tricuspid valve to the RV and then the aorta). Congenitally corrected transposition without associated cardiac lesions may go undetected for decades until the RV, which is the systemic ventricle, begins to fail. Patients with ccTGA are at risk of spontaneously developing complete heart block, at a rate of 2% per year, owing to the abnormal location of the atrioventricular node (AVN) and infranodal common bundle. The AVN is anteriorly situated in the right atrium at the lateral junction of pulmonary and mitral valves with the common bundle passing in the subendocardium anterior to the pulmonary valve annulus and inferiorly over the anterosuperior aspect of the membranous septum ( ; ).
). TGA can occur with an intact ventricular septum (IVS) or a VSD (40% to 45%), with or without subpulmonic (LVOT) obstruction (25% of those with a VSD), and variability in coronary artery pattern ( ; ). A much less common form of TGA is congenitally (physiologically) “corrected” TGA (ccTGA), in which there is both ventriculoarterial and atrioventricular discordance (i.e., a series circulation in which the RA connects via the mitral valve to the LV and then the PA, and the LA connects via the tricuspid valve to the RV and then the aorta). Congenitally corrected transposition without associated cardiac lesions may go undetected for decades until the RV, which is the systemic ventricle, begins to fail. Patients with ccTGA are at risk of spontaneously developing complete heart block, at a rate of 2% per year, owing to the abnormal location of the atrioventricular node (AVN) and infranodal common bundle. The AVN is anteriorly situated in the right atrium at the lateral junction of pulmonary and mitral valves with the common bundle passing in the subendocardium anterior to the pulmonary valve annulus and inferiorly over the anterosuperior aspect of the membranous septum ( ; ).
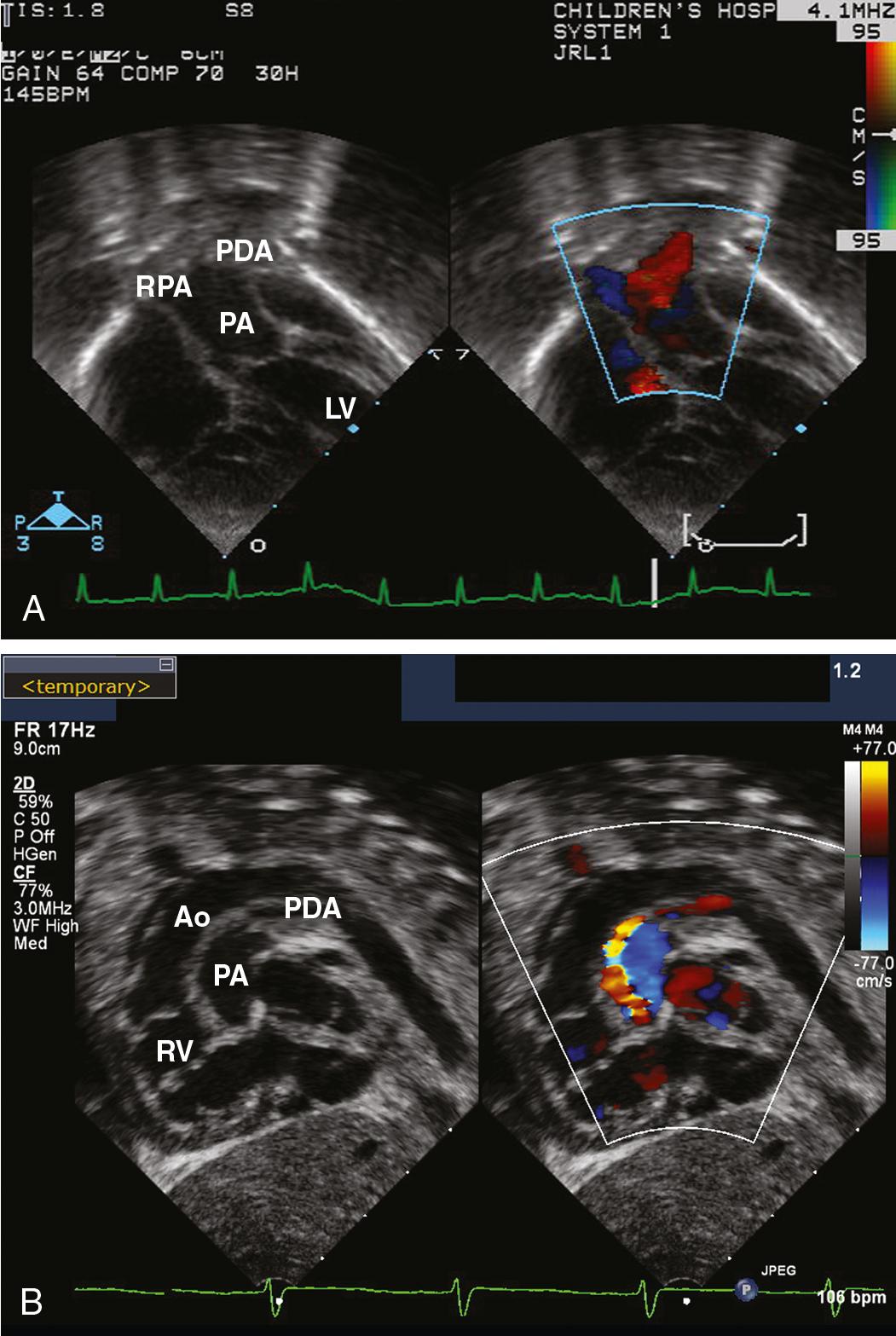
D-TGA produces two parallel circulations with recirculation of systemic and pulmonary venous blood. Clinically, these infants may present with severe hypoxemia shortly after birth and/or with pulmonary overcirculation and congestive heart failure. Adequate systemic oxygenation and short-term survival are dependent on one or more communications between the two circuits to allow intercirculatory mixing; these communications can be intracardiac (PFO, ASD, VSD), extracardiac (PDA, bronchopulmonary collaterals), or both. Initially, the infant is maintained on a prostaglandin E 1 (PGE 1 ) infusion to provide PBF via the PDA. With adequate mixing and a good-sized PDA, arterial oxygenation is satisfactory, but over time the decline in PVR leads to increased pulmonary blood flow. Patients with increased pulmonary blood flow, particularly those with a large VSD, are at risk of developing pulmonary overcirculation, having a large volume load imposed on the LA and LV, and congestive heart failure. Pulmonary overcirculation (e.g., tachypnea, systemic hypoperfusion, pulmonary congestion on chest x-ray, acidosis), although in the past managed by reduced Fio 2 via blending of atmospheric air with nitrogen or controlled ventilation with induced hypercarbia via added inspired CO 2 , is now managed with circulatory support as needed (e.g., dopamine), intubation and mechanical ventilation, and early surgical repair. Conversely, the more severe the LVOT obstruction, the more dependent the effective pulmonary blood flow will be on the presence of a PDA. In patients with reduced pulmonary blood flow or poor intercirculatory mixing despite PGE 1 and a widely patent atrial communication, hypoxemia can be lessened by increasing the cardiac output (volume loading, inotropic support), red blood cell (RBC) transfusion, and reducing oxygen consumption by sedation, paralysis and ventilation, and avoidance of fever.
If the atrial septum is restrictive (inadequate intracardiac mixing), the neonate will be hypoxemic with left atrial hypertension, and will have pulmonary edema and poor systemic perfusion with metabolic acidosis. A balloon atrial septostomy is then performed in the catheterization laboratory or at the bedside to improve mixing and to reduce left atrial pressure and pulmonary edema. This can be an emergent procedure performed shortly after delivery.
A general rule of thumb aims for arterial saturations in the 75% to 85% range in the nonintubated, spontaneously ventilating patient. Infants with evidence of multiple organ dysfunction resulting from severe hypoxemia and hypoperfusion are generally better managed with a period of stabilization and recovery, facilitated by the aforementioned measures to improve mixing and oxygen delivery, before undergoing corrective surgery. However, ongoing hypoxemia and longer time to surgery has been shown to increase preoperative brain injury ( ; ).
D-TGA/VSD and D-TGA/IVS are currently repaired anatomically by the arterial switch (Jatene) operation (ASO), which is usually performed 2 to 4 days after birth ( Fig. 30.14 , ![]() ) ( ; ). The pulmonary artery and aorta are transected distal to their respective valves. The coronary arteries are excised with a button of surrounding tissue and reimplanted into the proximal pulmonary artery (neoaorta). The great arteries are then switched, with the pulmonary artery brought anterior (Lecompte maneuver) and anastomosed to the proximal aorta (right ventricular outflow) and the aorta anastomosed to the proximal pulmonary artery (left ventricular outflow). Most patients with TGA have coronary anatomy that is suitable for coronary reimplantation. The exact coronary anatomy must be delineated because variants can contribute significantly to operative difficulty and the success of surgical repair. The “late” or two-stage repair for D-TGA with IVS can be used for neonates and infants in whom significant regression of LV mass has occurred ( ). These are generally infants on whom surgery was not performed during the first several weeks of life because of other factors (e.g., delayed diagnosis and referral, other anomalies, sepsis, or prematurity). The ASO is associated with an early hospital mortality of less than 5% in experienced centers ( ; ; ; ). Although the majority of patients reach adulthood with New York Heart Association functional class I, there are significant lifelong concerns. Postoperative sequelae have been reviewed and include neoaortic regurgitation, neoaortic root dilation, supravalvar pulmonary stenosis, supravalvar aortic stenosis, coronary artery disease, sudden cardiac death, and chronotropic incompetence ( ). The most common cause of mortality related to the ASO is coronary artery obstruction (5% to 7% of survivors); the pattern is bimodal, with a high early incidence related to technical difficulties with coronary reimplantation and a slow later incidence ( ). In adults, coronary artery occlusion (2% to 7% of cases) is generally asymptomatic, with the arteries displaying varying degrees of proximal eccentric intimal proliferation and concentric intimal smooth muscle hyperplasia with preserved tunica media, suggesting the potential for the development of early atherosclerosis in the reimplanted coronary arteries ( ; ; ; ). Detection of subclinical myocardial ischemia is still a challenge ( ). Noninvasive tests are not sufficiently sensitive to detect delayed coronary artery stenosis. ECG-gated CT angiography provides excellent spatial resolution and is an alternative to coronary artery angiography or intracoronary ultrasound. Stress echocardiography, cardiac MRI, or positron emission tomography may detect regional wall motion abnormalities and perfusion defects. It is still unclear whether invasive testing should be a mandatory component of surveillance in all patients who have undergone an ASO.
