Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Continuous improvement in burn care since World War II has resulted in a steady increase in the rate of survival after large burn injury. These improvements have been attributed to aggressive fluid resuscitation, early excision and grafting of burn wounds, more effective antimicrobials, advances in nutritional support, and development of burn centers. Today most patients with more than 80% total body surface area (TBSA) burned will survive if promptly treated in a modern burn unit with adequate resources. In their study of risk factors for death following burn injuries Ryan et al. identified three variables that can be used to estimate the probability of death: age greater than 60 years, burns of more than 40% TBSA, and the presence of inhalation injury. Mortality increased in proportion to the number of risk factors present: 0.3%, 3%, 33%, or approximately 90% mortality depending on whether zero, one, two, or three risk factors were present, respectively. The incidence of mortality is also influenced by significant coexisting disease or delays in resuscitation. O'Keefe et al. observed an approximately twofold higher death risk of death in women aged 30–59 years compared with men with similar burns and age. Although it has been assumed that very young children are also at increased risk of death from burn injuries, Sheridan et al. found very low rates of mortality in children younger than 48 months who had suffered large burns. One group that has not benefited as much from advances in burn care is the elderly. The morbidity and mortality (as expressed as lethal dose 50 for burns) associated with serious burns in elderly patients has not improved over the past three decades. Some burn patients develop refractory burn shock soon after injury and cannot be resuscitated.
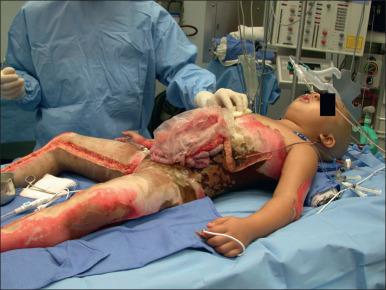
Major burn injury results in pathophysiological changes in virtually all organ systems. Box 13.1 lists and Figs. 13.1 and 13.2 illustrate some of the challenges presented by the acutely burned patient during the perioperative period. In addition to the predictable challenges relating to airway management, monitoring, and vascular access, patient positioning requires close communication and teamwork. Burns involving posterior areas may require turning the patient to the prone position for optimal access ( Fig. 13.1 ). Vascular catheters and the endotracheal tube must be secured with confidence and due care given to these life lines during patient turning. Several highly informative reviews of anesthetic management for burn surgery have been written during the past decade, each with its own special areas of concentration.
Compromised airway
Pulmonary insufficiency
Altered mental status
Associated injuries
Limited vascular access
Rapid blood loss
Impaired tissue perfusion due to:
Hypovolemia
Decreased myocardial contractility
Anemia
Decreased colloid osmotic pressure
Edema
Dysrhythmia
Impaired temperature regulation
Altered drug response
Renal insufficiency
Immunosuppression
Infection/sepsis
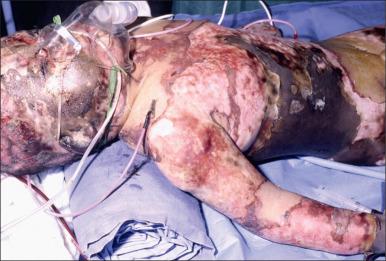
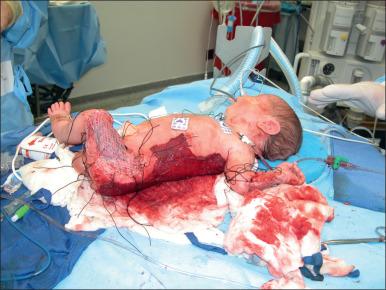
Patients suffering burn injuries often require surgical treatment for years after the initial injury in order to correct functional and cosmetic sequelae. Anesthetic management for reconstructive burn surgery presents many special problems but this chapter will concentrate on the care of acute burn patients. The acute phase of burn injury is defined as the period from injury until the wounds have been excised, grafted, and healed.
Modern burn care depends on coordination of a multidisciplinary team including surgeons, intensivists, nurse clinicians, nutritionists, rehabilitation therapists, pulmonary care therapists, and anesthesia providers. Rational and effective anesthetic management of acute burn patients requires an understanding of this multidisciplinary approach so that perioperative care is compatible with the overall treatment goals for the patient. The current standard of surgical treatment calls for early excision and grafting of nonviable burn wounds, which may harbor pathogens and produce inflammatory mediators with systemic effects resulting in cardiopulmonary compromise. After an extensive burn injury, the systemic effects of inflammatory mediators on metabolism and cardiopulmonary function reduce physiological reserve, and the patient's tolerance to the stress of surgery deteriorates with time. Assuming adequate resuscitation, extensive surgery is best tolerated soon after injury, when the patient is most fit. However it must be recognized that the initial resuscitation of patients with large burns results in large fluid shifts and may be associated with hemodynamic instability and respiratory insufficiency. Reynolds et al. reported that more than half of deaths after burn injuries occur due to failed resuscitation.
Effective anesthetic management of patients with extensive burn injuries requires an understanding of the pathophysiological changes associated with large burns and careful preoperative evaluation to assure that resuscitation has been optimized and an appropriate anesthetic plan has been formulated.