) ( ; ). The pulmonary artery and aorta are transected distal to their respective valves. The coronary arteries are excised with a button of surrounding tissue and reimplanted into the proximal pulmonary artery (neoaorta). The great arteries are then switched, with the pulmonary artery brought anterior (Lecompte maneuver) and anastomosed to the proximal aorta (right ventricular outflow) and the aorta anastomosed to the proximal pulmonary artery (left ventricular outflow). Most patients with TGA have coronary anatomy that is suitable for coronary reimplantation. The exact coronary anatomy must be delineated because variants can contribute significantly to operative difficulty and the success of surgical repair. The “late” or two-stage repair for D-TGA with IVS can be used for neonates and infants in whom significant regression of LV mass has occurred ( ). These are generally infants on whom surgery was not performed during the first several weeks of life because of other factors (e.g., delayed diagnosis and referral, other anomalies, sepsis, or prematurity). The ASO is associated with an early hospital mortality of less than 5% in experienced centers ( ; ; ; ). Although the majority of patients reach adulthood with New York Heart Association functional class I, there are significant lifelong concerns. Postoperative sequelae have been reviewed and include neoaortic regurgitation, neoaortic root dilation, supravalvar pulmonary stenosis, supravalvar aortic stenosis, coronary artery disease, sudden cardiac death, and chronotropic incompetence ( ). The most common cause of mortality related to the ASO is coronary artery obstruction (5% to 7% of survivors); the pattern is bimodal, with a high early incidence related to technical difficulties with coronary reimplantation and a slow later incidence ( ). In adults, coronary artery occlusion (2% to 7% of cases) is generally asymptomatic, with the arteries displaying varying degrees of proximal eccentric intimal proliferation and concentric intimal smooth muscle hyperplasia with preserved tunica media, suggesting the potential for the development of early atherosclerosis in the reimplanted coronary arteries ( ; ; ; ). Detection of subclinical myocardial ischemia is still a challenge ( ). Noninvasive tests are not sufficiently sensitive to detect delayed coronary artery stenosis. ECG-gated CT angiography provides excellent spatial resolution and is an alternative to coronary artery angiography or intracoronary ultrasound. Stress echocardiography, cardiac MRI, or positron emission tomography may detect regional wall motion abnormalities and perfusion defects. It is still unclear whether invasive testing should be a mandatory component of surveillance in all patients who have undergone an ASO.
The variant of D-TGA/VSD and severe LVOT obstruction precludes the ASO. In this setting, the Rastelli procedure is performed whereby the VSD is repaired by a patch that directs blood from the left ventricle through the defect into the aorta (and also closes the VSD), and a valved conduit is placed from the right ventricle to the main pulmonary artery. With the Rastelli procedure, the LV functions as the systemic ventricle, but conduit stenosis and valve degeneration are inevitable ( ). The Nikaidoh procedure involves translocation of the root of the aorta posterior to overlie the LV and translocation of the pulmonary artery anteriorly prior to creating RV-to-PA continuity ( ).
The atrial switch procedure (Mustard, Senning), which revolutionized the early management of infants with TGA, is now rarely performed. The sole indication in the current era is for repair of ccTGA ( ). The atrial switch is a physiologic repair in which a baffle in the atrium directs systemic venous blood to the mitral valve (and consequently to the LV and pulmonary artery) and pulmonary venous blood to the tricuspid valve (and consequently to the RV and aorta) ( Fig. 30.15 ). The atrial switch procedures provide excellent midterm results (15-year survival, 77% to 94%; 20-year survival, 80%), with many patients able to lead fairly normal lives into their third and fourth decades ( ). In the long term, there is progressive deterioration in RV function and development of tricuspid regurgitation, with 25% to 67% of patients having CHF by 45 years of age. Even in asymptomatic patients, exercise testing demonstrates chronotropic incompetence and moderate to severe limitations in RV function and maximal aerobic capacity. Sinus node dysfunction and atrial bradyarrhythmias and tachyarrhythmias are common. At 20 years follow-up, sinus rhythm was present in only 40% of patients ( ). Atrial flutter parallels the development of ventricular dysfunction, is experienced in one-third or more of patients 20 years after surgery, and is a probable marker for sudden death ( ; ; ; ). Intraatrial baffle leaks can result in shunting and hypoxemia. Baffle obstruction of the systemic venous return can cause superior vena cava (SVC) syndrome, hepatic congestion, ascites, and peripheral edema, whereas pulmonary venous baffle obstruction can cause pulmonary edema and pulmonary hypertension. Pulmonary hypertension unrelated to baffle obstruction can develop in about 7% of patients who survive to adulthood ( ).
Several management principles pertain to all infants, while some are specific for the individual subtypes.
Precardiopulmonary Bypass: For all patients, promoting overall filling and ventricular output are the most reliable ways to maintain intercirculatory mixing, oxygenation, and systemic oxygen delivery. It is therefore important to select drugs and other strategies that maintain cardiac output by their effects on heart rate, contractility, and preload, because decreases in cardiac output decrease systemic venous saturation, with a resultant decrease in arterial saturation. When a patient has evidence of pulmonary overcirculation and signs of congestion, maneuvers (particularly ventilatory) that further decrease PVR should be avoided. It is also important to avoid significant increases in PVR relative to SVR, which will decrease pulmonary blood flow via the PDA and reduce intercirculatory mixing. Similarly, reductions in SVR relative to PVR should also be avoided, because lower SVR promotes recirculation of systemic venous blood and decreases arterial saturation. Positive pressure ventilation needs to be sufficient to ensure adequate lung volume and gas exchange without promoting unwanted metabolic effects (e.g., respiratory alkalosis in patients with already low PVR and pulmonary overcirculation or respiratory acidosis with increased PVR) and mechanical compromise of venous return and ventricular function.
The PGE 1 infusion (0.01 to 0.05 mcg/kg per min) should be continued until shortly before CPB to ensure sufficient intercirculatory mixing. Anesthetic-related myocardial depression is more likely because of limited cardiac reserve relating to myocardial immaturity and preexisting ventricular dysfunction. Inhaled agent–based induction and maintenance techniques are not favored. Instead, anesthesia is usually induced and maintained using high-dosage fentanyl or sufentanil to provide hemodynamic stability and blunting of the stress response without adversely affecting intercirculatory mixing. In addition to muscle relaxation, judicious low-concentration inhaled agent or divided doses of benzodiazepines can be added to promote amnesia.
In patients with reduced pulmonary blood flow or poor intercirculatory mixing, efforts to reduce PVR should be made, again with the caveat of not impeding PBF or cardiac output by excessive ventilatory maneuvers. Volume expansion and inotropic support (e.g., dopamine, 5 to 10 mcg/kg per min) may be indicated to improve overall mixing and cardiac output and to some extent offset the alterations imposed by anesthetic agents and mechanical ventilation. Hypercarbia, acidosis, and hypoxemia further increase PVR and should be avoided, particularly in neonates with TGA/IVS where systemic oxygen delivery is tenuous. The converse is true for patients with decreased PVR. Myocardial ischemia with ST segment changes on ECG is a risk in the setting of extreme tachycardia, decreased coronary blood flow (hypotension, low cardiac output), and ventricular volume overload (increased end-diastolic pressure). It is important to be aware of the reactive pulmonary vasculature in the neonate whereby increases in PVR may severely compromise pulmonary blood flow.
Postcardiopulmonary Bypass: Ventilation should aim for mild respiratory alkalosis to lower PVR. Systemic ventricular dysfunction may necessitate inotropic and vasodilator therapy to terminate CPB. Dopamine (5 to 10 mcg/kg per min) and/or epinephrine (0.05 to 0.1 mcg/kg per min) are most often used. Milrinone (loading dose of 50 mcg/kg followed by 0.25 to 1.0 mcg/kg per min) may be used if SVR is high and because of its inotropic, lusitropic, and PVR-reducing effects. Although the vasodilation in infants that accompanies milrinone is substantially less than in adult patients, it is nonetheless advisable to administer the loading dose on CPB or post-CPB over 10 to 15 minutes or longer. If the quality of the repair is good and it was accomplished relatively swiftly, then it is unusual to require significant inotropic support or artificial pacing (for anything other than a relative sinus bradycardia). After CPB and surgical repair with the ASO, the two most significant problems are myocardial dysfunction and bleeding. In addition to poor myocardial protection and ischemia-reperfusion injury (nadir cardiac index at 9 to 12 hours post-CPB), coronary insufficiency related to poor implantation (kinking, torsion, ostial occlusion) and/or external compression by clot or hemostatic packing material while trying to control bleeding should be assumed until proven otherwise ( ). Evidence in support of this is a dusky myocardium, severe myocardial dysfunction (epicardial echo, need for higher than anticipated vasoactive support), ECG changes, need for atrial pacing, and ventricular arrhythmias. Evidence of myocardial ischemia after coronary reimplantation should be treated aggressively with prompt reevaluation of the anastomoses. Ischemia caused by coronary air emboli is transient and managed with maintenance of high perfusion pressures on CPB to promote distal migration and eventual dissipation. Echocardiography can help ensure adequate deairing of the left atrium and ventricle prior to separating from CPB, and also detect and distinguish between regional (i.e., potentially due to coronary insufficiency) and global (more likely due to ischemia-reperfusion injury) myocardial dysfunction, and assess the presence of flow in the proximal portions of the reimplanted coronary arteries.
Bleeding can be profound and related to extensive and pressurized aortic and pulmonary suture lines, poor surgical technique, and the severe coagulopathy associated with neonatal CPB and hypothermia (i.e., clotting factor dilution, thrombocytopenia, and platelet dysfunction) ( ; ). Heart rate should be maintained at age-appropriate levels, with atrial pacing if necessary, because cardiac output is likely to be more heart rate–dependent post-CPB in the presence of ventricular dysfunction. Although blood pressure should also be kept at age-appropriate norms (especially after hypothermic CPB or deep hypothermic circulatory arrest when cerebral autoregulation is likely to be impaired), at times reducing aortic or pulmonary artery pressure (or both) may be used to reduce suture line bleeding after the ASO ( ; ).
In the current era, the atrial switch operation is performed beyond infancy in combination with an ASO or Rastelli for repair of ccTGA. Obstruction of pulmonary or systemic venous return can occur after atrial baffle (Mustard or Senning) repairs. Systemic venous obstruction will produce evidence of systemic venous congestion and a measurable gradient between catheters located in the SVC and RA. Pulmonary venous obstruction may result in pulmonary venous and pulmonary arterial hypertension, pulmonary edema, and hypoxemia. Efforts to reduce SVR will reduce the RV afterload and help prevent tricuspid regurgitation. Therapy for atrial dysrhythmias also may be necessary. Echocardiography can help assess the presence of pulmonary or systemic venous obstruction, as well as atrial baffle leaks; any of these will require surgical revision if significant.
After an uncomplicated ASO with good ventricular function and no apparent sequelae, most patients can be managed in the same way as those with a structurally normal heart but with an index of suspicion for coronary artery disease. Severe pulmonary artery stenosis can be managed with interventional cardiac catheterization prior to elective procedures. Anesthesia for patients after the atrial switch operation should be based on the knowledge that the circulation can become increasingly fragile with limited physiologic reserve as patients age. The potential for decreased ventricular function is substantial, particularly of the systemic right ventricle, as well as for arrhythmias and end organ dysfunction. Although some volatile anesthetic will be tolerated by most of these patients, many will benefit from the more hemodynamically stable induction and maintenance regimens that have been discussed earlier. As elsewhere, it may be prudent to start an inotrope infusion at the time of or shortly after induction in those with more severe degrees of ventricular dysfunction. A significant number of these patients have a pacemaker, and the appropriate guidelines need to be followed (see earlier) ( ). Baffle leaks can result in cyanosis and increase the risk for systemic embolization of air or debris; consideration should be given to device closure in the catheterization laboratory prior to elective procedures associated with a high risk for embolization.
Truncus arteriosus is a single arterial trunk arising from the base of the heart and giving origin to the aorta, pulmonary arteries, and coronary arteries ( ). Truncus arteriosus is classified based on the origin of the pulmonary arteries ( Fig. 30.16 , ![]() –G). There is a single semilunar valve and VSD in the vast majority of cases. The semilunar valve is sometimes dysplastic, usually tricuspid but can also be uni-, bi-, quadri-, and pentacuspid, and may be regurgitant or stenotic ( ; ). The infundibular septum is virtually absent superiorly so that the VSD is nonrestrictive. Like tetralogy of Fallot, it is frequently associated with 22q11.2 microdeletions, DiGeorge, and velocardiofacial syndromes ( ). Extracardiac anomalies are seen in approximately 30% of patients.
–G). There is a single semilunar valve and VSD in the vast majority of cases. The semilunar valve is sometimes dysplastic, usually tricuspid but can also be uni-, bi-, quadri-, and pentacuspid, and may be regurgitant or stenotic ( ; ). The infundibular septum is virtually absent superiorly so that the VSD is nonrestrictive. Like tetralogy of Fallot, it is frequently associated with 22q11.2 microdeletions, DiGeorge, and velocardiofacial syndromes ( ). Extracardiac anomalies are seen in approximately 30% of patients.
Truncus arteriosus is a single-ventricle-physiology lesion amenable to a two-ventricle repair. It is the quintessential parallel circulation, with the
p:
s ratio determined by the ratio of pulmonary to systemic vascular resistance ( ; ). As the pulmonary and systemic circulations are supplied in parallel from a single vessel, for a given ventricular output increases in flow to one circulatory system will produce reductions in flow to the other. Shortly after birth, the balance of PVR and SVR is such that pulmonary blood flow is high and the patient with truncus arteriosus has symptoms of CHF with mild cyanosis. After cardiac reserve has been exhausted, further decreases in PVR increase pulmonary blood flow at the expense of systemic and coronary perfusion. This produces a progressive metabolic acidosis. If truncal valve insufficiency is present, an additional volume load is imposed on the ventricles.
Surgical repair is performed in the neonatal period as delay in repair results in early development of PVOD and myocardial dysfunction. Definitive repair involves patch closure of the VSD and detachment of the pulmonary arteries from the truncus with establishment of right ventricle–to–pulmonary artery continuity with a valved homograft. The RV-to-PA conduit requires placement of the proximal end over a ventriculotomy in the RV free wall. In some instances, truncal valve repair (preferred) or replacement is necessary to address valvular insufficiency or stenosis. Valvuloplasty is preferred over valve replacement, and the RV-to-PA valved conduit requires replacement as the child grows.
Unrepaired truncus arteriosus is a nonductal-dependent lesion and poses a significant anesthetic challenge.
Precardiopulmonary Bypass: Control of pulmonary blood flow and support of ventricular function are central management tenets. Declines in PVR increase L-R shunting, leading to systemic (in particular diastolic) hypotension, coronary ischemia, and ventricular fibrillation. Increased PVR can result in R-L shunting with desaturation, systemic and myocardial hypoxia, myocardial depression, and cardiovascular collapse. These patients are managed according to the principles described later (see Single-Ventricle Lesions and Hypoplastic Left Heart Syndrome).
Postcardiopulmonary Bypass: This is similar to that for D-TGA as discussed above. Inotropic support of the left and right ventricles is common, with maintenance of heart rate (preferably sinus rhythm or atrial pacing) at an age-appropriate rate, because cardiac output is likely to be more heart rate–dependent in the post-CPB period. Some degree of RV dysfunction is likely to exist because of the presence of a ventriculotomy and a large VSD patch; this is particularly problematic in patients with high PVR. PVR should be reduced when necessary through ventilatory interventions (respiratory alkalosis, increased Fio 2 , small amount of PEEP to maintain FRC).
Late sequelae following repair include obstruction of the right ventricle–to–pulmonary artery conduit, conduit regurgitation, truncal (aortic) valve regurgitation, residual VSD, and persistent pulmonary hypertension (for repairs done later in childhood). Anesthetic management for the repaired truncus is similar to that for right ventricular outflow obstruction (the most frequent sequela).
Ebstein anomaly consists of displacement of the septal and posterior leaflets of the tricuspid valve toward the apex of the right ventricle ( Fig. 30.17 , ![]() ) ( ). The right ventricle between the true annulus and the level of attachment of the septal and posterior leaflets to the ventricular septum is thin and dysplastic (“atrialized”). The anterior tricuspid leaflet at the annulus is larger than normal, whereas the other leaflets may be redundant, contracted, or thickened. The tricuspid valve is usually incompetent but can be stenotic and the anterior leaflet can occasionally cause RVOT obstruction. The right ventricular cavity beyond the atrialized portion ranges from small to large, whereas the right atrium is invariably enlarged. There is virtually always an interatrial communication (PFO or ASD), and an accessory AV conduction pathway (e.g., Wolff-Parkinson-White syndrome) is present in 10% to 25% of cases ( ; ).
) ( ). The right ventricle between the true annulus and the level of attachment of the septal and posterior leaflets to the ventricular septum is thin and dysplastic (“atrialized”). The anterior tricuspid leaflet at the annulus is larger than normal, whereas the other leaflets may be redundant, contracted, or thickened. The tricuspid valve is usually incompetent but can be stenotic and the anterior leaflet can occasionally cause RVOT obstruction. The right ventricular cavity beyond the atrialized portion ranges from small to large, whereas the right atrium is invariably enlarged. There is virtually always an interatrial communication (PFO or ASD), and an accessory AV conduction pathway (e.g., Wolff-Parkinson-White syndrome) is present in 10% to 25% of cases ( ; ).