The preoperative evaluation of burn patients requires knowledge of the continuum of pathophysiological changes that occur in these patients from the initial period after injury through the time that all wounds have healed. The dramatic changes that occur in all organ systems following burn injury directly affect anesthetic management. The following discussion will describe the pathophysiological changes that occur in the acutely burned patient as they relate to the preoperative evaluation. In addition to the routine features of the preoperative evaluation, evaluation of acute burn patients requires special attention to airway management and pulmonary support, vascular access, adequacy of resuscitation, and associated injuries. The severely burned patient presents with numerous preoperative concerns, as listed in Box 13.2 . The preoperative evaluation must be performed within the context of the planned operative procedure, which will depend on the location, extent, and depth of burn wounds; time after injury; presence of infection; and existence of suitable donor sites for autografting.
Age of patient
Extent of burn injuries (total body surface area)
Burn depth and distribution (superficial or full thickness)
Mechanism of injury (flame, electrical, scald, or chemical)
Airway patency
Presence or absence of inhalation injury
Elapsed time from injury
Adequacy of resuscitation
Associated injuries
Coexisting diseases
Surgical plan
Destruction of skin by thermal injury disrupts the vital functions of the largest organ in the body. The skin provides several essential protective and homeostatic functions ( Box 13.3 ). Treatment of patients with burn injuries must compensate for loss of these functions until the wounds are covered and healed. As a barrier to evaporation of water, the skin helps maintain fluid and electrolyte balance. Heat loss through evaporation and impairment of vasomotor regulation in burned skin diminishes effective temperature regulation . The skin's barrier function also protects against infection by invading organisms. Wound exudate rich in protein depletes plasma proteins when large body surface areas are injured.
Protection from environmental elements (e.g., radiation, mechanical irritation, or trauma)
Immunological: antigen presentation, antibacterial products (sebum), barrier to entry of pathological organisms
Fluid and electrolyte homeostasis: helps maintain protein and electrolyte concentrations by limiting evaporation
Thermoregulation: helps control heat loss through sweating and vasomotor regulation of superficial blood flow
Sensory: extensive and varied sensory organs in skin provide information about environment
Metabolic: vitamin D synthesis and excretion of certain substances
Social: appearance of skin has strong influence on image and social interactions
In addition to loss of important functions of the skin, extensive burns result in an inflammatory response with systemic effects that alter function in virtually all organ systems. Preoperative evaluation of the burn patient is guided largely by a knowledge of these pathophysiological changes.
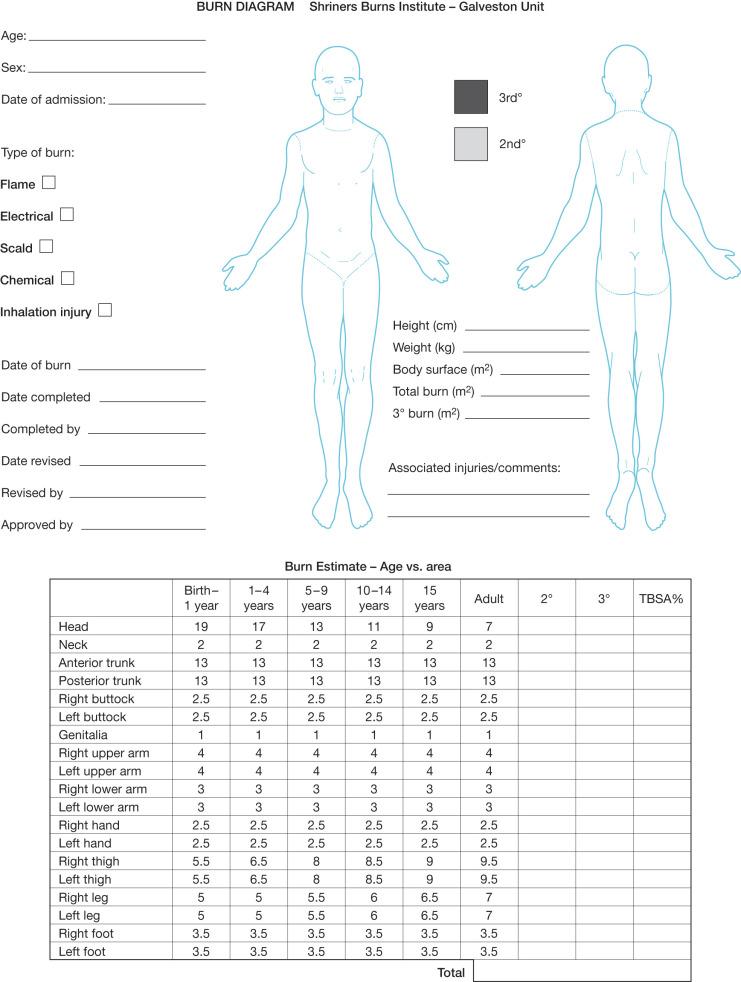
Much of the morbidity and mortality associated with burn injuries are related to the size of the injury. The extent of the burn injury is expressed as the TBSA burned. Estimates of TBSA burned are used to guide fluid and electrolyte therapy and to estimate surgical blood loss. These estimates can be made with Lund Browder charts developed from age-specific nomograms ( Fig. 13.3 ). Simplified “Rule of Nines” charts are available in emergency departments for rapid estimates of TBSA involvement. A knowledge of the burn depth is also critical to anticipating physiological insult as well as planned surgical treatment. First-degree or superficial second -degree burns may heal without scarring or deformity and do not require surgical excision. Deeper second-degree and third-degree burns require surgical debridement and grafting with associated surgical blood loss.
Accurate estimates of blood loss are crucial in planning preoperative management of burn patients. With extensive wound excision or debridement, large amounts of blood can be lost rapidly. Adequate preparation in terms of monitors, vascular access, and availability of blood products is essential. Surgical blood loss depends on area to be excised (cm 2 ), time since injury, surgical plan (tangential vs. fascial excision; Fig. 13.4 ), and presence of infection. Blood loss from skin graft donor sites will also vary depending on whether it is an initial or repeat harvest. These variables are valuable predictors of surgical blood loss, which is a critical factor in planning anesthetic management ( Table 13.1 ).
| Surgical Procedure | Predicted Blood Loss |
|---|---|
| <24 h since burn injury | 0.45 mL/cm 2 burn area |
| 1–3 days since burn injury | 0.65 mL/cm 2 burn area |
| 2–16 days since burn injury | 0.75 mL/cm 2 burn area |
| >16 days since burn injury | 0.5–0.75 mL/cm 2 burn area |
| Infected wounds | 1–1.25 mL/cm 2 burn area |
Special attention must be paid to the airway and pulmonary function during preoperative evaluation. Burn injuries to the face and neck can distort anatomy and reduce range of mobility in ways that make direct laryngoscopy difficult or impossible. Specific alterations include impaired mouth opening and edema of the tongue, oropharynx, and larynx, as well as decreased range of motion of the neck. The tissue injury and sloughing present after severe facial burns may make mask ventilation difficult. Inhalation injury may impair pulmonary gas exchange and lead to respiratory insufficiency or failure. The level of respiratory support must also be assessed. The level of required support may range from supplemental blow-by or mask oxygen to intubation and ventilation with high positive end-expiratory pressure (PEEP) and FIO 2 . Acute lung injury can occur from inhalation of chemical irritants, systemic inflammation from burn wounds or difficulties with resuscitation, or ventilator-induced injury. Common pathologies include upper airway thermal injury, pulmonary parenchyma damage from chemical irritants or inflammation, and lower airway obstruction from mucus plugs and epithelial casts, as well as pulmonary edema due to acute lung injury or volume overload. With very high levels of PEEP or peak inspiratory pressure, it must be determined if the anesthesia ventilator is adequate or if an ICU ventilator will need to be brought to the operating room. If the patient is intubated at the time of the preoperative evaluation, it is essential to know what the indications for intubation were so that an appropriate plan for postoperative support can be made.
There is general recognition that smoke inhalation injury increases morbidity and mortality for burn patients. The presence of an inhalation injury in combination with a cutaneous burn increases the volume of fluid required for resuscitation by as much as 44%. Numerous studies have also shown an increased incidence of pulmonary complications (pneumonia, respiratory failure, or acute respiratory distress syndrome [ARDS]) in patients with burns and inhalation injury when compared with burns alone. Sequelae of inhalation injury include upper airway distortion and obstruction from direct thermal injury as well as impaired pulmonary gas exchange due to effects of irritant gases on lower airways and pulmonary parenchyma. These two components of the inhalation injury have separate time courses and pathophysiological consequences.
Foley described the findings of 335 autopsies performed on patients who died from extensive burns. Intraoral, palatal, and laryngeal burns were not uncommon among patients with inhalation injuries. The most common sites of laryngeal injury were the epiglottis and vocal folds where their edges were exposed. In contrast, thermal necrosis below the glottis and upper trachea was not observed in any of these patients. The lower airways are nearly always protected from direct thermal injury by the efficiency of heat exchange in the oro- and nasopharynx unless the injury involves steam or an explosive blast. This has been demonstrated in an experimental model. Inhalation injury to the lower airways and pulmonary parenchyma is, therefore, almost always due to the effect of toxic or irritant gases.
Clinical suspicion of inhalation injury is aroused by the presence of certain risk factors such as history of exposure to fire and smoke in an enclosed space or a period of unconsciousness at the accident scene, burns including the face and neck, singed facial or nasal hair, altered voice, dysphagia, oral and/or nasal soot deposits, or carbonaceous sputum. The earliest threat from inhalation injury, aside from asphyxia or systemic poisoning, is upper airway obstruction due to edema. Early or prophylactic intubation is recommended when this complication threatens. However exposure to smoke does not always lead to severe injury and, in the absence of overt evidence of respiratory distress or failure, it may be difficult to identify patients who will experience progressive inflammation and ultimately require intubation of the trachea. In a retrospective study, Clark et al. reported that 51% of their patients exposed to smoke inhalation did not require intubation. Unnecessary intubation in the presence of an inflamed laryngeal mucosa risks further damage to the larynx and subglottic area.