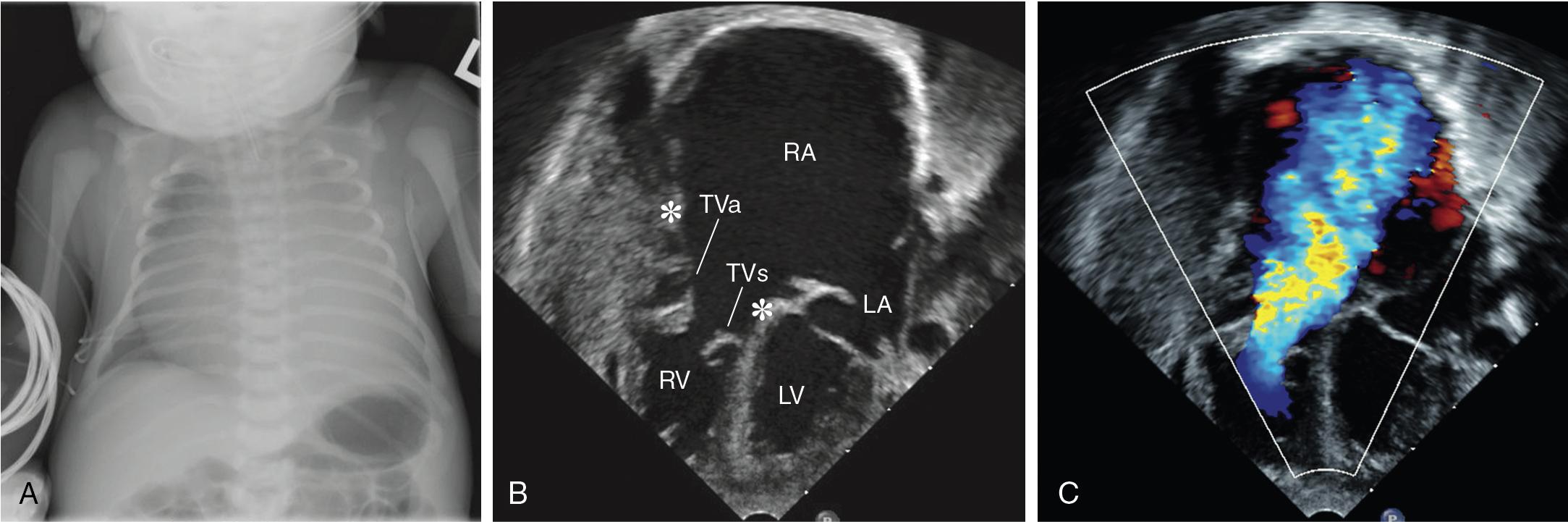
The clinical spectrum of Ebstein anomaly is extremely variable, ranging from the critically ill neonate with minimal to no anterograde flow out the right ventricle to the pulmonary artery, with severe tricuspid regurgitation and heart failure, to the child with minimal or no symptoms ( ). The severity of cyanosis depends on the magnitude of the atrial R-L shunt. Interventions for significant lesions include tricuspid valve repair (cone procedure) or replacement, closure of interatrial communications, and catheter ablation of accessory pathways ( ; ).
The major hazards during anesthesia in patients with Ebstein anomaly include depression of RV function and reduced forward flow into the pulmonary artery, accentuation of arterial hypoxemia because of increases in the magnitude of the R-L intracardiac shunt, and the development of supraventricular tachydysrhythmias ( ). Increased right atrial pressures may indicate the presence of right ventricular failure. Delayed onset of intravenous agents can be expected, which may result in part from pooling and dilution in an enlarged right atrium with tricuspid regurgitation. Both ventilatory and pharmacologic measures should focus on minimizing mechanical and metabolic effects of ventilation on RV afterload and on maintaining RV contractility. In the presence of a PFO, an increase in right atrial pressure above the pressure in the left atrium can lead to a R-L intracardiac shunt through the foramen ovale. Unexplained arterial hypoxemia or paradoxical air embolism during the perioperative period may result from shunting of blood or air through a previously closed foramen ovale.
A single ventricle is defined as the presence of two atrioventricular valves with one ventricular chamber or a large dominant ventricle with a diminutive opposing ventricle ( ). Single-ventricle or univentricular hearts encompass a wide variety of lesions that include hypoplastic left heart syndrome (HLHS) ( ![]() – ), tricuspid atresia or severe tricuspid stenosis, double-inlet single ventricle (usually left), single ventricle with common atrioventricular valve (unbalanced complete AV canal, heterotaxy variants), and some forms of double-outlet right ventricle and pulmonary atresia with intact ventricular septum. Initial management is aimed at optimization of systemic oxygen delivery and perfusion pressure. In the presence of approximately the right amount of pulmonary obstruction to balance the circulations, no procedure will be required in the neonatal period. Any surgical procedure required varies with the anatomy and may include the Norwood operation, a stage 1 hybrid procedure, pulmonary artery band, or systemic–to–pulmonary artery shunt. Subsequent management is aimed at reducing the volume load on the ventricle (superior cavopulmonary connection or bidirectional Glenn procedure) and finally achieving a series circulation with fully saturated systemic arterial blood (Fontan procedure). This is usually achieved through a staged approach. Although, collectively, single-ventricle defects are relatively rare, they account for a disproportionate share of repeated procedures with increased anesthesia risk, with resultant morbidity and mortality ( ).
– ), tricuspid atresia or severe tricuspid stenosis, double-inlet single ventricle (usually left), single ventricle with common atrioventricular valve (unbalanced complete AV canal, heterotaxy variants), and some forms of double-outlet right ventricle and pulmonary atresia with intact ventricular septum. Initial management is aimed at optimization of systemic oxygen delivery and perfusion pressure. In the presence of approximately the right amount of pulmonary obstruction to balance the circulations, no procedure will be required in the neonatal period. Any surgical procedure required varies with the anatomy and may include the Norwood operation, a stage 1 hybrid procedure, pulmonary artery band, or systemic–to–pulmonary artery shunt. Subsequent management is aimed at reducing the volume load on the ventricle (superior cavopulmonary connection or bidirectional Glenn procedure) and finally achieving a series circulation with fully saturated systemic arterial blood (Fontan procedure). This is usually achieved through a staged approach. Although, collectively, single-ventricle defects are relatively rare, they account for a disproportionate share of repeated procedures with increased anesthesia risk, with resultant morbidity and mortality ( ).
As discussed early in this chapter, unoperated single-ventricle physiology is a parallel circulation characterized by complete mixing of systemic and pulmonary venous blood at the atrial level, ventricular level, or both. With some lesions, systemic or pulmonary perfusion is dependent on a PDA. If there is no obstruction to pulmonary or systemic outflow, the amount of flow to each circulation is determined by the relative resistances of the pulmonary and systemic vascular beds. With no obstruction to pulmonary blood flow and the normal postnatal regression in PVR, PBF gradually increases and leads to congestive heart failure. Severe obstruction to PBF will result in progressive cyanosis in the absence of a PDA, while moderate obstruction can be associated with suitable balancing of the systemic and pulmonary blood flows. Systemic outflow obstruction will result in increased PBF, CHF, and systemic hypoperfusion. In single ventricle physiology, the effects of pulmonary and systemic blood flows on aortic and mixed venous oxygen saturations are shown in Table 30.5 . An arterial saturation of 75% to 80% is felt to be indicative of a relatively balanced circulation (maximum systemic O 2 delivery) with a
p:
s at or near 1:1 (assuming pulmonary venous saturation of 95% to 100% and mixed venous saturation of 50% to 55%) ( ). However, even with a “balanced”
p:
s of 1:1, the systemic ventricle is essentially pumping double the normal cardiac output. This gives some appreciation for the even greater degrees of systemic ventricular volume overload that is imposed by increased amounts of PBF. The addition of nitrogen to the inspired gas mixture in order to lower Fio 2 in order to increase PVR is rarely done ( ; ).
| Aortic Saturation (%) | Svo 2 (mm Hg) | p: s |
Clinical Considerations |
|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Norwood operation, also referred to as stage I single-ventricle palliation, is performed for patients with HLHS and its variants or when the pathway to the aorta from the systemic ventricle is obstructed ( ). It is a palliative procedure that eliminates the need for continued patency of the ductus arteriosus. Prior to the stage 1, a nonrestrictive atrial septum is essential for pulmonary venous blood to reach the systemic right ventricle. Creating a nonrestrictive atrial septum can be achieved in the cardiac catheterization laboratory with a Rashkind-Miller balloon atrial septostomy, or a surgical atrial septectomy can be performed at the time of initial palliation. The stage 1 procedure is performed during the first several days to a week of life using CPB in conjunction with 40 to 60 minutes of deep hypothermic circulatory arrest or regional cerebral perfusion during reconstruction of the aortic arch (see later) ( ). The procedures necessary for palliation of HLHS are outlined in Fig. 30.18 . The Norwood operation consists of establishing unimpeded flow of venous return from both the systemic and pulmonary circulations into the right ventricle and then into the (neo)aorta and creating a stable conduit for pulmonary blood flow. In practice, this requires a freely patent atrial septum (an atrial septectomy is often necessary), transection of the main pulmonary artery just proximal to its bifurcation and anastomosis end to side to the ascending aorta (the Damus-Kaye-Stansel procedure) so the combined venous return is ejected by the right ventricle into the aorta, and reconstruction and enlargement of the ascending aorta and aortic arch (using both the proximal pulmonary artery and homograft material). A well-functioning tricuspid (or atrioventricular valve) is very important for the single ventricle circulation. Pulmonary blood flow is established with a systemic-to-pulmonary artery shunt in the form of a modified BT shunt (the proximal insertion typically into the innominate artery) or an RV-PA conduit (the Sano modification) ( ; ). A superior cavopulmonary connection cannot be performed in lieu of a systemic–to–pulmonary artery shunt in neonates and early infancy because the elevated pulmonary artery pressure and PVR necessitates a high perfusion pressure. The Norwood operation still leaves the infant with a single-ventricle parallel circulation.
Precardiopulmonary Bypass: Management of anesthesia for HLHS and other SV physiology pre-CPB is described in Box 30.6 . A continuous infusion of PGE 1 is used to maintain ductal patency for lesions associated with ductal-dependent blood flow (either systemic or pulmonary). In left-sided obstructive lesions (e.g., HLHS, aortic arch hypoplasia), an adequate amount of R-L flow through the duct is needed for systemic and coronary perfusion, while in right-sided obstructive lesions (e.g., pulmonary or tricuspid atresia), L-R flow through the duct is needed for pulmonary perfusion. Use of invasive monitoring in shunt-dependent patients for noncardiac operations is usually determined by the nature of the surgical procedure.
A continuous infusion of PGE 1 is used to maintain ductal patency in patients with ductal-dependent blood flow (either systemic or pulmonary).
A target Pao 2 of 40–45 mm Hg and an Sao 2 of 70%–80% are associated with adequate systemic O 2 delivery.
In SV patients with unrestricted pulmonary blood flow,
PVR manipulation is essential and is accomplished most reliably with ventilatory interventions.
Hypercarbia in combination with a 21% Fio 2 is used to increase PVR and reduce pulmonary blood flow.