Traditional clinical predictors of airway obstruction have been found to be poor predictors of airway compromise in patients with risk factors for inhalation injury. More objective criteria for evaluation of the risk of airway obstruction are often needed. Hunt et al. found fiberoptic bronchoscopy to be a safe and accurate method for diagnosis of acute inhalation injury. They described observations of severe supraglottic injuries associated with mucosal edema obliterating the piriform sinuses and causing massive enlargement of the epiglottis and arytenoid eminence. Haponic et al. made serial observations by nasopharyngoscopy in patients at risk for inhalation injury and found distortions of the upper airway described as compliant, edematous mucosa of the aryepiglottic folds, and arytenoid eminences that prolapsed to occlude the airway on inspiration. Progressive upper airway edema in these patients was correlated with body surface area burned, resuscitative volume administered, and rate of infusion of resuscitative fluids. For patients who are at risk for inhalation injury but lack definitive indications for intubation, fiberoptic nasopharyngoscopy is effective in identifying impending airway compromise. Serial exams may help avoid unnecessary intubations and at the same time identify progressive inflammatory changes and allow intubation before severe airway obstruction and emergent conditions develop.
Lower airway and parenchymal injuries develop more slowly than upper airway obstruction. Prior to resuscitation clinical signs and symptoms relating to respiratory function, chest x-ray, and blood gas analysis may be within normal limits despite significant injury that eventually progresses to respiratory failure requiring intubation and mechanical ventilation.
Linares et al. studied the sequence of morphological changes following smoke inhalation in an experimental sheep model. They observed four discrete but overlapping phases of injury described as exudative, degenerative, proliferative, and reparative. During the first 48 hours the exudative phase was characterized by polymorphonuclear (PMN) infiltration, interstitial edema, loss of type I pneumocytes, and damage to the tracheobronchial epithelium in the form of focal necrosis, hemorrhage, and submucosal edema. The degenerative phase occurred between 12 and 72 hours and was characterized by progressive epithelial damage with shedding of necrotic tissue and formation of pseudomembranes and casts. Hyaline membranes developed over alveolar surfaces. Macrophages began to accumulate to begin absorption of necrotic debris. A proliferative phase was described between days 2 and 7, during which type II pneumocytes and macrophages proliferated. After the fourth day, reparative changes were observed with regeneration of epithelium from spared epithelium from the orifices of glands.
Several reviews provide lucid descriptions of pathophysiological changes resulting from smoke inhalation. Decreased dynamic compliance increases the work of breathing. Increased closing volume and decreased functional residual capacity lead to atelectasis and shunt resulting in hypoxia. Airways become plugged by sloughed epithelium, casts, and mucus. Impaired ciliary action exacerbates the airway obstruction by decreasing the clearance of airway debris. These changes lead to further shunt and allow colonization and pneumonia. Treatment for inhalation injury is empiric and supportive with tracheal intubation and mechanical ventilation. The application of aggressive pulmonary toilet, high-frequency percussive ventilation, and respiratory therapy protocols designed to mobilize obstructing debris are also highly beneficial. As with treating ARDS, it has been suggested that reduced tidal volumes and airway pressures may limit ventilator-induced lung injury in patients with smoke inhalation injury. Recently, however, Sousse et al. examined the effect of reduced tidal volumes on clinical outcomes in patients with inhalation injury requiring mechanical ventilation. Historical controls were patients previously ventilated with tidal volumes of 15 mL/kg. Outcomes of these patients were compared with current patients receiving 9 mL/kg. Patients in the group with lower tidal volumes required fewer ventilator days and had a lower incidence of atelectasis and ARDS. Where the pathophysiology of ARDS relates primarily to alveolar flooding with protein rich fluid, smoke inhalation injury involves more small airway obstruction (cast and necrotic debris) resulting in increased airway resistance, decreased compliance, and V/Q mismatch. It may be that these two disease processes require different treatments.
Carbon monoxide (CO) and cyanide are two major toxic components of smoke. The burn patient with evidence of smoke inhalation injury should be evaluated for the presence of toxicity resulting from these compounds. CO binds hemoglobin 200 times more avidly than oxygen. Therefore CO markedly impairs the association of oxygen with hemoglobin and decreases oxygen-carrying capacity. CO also shifts the oxy-hemoglobin dissociation curve to the left, thus decreasing the release of oxygen into tissues. These factors result in decreased oxygen delivery to tissues and, at critical levels, lead to anaerobic metabolism and metabolic acidosis. Signs and symptoms of CO poisoning include headache, mental status changes, dyspnea, nausea, weakness, and tachycardia. Patients suffering CO poisoning have a normal Pa o 2 and oxygen saturation by routine pulse oximetry. They are not cyanotic. Carboxyhemoglobin must be detected by co-oximetry. Carboxyhemoglobin levels above 15% are toxic and those above 50% are often lethal. The major treatment approach is administration of 100% oxygen and, in severe cases, hyperbaric treatment to increase the partial pressure of oxygen in blood.
Cyanide is also a component of smoke, resulting from the burning of certain plastic products. Cyanide directly impairs the oxidative apparatus in mitochondria and decreases the ability of cells to utilize oxygen in metabolism. These alterations result in conversion to anaerobic metabolism and the development of metabolic acidosis. Signs and symptoms include headache, mental status changes, nausea, lethargy, and weakness. Hydrogen cyanide levels above 100 ppm are generally fatal.