Hypercarbia (Paco 2 of 45–55 mm Hg) can be achieved with alveolar hypoventilation or with increased inspired CO 2 (3% Fico 2 ).
Avoid a high Fio 2 unless the Pao 2 is less than 35–40 mm Hg.
Because ventilatory interventions are incapable of reducing
p:
s much below 2:1, increased cardiac output is often necessary to ensure adequate systemic oxygen delivery and coronary perfusion pressure.
Increased stroke volume with preload augmentation is limited.
Increased heart rate (>140–150 bpm) can cause myocardial ischemia in patients with aortic diastolic blood pressures in the range of 20–30 mm Hg.
Inotropic support may be necessary.
After sternotomy, the surgeon can limit pulmonary blood flow and reduce with a vessel loop around a PA (usually the right).
Infants with SV physiology who arrive in the operating room unintubated from the ICU have a balanced circulation, but after induction, intubation, and mechanical lung expansion, these patients may have a precipitous decrease in PVR and immediate compromise of systemic circulation.
An intravenous induction with ketamine, etomidate, or high-dosage narcotic (will necessitate postoperative ventilation) and muscle relaxant is recommended. Denitrogenation with 100% O 2 is recommended prior to laryngoscopy and tracheal intubation so as to prevent hypoxemia during this interval ( ). Once the airway is secured, the Fio 2 should be reduced. Inhalation agents should be used with caution, and a benzodiazepine (usually midazolam) can be added as tolerated. An inotrope (dopamine 3 to 10 mcg/kg per min or epinephrine 0.05 to 0.1 mcg/kg per min) can be used to support hemodynamics as necessary, particularly if a volatile agent is being used to achieve an adequate depth of anesthesia. It should be noted that the ability to recruit stroke volume with preload augmentation is limited in neonates, particularly in the presence of already volume-overloaded ventricles. Although cardiac output in these circumstances has increasing dependency on contractile state and heart rate, very high heart rates (greater than about 160 beats per min) can potentially induce myocardial ischemia, particularly when aortic diastolic blood pressure is low (<20 to 30 mm Hg and resulting from runoff via the PDA).
Postcardiopulmonary Bypass: The post bypass management is summarized in Box 30.7 . The major pathophysiologic perturbations after CPB include hypoxemia, hypotension, low systemic perfusion, difficulties with gas exchange, and bleeding (see Bleeding After Pediatric Cardiopulmonary Bypass). End organ injury, whether present preoperatively or post-CPB hypoperfusion, is also relatively common with myocardial, renal, and cerebral dysfunction and damage predominating ( ). Myocardial dysfunction contributing to a low systemic cardiac output state is quite common after stage I HLHS repairs. Given adequate filling pressures (common atrial pressure of ∼8 to 12 mm Hg), dopamine (5 to 10 mcg/kg per min), and/or epinephrine (0.03 to 0.1 mcg/kg per min) may be necessary. The need for artificial pacing (for other than a relative sinus bradycardia), the presence of ST-T wave changes or dysrhythmias, or the need for substantially greater degrees of vasoactive support should prompt consideration of potential technical problems with the repair (e.g., coronary insufficiency arising from the construction of the Stansel anastomosis). In a somewhat oversimplified and artificial fashion, one can describe three general clinical situations in the post-CPB period after stage I repairs:
Too much PBF or shunt too large. This is characterized by higher-than-expected arterial oxygen saturations (90% or higher), frequently in combination with low systolic and particularly diastolic blood pressure, systemic hypoperfusion, acidosis, and oliguria. ECG evidence of ischemia may also be present if of sufficient magnitude. A relatively large shunt is a major factor. Although ventilatory maneuvers to increase PVR (e.g., hypoventilation, low Fio 2 , possibly adding inspired CO 2 or N 2 to create a hypoxic gas mixture [17% to 18% O 2 ]) or increased levels of inotropic support can be attempted, narrowing of the shunt is usually required.
Too little PBF or shunt too small. These patients display arterial oxygen saturations substantially lower than expected, often with normal or even elevated systemic blood pressures and perfusion (if myocardial performance is adequate). Causes include insufficient shunt diameter, narrowing at the proximal or distal insertion sites, shunt compression/kinking, more distal PA narrowing, increased intrinsic pulmonary vascular resistance, and lung pathology (resulting from CPB, preoperative overcirculation, or other processes). In the absence of technical difficulties or mechanical obstruction, maneuvers that decrease PVR, improve ventilation, or increase systemic arterial blood pressure and cardiac output can be attempted.
Low arterial oxygen saturation or low systemic blood pressure. This may be the most common symptom complex in most institutions, and it can be the most difficult to manage. Some degree of myocardial injury occurs in all of these patients; significant myocardial dysfunction leads to low cardiac output, low shunt perfusion, and reduced venous oxygen saturation, which, taken together, result in low oxygenation. Alternatively, poor oxygenation (see #2) in at least some patients (perhaps including those who were not particularly cyanotic preoperatively and thus have less myocardial tolerance to reduced saturations postoperatively) results in compromised myocardial function, which then follows the low cardiac output scenario just described. Measuring SVC saturation can be helpful in making this determination, because a value that is more than about 25 saturation points lower than the arterial value (e.g., an SVC saturation of 25% when the arterial is 68% equals 43 saturation points) is indicative of some contribution of low oxygen delivery to the reduced arterial oxygen saturation. Echocardiography and measuring the saturation of pulmonary venous blood (catheterization) can at times be helpful in delineating myocardial versus pulmonary etiology. From a practical standpoint (once the possibility of technical problems has been eliminated), treatment begins with ensuring appropriate ventilation parameters, oxygen-carrying capacity (hematocrit at least 35% to 40%), and optimizing cardiac output (adequate filling, ionized calcium, and inotropic support) and arterial blood pressure (to ensure adequate PBF via the shunt). Cerebral oximetry has been shown to be insensitive in detecting a mixed venous O 2 saturation below 30% ( ; ).
After CPB, high PVR, reduced pulmonary blood flow, and hypoxemia (Pao 2 <40 mm Hg) are common.
The modified Blalock-Taussig shunt or RV-PA conduit may be too large (resulting in pulmonary overcirculation and systemic hypoperfusion) or too small (inadequate pulmonary blood flow, excessive cyanosis) for prevailing physiologic conditions.
Ventricular dysfunction may exist. Low venous oxygen saturation may contribute to increased arterial desaturation, which can further exacerbate myocardial dysfunction.
Myocardial and tissue edema may be significant and preclude chest closure.
Bleeding may be significant.
Inhaled pulmonary vasodilators such as NO may be necessary to improve Sao 2 or reduce the transpulmonary gradient in these patients. This is particularly true in the subgroup of patients who have repair of partially obstructed pulmonary venous drainage at the time of their initial repair, and perhaps those with left atrial hypertension (e.g., restrictive atrial septum) prior to surgery.
The major differences between a modified BT and Sano-type shunt for HLHS in the immediate postoperative period relate to interactions between RV function, RV output, PBF, and oxygenation. In both situations, systemic and pulmonary blood flows and oxygenation are governed by RV function and output and the balance of SVR to PVR. The PVR is affected by factors related to shunt- and lung-based resistances. The Sano, for example, is a wider (5 mm vs. 3.5 mm) but substantially longer shunt. However, a major physiologic difference in the Sano is that there is no diastolic runoff from the aorta into the lungs. As a result, for a given degree of RV output, blood pressures (particularly diastolic) tend to be higher in the Sano than in the BT shunt patients, with resultant benefits to coronary perfusion and perhaps myocardial performance. The two types of shunts have been compared with regard to death, cardiac transplantation, cardiovascular interventions, and RV size and function in a large multicenter RCT ( ). Although transplantation-free survival at 12 months was better with an RV-to-PA shunt, at 3 and 6 years the combined death and cardiac transplantation rates were not different between the shunt groups ( ).
The principles of anesthetic management and balancing systemic and pulmonary blood flow prior to and following stage 1 palliation are the same, with the exclusion of CPB ( Box 30.6 ).
Surgically created shunts early in their course are typically large so as to allow for growth, and thus the major issues are controlling excessive PBF, ensuring adequate systemic perfusion, and managing congestive heart failure. On the other hand, the child can outgrow the shunt (or PA band) at around 3 to 6 months of age, so the
p:
s falls and eventually becomes less than 1:1 with a lower arterial oxygen saturation. Shunt calcification or stenosis also plays a role in limiting shunt flow, can occur at any time, and can result in increasing cyanosis. Clearly, severe or total shunt occlusion (thrombosis) can be catastrophic. In patients with evidence of shunt flow that is beginning to be critically limited (decreased saturations, increasing hemoglobin, need for supplemental O 2 , echocardiographic evidence), it is reasonable to assume that the effective resistance to PBF is relatively fixed (i.e., pulmonary overcirculation is unlikely to be possible) and therefore primary attention should be directed toward maneuvers that maintain or in fact increase cardiac output and systemic blood pressure in order to support adequate PBF. It is also reasonable to try to avoid increases in intrinsic and reactive components of PVR by using increased inspired oxygen concentrations and avoiding hypercarbia, acidosis, and other factors that increase PVR. Although infants with preserved myocardial function will tolerate a judicious inhalation induction, intravenous induction is generally preferred because loss of the airway and hypotension can lead to profound hypoxia and cardiac arrest if not promptly corrected. Vascular access may be difficult as many of these patients have had long ICU stays. Many of these patients are tolerant to opioids and benzodiazepines, and this should be considered if premedication is planned prior to placement of an intravenous catheter. Intramuscular induction with ketamine (plus or minus a muscle relaxant) can be a safe alternative in the absence of a difficult airway.