Treatment of cyanide toxicity begins with a high inspired oxygen concentration, which may increase intracellular oxygen tension enough to cause nonenzymatic oxidation of reduced cytochromes or displace cytochrome oxidase and potentiate the effects of administered antidotes. Pharmacological intervention includes methemoglobin generators such as the nitrates (amyl nitrite inhalation 0.2 mL, or sodium nitrite, intravenous 10 mL of 3% solution for adults and 0.13–0.33 mL/kg of 3% solution for pediatrics) and dimethylaminophenol (3.25 mg/kg) to increase methemoglobin levels. Methemoglobin competes with cytochrome oxidase for cyanide. However excessive levels of methemoglobin lead to decreased oxygen-carrying capacity and may be toxic. Direct binding agents have a high affinity for cyanide. Di-cobalt edetate (20 mL of 15% solution for adults or 0.3–0.5 mL/kg of 15% solution for pediatrics) is extremely rapid in action but has significant toxicity, whereas hydroxocobalamin (adults 5–10 g or pediatrics 70 mg/kg), the precursor of vitamin B 12 , has been shown to be safe with few systemic side effects, is actively metabolized by the liver, and avoids renal absorption. Sulfur donors such as sodium thiosulphate (adults 25 mL of 50% solution or pediatrics 1.65 mL/kg of 25% solution) accentuate the bodies' enzymatic conversion of cyanide to thiocyanate in the presence of the mitochondrial enzyme rhodanese, thus decreasing its toxicity and increasing elimination.
Thermal injury has profound effects on the systemic circulation, and hemodynamic management is a major component of perioperative care. The skills and clinical experience of anesthesiologists match very well with the needs of patients presenting with serious burn injury, and anesthesiologists are often asked to participate in the initial resuscitation. Patients may also require surgical intervention such as escharotomy, fasciotomy, or wound excision during the first 24 hours after injury; that is, during the initial fluid resuscitation. Because of this, it is important for anesthesiologists to understand the fundamentals of burn resuscitation. It is also critical for the anesthesiologist to be able to evaluate the quality of the resuscitation and assess the hemodynamic status and physiological reserve of the patient after the initial acute resuscitation. These require familiarity with phasic changes in cardiovascular function that follow major burn injuries.
During the first few days after a large burn injury there is a biphasic change in cardiovascular function. With loss of fluid from the vascular space, hypovolemia develops quickly without aggressive replacement. This is associated with decreased cardiac output and increased systemic resistance. Over the next 2–3 days, if resuscitation is successful, this pattern is reversed. A hyperdynamic pattern develops with significantly elevated cardiac output and decreased systemic vascular resistance. Evaluation of physiological status and planning for perioperative care requires knowledge of these changes.
After massive thermal injury, a state of burn shock develops due to intravascular hypovolemia and, in some cases, myocardial depression. This state of burn shock is characterized by decreased cardiac output, increased systemic vascular resistance, and tissue hypoperfusion. Intravascular hypovolemia results from alterations in the microcirculation in both burned and unburned tissues, leading to the extensive loss of intravascular fluid to the interstitium. Cutaneous lymph flow increases dramatically in the immediate post burn period and remains elevated for approximately 48 hours. The forces responsible for this massive fluid shift involve all components of the Starling equilibrium equation,
where K f is the capillary filtration coefficient, P c is the capillary pressure, P if is the interstitial hydrostatic pressure, σ s the reflection coefficient for protein, π c is the plasma colloid osmotic pressure, and π i the interstitial colloid osmotic pressure. The specific alterations include:
Increase in microvascular permeability (k f and σ) due primarily to the release of local and systemic inflammatory mediators;
Increase in intravascular hydrostatic pressure (P c ) due to microvascular dilatation;
Decrease in interstitial hydrostatic pressure (P i );
Decrease in intravascular oncotic pressure (π c ) due to leakage of protein from the intravascular space
Relative increase in interstitial oncotic pressure due to a smaller decrease in interstitial oncotic pressure (π i ) compared to intravascular oncotic pressure (π c ).
The leakage of protein and fluid into the interstitial space often results in a washout of interstitial colloid and markedly increased lymph flow. The net effect of these changes is the development of massive edema during the first 24–48 hours after thermal injury with a concomitant loss of intravascular volume. The hypotension associated with burn injury is also due, in part, to myocardial depression. It has been 50 years since Baxter and colleagues first described burn-induced cardiac dysfunction. They postulated that a circulating depressant “factor” was present in the plasma, resulting in reduced myocardial contractility. Since then, numerous groups have confirmed similar findings. Although, this “factor” has not been specifically isolated, cytokines including tumor necrosis factor-α (TNF-α), interleukin 1-β (IL-1β), gut-derived factors from plasma and mesenteric lymphatics, and other neurohumoral mediators have been shown to reduce contractility and relaxation properties of the heart. Both systemic activation and local tissue levels of cytokine expression are responsible for early burn-induced myocardial depression (first 2–6 hours) that begins to resolve days within days after injury. There is pronounced synergy among burn injury, sepsis, and other forms of shock with regard to myocardial depression, suggesting a common pathway(s) in cardiac dysfunction.
Clinically the incidence of myocardial dysfunction following severe burn injury and its related sequelae remain somewhat controversial. Studies have often been small, underpowered, and use differing methodologies, and data have been collected at different time periods. Indirect assessments of ventricular function throughout the acute resuscitation period using right heart catheterization found that left ventricular function was hypocontractile in the context of high circulating catecholamine levels. On the other hand, normal cardiac function has also been reported in the initial resuscitation period following burn injury. Goodwin et al., reported an increase in internal fiber shortening using M-mode echocardiography following burn injury. Others reported similar findings at similar time after injury. More recently diastolic dysfunction in the resuscitation period was found to be associated with increased cytokine levels and death. Myocardial injury, indicated by increased plasma troponin, has also been described and associated with reduced stroke work and diastolic dysfunction. Autopsy data in burns show evidence of myocardial ischemia (30–60% cases, for all age groups), suggesting non–coronary artery causes for ischemia. Myocardial ischemia is likely from reduced supply (tachycardia) and increased demand (tachycardia + contractility) due to catecholamine surge and sympathetic activation. Thus cardiac dysfunction in the early (ICU) phase is likely due to myocardial stunning since fibrosis would take longer (e.g., months).
If the patient survives the initial burn shock and is adequately resuscitated, a state of hyperdynamic circulation develops over the following 2–3 days that is supported by a variety of inflammatory mediators. This state of systemic inflammation has been termed the systemic inflammatory response syndrome (SIRS) and is characterized in burned patients by tachycardia, a marked decrease in systemic vascular resistance, and increased cardiac output. SIRS is expressed as a continuum of severity ranging from the presence of tachycardia, tachypnea, fever, and leukocytosis to refractory hypotension, and, in its most severe form, shock and multiple organ system dysfunction. In thermally injured patients, the most common cause of SIRS is the burn itself; however, sepsis (SIRS with the presence of infection or bacteremia) is also a common occurrence.
As a result of these pathophysiological mechanisms, burns involving more than 20% of the patient's TBSA will produce a state of burn shock. In patients with reduced physiologic reserve this can occur with less extensive burns (e.g., 10% TBSA burned). Initial survival from this insult requires aggressive replacement of intravascular fluid. At the same time overresuscitation can cause serious morbidity which, in extreme cases, can be lethal. Several resuscitation protocols utilizing various combinations of crystalloids, colloids, or hypertonic fluids have been developed as guides for administration of the large amounts of fluid needed by patients with acute burns ( Table 13.2 ). Alvarado and colleagues have provided a history of the evolution of these protocols. As these authors point out, little theoretical progress has been made in our understanding of burn resuscitation since current protocols were introduced in the 1970s. As a result, considerable controversy continues regarding such basic decisions as to what fluids to use or what physiological variables to use to titrate volume replacement. Whereas in the past colloid solutions were often avoided during the first 24 hours after burn injury, colloids, especially as albumin or plasma, are returning into favor.
| Colloid formulas | Electrolyte | Colloid | D5W |
|---|---|---|---|
| Evans | Normal saline 1.0 mL/kg/% burn | 1.0 mL/kg/% burn | 2000 mL/24 h |
| Brooke | Lactated Ringer's 1.5 mL/kg/% burn | 0.5 mL/kg | 2000 mL/24 h |
| Slater | Lactated Ringer's 2 liters/24 h | Fresh frozen plasma | 75 mL/kg/24 h |
| Crystalloid formulas | |||
| Parkland | Lactated Ringer's | 4 mL/kg/% burn | |
| Modified Brooke | Lactated Ringer's | 2 mL/kg/% burn | |
| Hypertonic saline formulas | |||
| Hypertonic saline solution (Monafo) | Volume to maintain urine output at 30 mL/h Fluid contains 250 mEq Na/liter |
||
| Modified hypertonic (Warden) | Lactated Ringer's +50 mEq NaHCO 3 (180 mEq Na/liter) for 8 hours to maintain urine output at 30–50 mL/h Lactated Ringer's to maintain urine output at 30–50 mL/h beginning 8 hours postburn |
||
| Dextran formula (Demling) | Dextran 40 in saline—2 mL/kg/h for 8 hours Lactated Ringer's—volume to maintain urine output at 30 mL/h Fresh frozen plasma—0.5 mL/kg/h for 18 hours beginning 8 hours postburn |
||
Isotonic crystalloid is still the most commonly used fluid for resuscitation in U.S. burn centers. The most popular fluid resuscitation regimen, the Parkland Formula, uses isotonic crystalloid solutions and estimates the fluid requirements in the first 24 hours to be 4 mL/kg per TBSA burned. Another popular formula is the modified Brooke formula recommending 2 mL/kg per TBSA burned. With both of these protocols, half is given during the first 8 hours and half given over the next 16 hours. The American Burn Association “consensus formula” recommends resuscitation with 2–4 mL/kg/TBSA burned.
These formulas are only estimates, and fluid requirements will vary considerably among patients with similar percentage of TBSA burned for a variety of reasons; rates and volumes must be titrated according to the patient's response. Fluid administration is generally titrated to maintain mean blood pressure above 70 and urine output at 30–50 mL/h in adults and 0.5–1.0 mL/kg per hour in pediatric patients. The use of invasive hemodynamic monitors allows targeting of determinants of oxygen delivery, which should intuitively lead to more physiologically precise resuscitation. Such efforts, however, have not improved clinical outcome and have generally resulted in more aggressive fluid administration and consequent overresuscitation.