When the risks of CPB are higher than normal, such as with very low birth weight, intracerebral hemorrhage, and necrotizing enterocolitis, or as a means of resuscitation, the hybrid procedure is an alternative strategy to the Norwood operation ( ). The hybrid procedure is a combined catheter-based and surgical approach that comprises pulmonary artery banding via a median sternotomy and placement of a stent in the PDA. Transcatheter creation of an ASD with stenting is performed when necessary ( ) ( Fig. 30.19 ). The initial hybrid procedure is then followed by a comprehensive stage II, which combines components of both the Norwood and the traditional stage II (cavopulmonary connection) ( ). Although some centers perform the hybrid procedure routinely, there is insufficient evidence as to whether this is superior for the majority of patients ( ). (For a discussion of anesthetic management for the hybrid procedure, see Anesthesia for Cardiac Catheterization.)
Glenn’s original description of a superior cavopulmonary connection was an end-to-side anastomosis of the cranial end of the transected SVC to the distal end of the transected right pulmonary artery (RPA) performed through a right thoracotomy without the use of CPB ( ![]() ) ( ). Both the proximal SVC and the RPA were oversewn. The typical Glenn has been replaced by the modified bidirectional Glenn (BDG) procedure, in which the cranial end of the transected SVC is anastomosed in an end-to-side fashion to the RPA (which is left in continuity with the main and left PAs) and the cardiac end of the SVC is oversewn ( Fig. 30.18 ). This creates SVC continuity with both the left and right pulmonary arteries and bidirectional pulmonary blood flow (i.e., the BDG directs systemic venous blood from the SVC directly into the pulmonary circulation). The BDG procedure is usually performed with the patient on CPB and through a median sternotomy. If there were prior palliative procedures, the aortopulmonary shunt is ligated or the PA band taken down. The azygos vein is usually ligated so the SVC does not decompress retrograde to the inferior vena cava (IVC), thereby reducing the quantity of blood delivered to the PAs (unless, for example, the IVC is interrupted with azygous continuation, as occurs frequently in patients with heterotaxy syndrome and some others). Normally, all upper extremity and cerebral venous return drains via the SVC. In some patients (particularly those with heterotaxy syndrome), there may be bilateral SVCs that are not in continuity via a connecting vein. In this case a bilateral BDG must be done with one SVC anastomosed in an end-to-side fashion to the RPA and the other SVC anastomosed in an end-to-side fashion to the LPA.
) ( ). Both the proximal SVC and the RPA were oversewn. The typical Glenn has been replaced by the modified bidirectional Glenn (BDG) procedure, in which the cranial end of the transected SVC is anastomosed in an end-to-side fashion to the RPA (which is left in continuity with the main and left PAs) and the cardiac end of the SVC is oversewn ( Fig. 30.18 ). This creates SVC continuity with both the left and right pulmonary arteries and bidirectional pulmonary blood flow (i.e., the BDG directs systemic venous blood from the SVC directly into the pulmonary circulation). The BDG procedure is usually performed with the patient on CPB and through a median sternotomy. If there were prior palliative procedures, the aortopulmonary shunt is ligated or the PA band taken down. The azygos vein is usually ligated so the SVC does not decompress retrograde to the inferior vena cava (IVC), thereby reducing the quantity of blood delivered to the PAs (unless, for example, the IVC is interrupted with azygous continuation, as occurs frequently in patients with heterotaxy syndrome and some others). Normally, all upper extremity and cerebral venous return drains via the SVC. In some patients (particularly those with heterotaxy syndrome), there may be bilateral SVCs that are not in continuity via a connecting vein. In this case a bilateral BDG must be done with one SVC anastomosed in an end-to-side fashion to the RPA and the other SVC anastomosed in an end-to-side fashion to the LPA.
The BDG (Stage II palliation) is normally undertaken at about 4 to 6 months of age, at which point the PVR has decreased sufficiently such that systemic venous pressure (SVC pressure) is sufficient to drive pulmonary blood flow ( ). The BDG may be done at an earlier age in those patients who have outgrown a PA band, modified BT shunt, or RV-to-PA conduit, or those who have a low Sao 2 , as well as those who are not tolerating the additional volume load on their ventricle because of a loose PA band or large modified BT shunt.
There are big physiologic differences between a circulation with a systemic–to–pulmonary artery shunt and that with a BDG. The goals of the BDG are to reduce the volume load on the single ventricle with maintenance of a viable level of oxygen saturation. Although the effective PBF in both circulations is essentially equal for a given cardiac output, Sao 2 , Spvo 2 , and Svo 2 , the volume load on the systemic ventricle is twice as large for the circulation with a systemic–to–pulmonary artery shunt. This is because in the shunt circulation there is recirculated pulmonary blood (physiologic L-R shunt), whereas in the BDG circulation there is no recirculated pulmonary blood flow. As a result, a child with a BDG will have the same Sao 2 as a child with a systemic–to–pulmonary artery shunt (typically in the range of 80% to 85%) but with a significant reduction in the volume load on the systemic ventricle.
An alternative procedure to the BDG, albeit with the same goal of subsequent conversion to a Fontan circulation, is the hemi-Fontan. In a hemi-Fontan, an atriopulmonary anastomosis is constructed between the dome of the right atrium at the RA/SVC junction and the inferior surface of the right pulmonary artery, and a polytetrafluoroethylene (PTFE; Gore-Tex) baffle is used to isolate the cavopulmonary connection from the RA and to supplement the central pulmonary artery area.
Precardiopulmonary Bypass: The principles outlined previously regarding the management of single-ventricle physiology ( Box 30.6 ) apply here, with some modifications. When these infants present for BDG (4 to 6 months of age), they have generally grown and gained weight such that many will have outgrown and/or developed a narrowed mBTS or RV-PA conduit. As a result, these infants are much less likely to develop an excess of pulmonary blood flow and thus compromised systemic O 2 delivery (unless they are in the small subset being done early for excessive PBF and systemic ventricular volume overload). Most importantly, some of these patients may have extremely limited PBF (
p:
s <0.5) and be very cyanotic. This subset is at even higher anesthetic risk in that an increase in PVR (as with loss of an airway) and/or a fall in cardiac output, blood pressure, and SVR (the driving forces for flow through the narrowed or obstructed conduit) can lead to profound desaturation and cardiovascular collapse. As discussed previously, their baseline oxygen saturation is a reasonably good (albeit physiologically somewhat simplistic) indicator of which of the two general categories (pulmonary overcirculation or limited PBF) they belong in ( ). In a patient with a shunt-dependent circulation and requiring supplemental O 2 , it is safest to assume that the shunt is critically narrowed until proven otherwise.
In either case, an IV induction is generally preferred. Vascular access may be difficult as a result of prolonged ICU stays and other procedures (e.g., catheterizations, gastrostomy tubes) after the initial repair. In addition, some of these patients appear tolerant to opioids and benzodiazepines after their ICU stay. This should be considered if premedication is planned prior to IV placement. Once IV access is obtained, volume expansion (crystalloid or 5% albumin) is a reasonable consideration prior to or immediately following induction. Induction techniques that favor prompt and reliable airway control as well as hemodynamic stability are preferable to ones associated with decreased myocardial contractility or SVR (e.g., propofol, high sevoflurane concentrations). Patients with significant ventricular dysfunction, ventricular volume overload, or critically narrowed sources of PBF may also benefit from the inclusion of inotropic support as part of the induction regimen.
Postcardiopulmonary Bypass: The success of this circulation and the resultant arterial oxygen saturation depend on a technically satisfactory anastomosis, minimal to no pulmonary artery stenoses, and a number of hemodynamic and related parameters. The SVC return needs to flow unimpeded and at sufficient pressure to traverse the pulmonary vascular bed, where mechanical and intrinsic resistance must be low; follow by unimpeded egress via the pulmonary veins (here again without mechanical obstruction or increased pulmonary venous tone) to fill the common atrium; and then traverse a nonstenotic, nonregurgitant atrioventricular valve to fill a well-functioning systemic ventricle (e.g., the mitral valve and LV in the case of tricuspid atresia, the tricuspid valve and RV in HLHS). This ventricle must be in unobstructed continuity with the aorta. It is therefore critical to maintain sinus rhythm with a normal heart rate, contractility, and normal to increased preload to preserve cardiac output. Decreases in cardiac output result in reduced IVC O 2 saturation, which will reduce arterial saturation when IVC and pulmonary venous blood mix in the common atrium.
A central venous catheter in the internal jugular vein will measure SVC pressure (which also equals mean pulmonary artery pressure [mPAP] after a BDG). A surgically placed atrial catheter will measure common atrial pressure (a common atrium exists because of the large atrial septectomy). The difference between the SVC/mPAP and common atrial pressure is the transpulmonary gradient (TPG). A TPG of about 6 to 10 mm Hg is the norm in terms of driving adequate PBF and filling of the downstream systemic ventricle. Thus with a common atrial pressure of 6 to 10 mm Hg (the expectation in the presence of preserved ventricular function, adequate arterial blood pressure, and other evidence of adequate systemic cardiac output), the proximal pressure in the SVC will be in the range of 12 to 18 mm Hg. Increased PVR or lung disease can widen the TPG, so to obtain a comparable degree of ventricular filling and cardiac output, the SVC pressure must be increased to above 10 mm Hg (either by exogenous volume administration or by activation of adrenal and renal mechanisms that expand intravascular volume, which of course will be slower). Increased SVC pressure is also necessary to maintain preload and cardiac output in the case of systemic ventricular dysfunction. Here, the TPG is likely to be relatively normal (6 to 10 mm Hg), but the higher ventricular end-diastolic pressure (>10 mm Hg) necessitates an SVC pressure of >15 mm Hg to achieve comparable cardiac output.
In addition to adequate preload, inotropic support of the systemic ventricle is usually necessary because of ventricular dysfunction induced by chronic volume overload, prior injury, and the acute effects of CPB and ischemia-reperfusion injury. Typically, dopamine (3 to 5 mcg/kg per min, occasionally as high as 10 mcg/kg per min) is used. Milrinone (0.25 to 0.5 mcg/kg per min, at times up to 1.0 mcg/kg per min) can benefit this circulation, although others reserve it for use in combination with dopamine or for patients who have hypertension or poor perfusion with normal or elevated blood pressure.