When evaluating the quality of resuscitation, estimates of fluid requirements based on these protocols can reveal significant departure from predicted needs. Review of the patient's physiological status provides evidence of over- or underresuscitation. Crystalloid solutions generally provide adequate volume resuscitation; however the large volumes that are needed result in substantial tissue edema and hypoproteinemia. In addition, a trend toward administration of more fluid than the Parkland Formula would predict has been termed “fluid creep.” As mentioned earlier, overresuscitation can be associated with serious morbidity and even mortality. This has led to a search for interventions that can reduce the volume of fluid needed for resuscitation.
Although colloid was included in earlier resuscitation formulas, it was dropped during the 1970s. An overall clinical benefit was difficult to demonstrate for colloid solutions, especially when given during the first 12 hours after injury. Pruitt and colleagues reported that the addition of colloids to resuscitation fluid during the first 24 hours did not increase the intravascular volume more than crystalloid fluid alone. It was also suggested that colloid use could contribute to pulmonary edema during the post-resuscitation period. Because of the added cost with little established benefit, colloid solutions have not been used routinely for initial volume resuscitation in burned patients in the United States until recently. In 1998, a highly publicized Cochrane meta-analysis concluded that albumin administration increases mortality in critically ill patients, including patients with severe burn injuries. As relating specifically to burn-injured patients, there were serious methodological flaws with this meta-analysis sufficient for most clinicians to discount the Cochrane conclusion that albumin administration increases mortality among burn patients. In a recent review, Saffle reported that, in nearly all studies of burn resuscitation, the use of colloid solutions has reduced the volume required as well as the complications of overresuscitation in these patients. When administered specifically for the therapeutic goal of limiting the volume necessary for resuscitation, the use of colloid solutions is a rational choice for many clinicians.
In a prospective randomized study, the use of plasma for volume resuscitation was found to reduce volume infused and weight gain along with intra-abdominal pressure and the incidence of abdominal compartment syndrome (see later discussion). These outcome variables have not been used for comparing crystalloid and colloid resuscitation in the past. With the trend toward larger volumes for initial resuscitation, with associated morbidity, it may be that the use of colloid is beneficial for larger injuries. The use of plasma during resuscitation may involve more than just volume and increased colloid osmotic pressure. Kozar and colleagues demonstrated that plasma but not crystalloid resuscitation partially reverses hemorrhage-induced endothelial damage in an experimental animal model. Restoration of the endothelial glycocalyx may help recovery of the capillary function and reduce fluid extravasation.
Crystalloid solutions alone provide adequate intravascular expansion without unacceptable complications in many patients. However not all patients respond favorably to crystalloid resuscitation. Box 13.4 provides a list of a number of factors that can significantly increase the volume of fluid needed for resuscitation. For patients with these features, and in other patients for unknown reasons, very large volumes of crystalloid are needed to support blood pressure and maintain urine output. In these cases the excessive volume of fluid can result in dangerous morbidity, such as abdominal compartment syndrome ( Fig. 13.5 ). For example, patients who receive more than 250 mL/kg during the first 24 hours are at risk for abdominal compartment syndrome. It is important to recognize when resuscitation is difficult so that measures can be taken to limit morbidity. Extensive recent experience with burn injuries resulting from military conflicts has produced protocols that include administration of albumin for managing patients who do not have an adequate response to appropriate volumes of crystalloid fluid. Likewise many civilian burn centers have adopted “colloid rescue” for patients with inadequate response to resuscitation. Lawrence and colleagues evaluated responses of burn patients as an hourly ratio (I/O) of volume of fluid infused (mL/kg/%TBSA burned/h) to urine output (mL/kg/h). Patients who responded favorably to fluid administration maintained a ratio of less than 0.4, but poor responders had progressively increasing ratios to a maximum of 1.97. When patients were identified as poor responders, 5% albumin was added to their resuscitation fluid regimen. After addition of albumin each of the patients responded with a prompt decrease in the I/O ratio for the remainder of the resuscitation. In this study albumin rescue was initiated more than 12 hours after injury. The authors noted that these patients still received large volumes of fluid and that this volume might have been reduced if colloid had been given earlier. In the future it may be feasible to recognize poor responders early during resuscitation to allow earlier intervention. This study also emphasizes the heterogeneity of the burned patient population with regard to response to treatment. This heterogeneity may also explain in part why it has been difficult to show benefit from colloid administration. It would be difficult to show benefit from an intervention if it is given to a group of patients comprising both responders and nonresponders.