In general, normocarbia and a normal PVR should be the goal of post-CPB ventilatory management. Hypoventilation and atelectasis, by increasing PVR, can compromise pulmonary blood flow and consequently systemic ventricular filling and cardiac output. High mean airway pressure will mechanically limit nonpulsatile PBF, thereby impairing it. After construction of the BDG anastomosis, there is an acute increase in SVC pressure and presumably in intracranial pressure. The volume load reduction and elevated SVC pressure with transition to the BDG circulation are believed to contribute to the (reflex) systemic hypertension and postoperative irritability that is commonly seen in these patients. The majority of PBF is supplied via venous blood from the brain, which is the largest single source of venous drainage from the upper body. Hyperventilation and hypocarbia, although beneficial in reducing PVR, also reduce cerebral blood flow (CBF) and cerebral venous drainage. Maintaining normocarbia or slight hypercarbia after BDG has been demonstrated to provide maximal Sao 2 . These patients tend to have a large end-tidal CO 2 -Paco 2 gradient because of increased physiologic dead space from a low PA driving pressure (SVC pressure). Thus mean airway pressure should be kept at the minimal level that is compatible with delivery of an adequate tidal volume and normal lung expansion. This can usually be accomplished with a relatively large tidal volume (10 to 12 mL/kg), slow respiratory rates (10 to 15 breaths/min), and short inspiratory times (inspiratory-to-expiratory ratio of 1:3 or 1:4); positive end-expiratory pressure (PEEP) can be used beneficially (i.e., for maintenance of functional residual capacity and gas exchange) but with caution.
BDG patients have a Sao 2 of about 76% to 85%, similar to that prior to their shunt. However, their
p:
s ranges from 0.5:1 to 0.8:1, and as a result there is an acute reduction in volume load on the systemic ventricle. The most likely cause of a low Sao 2 after a BDG is low cardiac output with a low IVC saturation (IVC blood does not pass through the lungs). Comparison of the SVC and common atrial pressures, perhaps in combination with surface echocardiography or TEE, may help delineate the cause (e.g., primary ventricular dysfunction, hypovolemia, AV valve dysfunction). If a low Sao 2 persists despite optimization of filling status and ventricular function, other causes of reduced pulmonary blood flow should be considered, such as a stenotic anastomosis or the presence of decompressing venovenous collaterals (typically from the SVC to the IVC or the SVC to the pulmonary veins or left atrium).
Reduced pulmonary venous oxygen saturation (Ppvo 2 ) may also be a source of low Sao 2 . Ventilatory maneuvers to reduce V/Q mismatch and intrapulmonary shunt should be done. A high Fio 2 may be necessary in the presence of significant V/Q mismatch. Response to inhaled pulmonary vasodilators such as inhaled NO can be variable; the BDG (and Fontan) patients most likely to respond with improvements in arterial oxygen saturation and apparent cardiac output usually have significantly elevated proximal venous or TPG pressures (>18 to 20 mm Hg and >10 mm Hg, respectively) ( ; ).
The Fontan procedure was first performed in 1968 and was initially described for patients with tricuspid atresia ( ). The original version included a Glenn shunt, which drained the SVC into the distal right pulmonary artery. The proximal end of the right pulmonary artery was joined to the right atrial appendage via an aortic valve homograft, and a pulmonary valve homograft valve was placed at the IVC-RA junction. The main pulmonary artery was ligated, and the ASD was closed. In subsequent early iterations, creation of the Glenn shunt and placement of homograft valves at the RA-PA and IVC-RA junctions were eliminated, resulting in creation of a direct atriopulmonary connection. Experience with direct atriopulmonary connections has found that elevated systemic venous pressure leads to progressive and severe atrial dilation (volumes as large as 300 to 500 mL) that is believed to contribute to stasis (and thus thromboembolism) and atrial arrhythmias. Since that time, many additional modifications have been described, but the goals of the procedure remain the same: total cavopulmonary connection delivers all systemic venous blood directly to the pulmonary arteries with the presumption that a subpulmonary ventricular pump is not essential for venous return to cross the pulmonary vascular bed ( ).
The Fontan procedure is generally performed at 2 to 3 years of age and is normally the final operation in the staged correction of single-ventricle defects ( ![]() – ). The Fontan places the systemic and pulmonary circulations in series (thereby correcting cyanosis) and driven by the single ventricle ( ). The principles for success of a Fontan are the same as those discussed for the BDG; that is, total cavopulmonary continuity without obstruction (absolute and relative), a well-functioning AV valve, and a single ventricle in unobstructed continuity with the aorta and sufficient systolic and diastolic function to support the systemic circulation.
– ). The Fontan places the systemic and pulmonary circulations in series (thereby correcting cyanosis) and driven by the single ventricle ( ). The principles for success of a Fontan are the same as those discussed for the BDG; that is, total cavopulmonary continuity without obstruction (absolute and relative), a well-functioning AV valve, and a single ventricle in unobstructed continuity with the aorta and sufficient systolic and diastolic function to support the systemic circulation.
The most commonly performed procedures in the current era are the lateral tunnel and extracardiac Fontan procedures (see Figs. 30.4 and 30.5 ). The lateral tunnel Fontan incorporates a small amount of systemic venous atrium in the cavopulmonary pathway, whereas the extracardiac Fontan does not incorporate any atrial tissue. It is still unknown which of these two procedures is superior ( ). In theory, the extracardiac Fontan may offer additional protection from development of atrial arrhythmias because of a lack of suture lines in the atrium. Research using computational fluid dynamics found that an 18 to 20 mm extracardiac conduit leads to lower power loss ( ; ). Fenestration of the Fontan circuit was described in 1990, and although not used routinely in all centers, it has been shown to be associated with reduced morbidity (e.g., low cardiac output, increased pleural effusions, increased volume requirements) and improved outcomes in standard and high-risk patients ( ; ). Fenestration is discussed in more detail after post-CPB management.
Factors that make a patient unsuitable for a Fontan procedure include nonmodifiable conditions such as early infancy, chronic lung disease with pulmonary hypertension, severe ventricular systolic or diastolic dysfunction, and recalcitrant pulmonary vein stenosis ( ; ). Many coexisting lesions previously believed to be at least relative contraindications (AV valve regurgitation or stenosis, significantly narrowed or distorted pulmonary arteries) are now corrected at the time of the BDG and/or Fontan.
One reason for interposing a BDG (or hemi-Fontan) procedure in the staged single-ventricle pathway is that acute ventricular volume reduction is better tolerated in the BDG than in Fontan physiology, and results in reduced morbidity and mortality. The rationale is that the acute reduction of systemic ventricular volume that occurs in the univentricular heart if one transitions directly to a Fontan results in impaired ventricular compliance because wall thickness does not regress as quickly as ventricular volume. The result is impaired ventricular diastolic function and elevated end-diastolic pressure, which impedes pulmonary venous return and hence reduced pulmonary blood flow. The cardiac output falls significantly because systemic ventricular output can only equal the quantity of blood that traverses the pulmonary vascular bed. Performing the BDG at 3 to 6 months of age allows early reduction in the volume overload to the systemic ventricle and thereby promotes remodeling of the ventricle at lower end-diastolic volumes. When there is impairment of pulmonary venous return after the BDG (or hemi-Fontan), blood from the IVC will continue to provide systemic ventricular filling and cardiac output at a lower pressure than that required to traverse the pulmonary vascular bed; similar conceptual thinking underlies the creation of a fenestration in the Fontan pathway (see Fenestrating the Fontan).
One risk of the staged procedures is the development of pulmonary arteriovenous malformations (AVMs), which develop as a result of diversion of hepatic venous blood flow away from the pulmonary capillary bed ( ). This of course is the blood flow pattern in BDG patients, approximately 10% of whom will develop significant pulmonary AVMs resulting in pulmonary venous desaturation and therefore lower arterial saturation. These lesions usually resolve after the BDG is converted to a Fontan and when hepatic-to-pulmonary blood flow “continuity” is restored.
Although it is often stated that pulmonary blood flow is passive and driven by the components of the TPG (systemic venous pressure = mean pulmonary artery pressure > pulmonary venous atrial pressure), the systemic ventricle generates the energy necessary to provide flow through the pulmonary capillary bed because the systemic vascular bed and pulmonary vascular bed are in series, without an intervening atrium and ventricle. As a result, the single ventricle in a Fontan circulation is faced with higher afterload than the systemic ventricle in a two-ventricle series circulation. Additional circulatory abnormalities that contribute to progressive impairment of ventricular function, exercise capacity, and altered hemodynamic behaviors (e.g., in response to blood loss, stress, or exercise) include increased arteriolar tone and venous capacitance, limited preload augmentation ability, limited ability to increase heart rate, increased resistance across the total cavopulmonary connection due to increasing PVR, and decreased diastolic function of the systemic ventricle ( ). The physiology and clinical implications of the Fontan circulation have been reviewed in detail ( ).
Precardiopulmonary Bypass: Patients presenting for the Fontan procedure are typically 2 to 3 years of age with a superior cavopulmonary connection (BDG or hemi-Fontan). The principles outlined for managing BDG physiology post-CPB generally apply here, with the knowledge that the cardiovascular system is more stable with a BDG. Arterial Sao 2 is 76% to 85% unless there are significant decompressing venovenous collateral vessels from the SVC to either the IVC or the pulmonary venous system. These are usually addressed by coil occlusion in the cardiac catheterization laboratory prior to the Fontan.
Liberal clear fluids up to 2 hours preoperatively should be the norm, and in hospitalized patients maintenance intravenous fluids should be begun while the patient is fasting. Volume expansion may be necessary before or during induction to ensure adequate preload.
Premedication is recommended because these patients and their families have generally had multiple hospital exposures and procedures. An upset and crying child leads to higher PVR and SVR (afterload) with a reduction in Sao 2 and cardiac output. Heavy premedication allows for lower concentrations of volatile agent if an inhalational induction is chosen. Although patients with preserved ventricular function will tolerate a judicious (<3% to 4% sevoflurane) mask induction from a cardiovascular standpoint, several factors need to be considered: coughing, breath-holding, laryngospasm, or high intrathoracic pressure will lead to increased PVR, which impedes pulmonary blood flow and thereby cardiac output; hypoxemia develops rapidly, which itself increases PVR (vicious circle); slower rate of induction from the reduced
p:
s (0.5:1 to 0.8:1); possible difficult IV access; and venous congestion of the head and tongue from elevated SVC pressure. Based on these factors, an IV induction is preferred by many.
The key physiologic principles in patients with a superior cavopulmonary connection (Fontan physiology) are control of the airway and maintenance of filling and function of the systemic ventricle. Hemodynamic goals for induction and the pre-CPB period are sinus rhythm to maintain PBF and the atrial contribution to ventricular filling, normal to increased preload, avoiding significant myocardial depression and the early administration of an inotropic agent if necessary, and avoiding excessive tachycardia and bradycardia (adequate ventricular filling time, cardiac output dependent on relatively normal heart rate). Prevention of metabolic or mechanical events that significantly increase PVR is critical; these include airway obstruction, hypoxemia, hypoventilation with hypercarbia and acidosis, atelectasis, high intrathoracic pressure, and an excessive sympathetic response. From a practical standpoint, volume expansion (isotonic crystalloid or colloid) prior to or following hemodynamically stable induction regimens such as etomidate (0.2 to 0.3 mg/kg), ketamine (1 to 3 mg/kg), or high-dose opioid (e.g., fentanyl, 10 to 25 mcg/kg) supplemented with lower doses of either etomidate, ketamine, or a benzodiazepine, and muscle relaxant are frequently used. Maintenance of anesthesia typically includes additional opioid, appropriate concentrations of inhaled agent, a benzodiazepine, or a combination of these. Advantages of an internal jugular catheter (in addition to fluid and drug administration) are the measurement of central venous pressure pre- and post-CPB, calculation of the TPG in conjunction with a common atrial catheter, detection of potential cannulation and anastomotic issues, and postoperatively the SVC oxygen saturation (Svo 2 ) can provide a reasonable indication of cardiac output. Some centers avoid SVC catheters in most or all infants, particular in Glenn and Fontan patients, because of concerns for SVC thrombosis, infection, and other risks common to central vascular access.
Postcardiopulmonary Bypass: Management of the Fontan circulation is summarized in Table 30.6 and troubleshooting for low cardiac output in Table 30.7 . Many of the goals for hemodynamic management post-CPB are similar to those with a superior cavopulmonary connection with the exception that in a Fontan the whole systemic venous return now needs to cross the pulmonary vascular bed. It is important to maintain heart rate, contractility, and preload, as discussed above for pre-CPB management, for adequate cardiac output. Inotropic support of the systemic ventricle may be necessary as a result of ventricular dysfunction induced by chronic volume overload, prior surgical or ischemia-reperfusion injury, and the acute effects of CPB. Dopamine (3 to 5 mcg/kg per min) is usually sufficient, but occasionally higher dosages of dopamine or the addition of other inotropes such as epinephrine (0.03 to 0.05 mcg/kg per min) are needed. Milrinone (0.5 to 1.0 mcg/kg per min) may be useful for those patients who have high SVR and afterload/contractility mismatch; a loading dose (25 to 50 mcg/kg) can be given while the patient is on CPB. A difference between the Sao 2 and Svo 2 of >25% (e.g., 50% SVC saturation vs. 88% arterial saturation) is indicative of low cardiac output (assuming normal hemoglobin concentration). These patients frequently require substantial amounts of volume supplementation in the immediate post-CPB period, even in the absence of significant bleeding.
| Physiology | Causes | Clinical Presentation | Management |
|---|---|---|---|
|
|
|
|
| Low pulmonary blood flow and inadequate preload delivery to systemic (common) atrium TPG greater than 10 mm Hg |
Fenestration patent or baffle leak present | Sao 2 75%–80% Sa-vo 2 >20 mm Hg Common atrial pressure <10 mm Hg Hypotension Tachycardia Poor distal perfusion Metabolic acidosis |
Volume replacement to keep baffle pressure stable Reduce PVR Correct acidosis Inotropic support Afterload reduction consider catheterization Intervention to open or create baffle fenestration |
| Systemic ventricular dysfunction TPG of 5–10 mm Hg |
Systolic dysfunction Diastolic dysfunction AV valve regurgitation or stenosis Loss of AV synchrony Afterload-contractility mismatch |
No fenestration or premature fenestration closure Sao 2 85%–90% Sa-vo 2 >35%–40% Baffle pressure >20 mm Hg Common atrial pressure >15 mm Hg Pulmonary edema Hypotension Tachycardia Poor distal perfusion Metabolic acidosis |
Volume replacement to keep baffle pressure stable Correct acidosis PEEP may be necessary for pulmonary edema Inotropic support Afterload reduction Provide AV synchrony (antiarrhythmics or pacing) Mechanical support (postcardiotomy support or bridge to transplantation) Surgical intervention (takedown to BDG) |
| Systemic ventricular dysfunction TPG of 5–10 mm Hg |
Systolic dysfunction Diastolic dysfunction AV valve regurgitation or stenosis Loss of AV synchrony Afterload-contractility mismatch |
Fenestration patent or baffle leak present Sao 2 70%–75% Sa-vo 2 >35%–40% Baffle pressure >20 mm Hg Common atrial pressure >15 mm Hg Pulmonary edema Hypotension Tachycardia Poor distal perfusion Metabolic acidosis |
Volume replacement to keep baffle pressure stable Correct acidosis PEEP may be necessary for pulmonary edema Inotropic support Afterload reduction Provide AV synchrony (antiarrhythmics/pacing) Mechanical support (postcardiotomy support or bridge to transplantation) Surgical intervention (takedown to BDG) |
| Systemic Venous Pressure | Common Atrial Pressure | Transpulmonary Gradient | |
|---|---|---|---|
| Anatomic | |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
↑ |
|
|
|
|
|
|
| Physiologic | |||
|
|
|
|
|
|
|
|
* Superior vena cava–to–pulmonary artery pressure gradient greater than 2 mm Hg. Note: After the cavopulmonary anastomosis, because inferior vena caval blood flow enters the systemic ventricle without first passing through the lungs, any anatomic obstruction through the lungs will result in low oxygen saturation but a relatively preserved cardiac output.
In the absence of a pumping pulmonary ventricle, an essential goal is to maintain an adequate pressure gradient between the systemic venous return and the common atrium. The TPG is calculated as the SVC/mean PAP pressure minus the common atrial pressure (see Bidirectional Glenn or Superior Cavopulmonary Connection). Allowing for individual variations, expected values can be summarized as follows:
Mean PAP of about 12 to 18 mm Hg is usually seen in the presence of reasonably good systemic ventricular function, absence of lung injury, and appropriate ventilation.
Reasonably good ventricular function will be associated with an EDP (and thus common atrial pressure) of about 5 to 10 mm Hg.
TPG (SVC/mPAP: common atrial pressure) is usually less than 10 mm Hg with appropriate ventilation and no significant lung injury, mechanical, or anastomotic obstructions.
Pao 2 well below 100 mm Hg (50 to 70 mm Hg is not uncommon) with a fenestration.
Any significant departure from these requirements can result in severe circulatory compromise. In an oversimplified fashion, the Fontan circulation can be viewed as a waterfall with the systemic venous pressure as the top and the ventricular end-diastolic pressure as the bottom. Any downstream problem will necessitate a higher upstream pressure. For example, ventricular dysfunction with an end-diastolic pressure of 15 mm Hg mandates a venous pressure of 20 to 25 mm Hg (i.e., the TPG is preserved, but venous pressures must be proportionately higher) to achieve comparable pulmonary blood flow. In the absence of hyperventilation, a low end-tidal CO 2 is generally a good indication of reduced pulmonary blood flow. Where a fenestration (see below) has been created, the relatively sudden occurrence of hypotension, increased SVC pressure, or increased Pao 2 (i.e., >150 to 200 mm Hg on 100% oxygen) should raise the possibility of acute fenestration closure.
Ventilatory management is the same as discussed above for a BDG circulation. PVR needs to be kept normal or slightly low with optimization of ventilation and acid-base management. Mean airway pressure should be kept at the minimal level that is compatible with delivery of adequate minute ventilation and maintenance of normal lung expansion. This can usually be accomplished with a relatively large tidal volume (10 to 12 mL/kg), slow respiratory rates (10 to 15 breaths/min), and short inspiratory times (inspiratory-to-expiratory ratio of 1:3). Excessively high mean airway pressure will mechanically limit venous return and pulmonary blood flow. Similarly, PEEP should be used with caution; however, it may be beneficial at levels (4 to 5 cm H 2 O) sufficient to maintain functional residual capacity and support gas exchange without causing overdistention. As discussed previously, inhaled NO may benefit some patients with significantly increased SVC and TPG pressures (assuming the increased TPG is not the result of ventricular dysfunction, desaturation resulting from V/Q mismatch, or poor repair). It is commonly stated that early tracheal extubation and spontaneous ventilation enhance pulmonary gas exchange and hemodynamics in Fontan patients ( ). On the other hand, positive pressure ventilation can improve the function of a dysfunctional systemic ventricle by reducing afterload (Laplace’s law) and also potentially reducing the work of breathing. In addition, low lung volumes, hypercarbia, and hypoxemia, along with the potential effects on ventricular filling and function, have the potential to negate any advantages produced by spontaneous ventilation.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here