Inhalation injury
Delay in resuscitation
Crush injury
Electrical injury
Large full-thickness burns
Methamphetamine lab accidents
Associated injuries
The use of hypertonic saline, either alone or in conjunction with colloids, has also been advocated by some in the initial resuscitation of burned patients. Among the potential benefits are reduced volume requirements to attain similar levels of intravascular resuscitation and tissue perfusion compared to isotonic fluids. Theoretically, the reduced volume requirements would decrease the incidence of pulmonary and peripheral edema, thus reducing the incidence of pulmonary complications and the need for escharotomy. Hypertonic saline dextran solutions have been shown to expand intravascular volume by mobilizing fluids from intracellular and interstitial fluid compartments. Although hypertonic saline dextran solutions will transiently decrease fluid requirements, there is potential for a rebound in fluid resuscitation needs. Therefore most burn centers continue to employ isotonic crystalloid fluids rather than hypertonic solutions for initial resuscitation of patients in burn shock.
Unfortunately there is no single physiological variable that is always reliable as an end point to guide resuscitation in acute burn patients. Several variables are used to assess the adequacy of volume resuscitation in burned patients ( Box 13.5 ). The overall goal is early volume resuscitation and establishment of tissue perfusion. Traditionally urine output (0.5–1 mL/kg per h) and normalization of blood pressure (mean arterial blood pressure of greater than 70 mm Hg) have been used as endpoints. However, some studies indicate that these parameters may be poor predictors of adequate tissue perfusion. Jeng and colleagues showed that attaining urine outputs of greater than 30 mL/h and mean blood pressures of greater than 70 mm Hg correlated poorly with other global indicators of tissue perfusion such as base deficit and blood lactate levels. In order to maintain perfusion of vital organs such as heart and brain, blood flow is often redistributed away from splanchnic organs. Persistent hypoperfusion of these organs ultimately results in tissue injury and may be a contributing factor to multisystem organ dysfunction. Several studies have shown that normalization of blood pressure, heart rate, and urine output alone does not by itself correlate with improved outcome. Therefore during the preoperative assessment of the burn patient, the anesthesiologist should not base the cardiovascular assessment on one variable but use a more global approach to evaluating the patient's physiological status and reserve.
Normalization of blood pressure
Urine output (1–2 mL/kg/h)
Blood lactate (<2 mmol/L)
Base deficit (<−5)
Gastric intramucosal pH (>7.32)
Central venous pressure
Cardiac index (Cl) (4.5 L/min/m 2 )
Oxygen delivery index (DO 2 L) (600 mL/min/m 2 )
When vital signs and urine output are within normal limits, measurements of metabolic function may provide more subtle evidence of impaired perfusion. In burn patients, tissue perfusion is not uniform. Perfusion of the splanchnic beds may be sacrificed in order to maintain the perfusion of heart, brain, and kidneys. Blood lactate and base deficit provide indirect metabolic global indices of tissue perfusion. Lactic acid is a byproduct of anaerobic metabolism and is an indicator of either inadequate oxygen delivery or impaired oxygen utilization. In the absence of conditions such as cyanide poisoning or sepsis that alter oxygen utilization at the cellular level, lactate level may be a useful marker of oxygen availability. Wo and colleagues found serum lactate to be the most predictive index of adequate tissue perfusion, and a lactate level of less than 2 mmol/L in the first 24–72 hours after burn injury correlated with increased survival. Base deficit is another indirect indicator of global tissue perfusion. The base deficit is calculated from the arterial blood gas using the Astrup and Siggard-Anderson nomograms. Although it is a calculated and not directly measured variable, base deficit provides a readily obtained and widely available indicator of tissue acidemia and shock. Base deficit has been shown to correlate closely with blood lactate and provides a useful indicator of inadequate oxygen delivery. A retrospective study by Kaups et al. showed that base deficit was an accurate predictor of fluid requirements, burn size, and mortality rate.
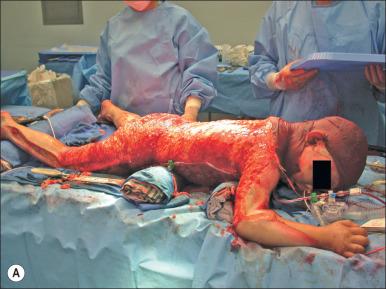
Overresuscitation can also be a serious complication of fluid administration to acute burn patients. Blindness due to ischemic optic neuropathy has been reported as a complication of burn resuscitation. Greenhalgh and Warden first described the association of increased abdominal pressures and compartment syndrome with burn resuscitation. Several studies since then have described the common occurrence of increased intra-abdominal pressure with large-volume burn resuscitation. Intra-abdominal hypertension is termed abdominal compartment syndrome when it is associated with impaired respiration, circulation, and urine output. Mechanical ventilation is impaired by pressure on the diaphragm, circulation is impaired by restricted venous return due to caval compression, and urine output is impaired by compression of renal vessels. When this pattern presents, the patient should be examined for elevated intra-abdominal pressure. This can be accomplished by measuring bladder pressure: 50 mL of saline is instilled into the bladder through the Foley catheter and the height of the saline column above the symphysis pubis is measured (1.36 cm of H 2 O = 1 mm Hg). Conservative treatment of elevated intra-abdominal pressure includes attempts to limit the volume of intravenous fluid needed for resuscitation. The inclusion of plasma with resuscitation fluids has been found to reduce the volume required and was associated with significantly lower intra-abdominal pressures. When intra-abdominal hypertension occurs it can be relieved to a degree by optimizing sedation and analgesia. Diuresis with furosemide and muscle relaxants to reduce muscle tone have been used to reduce intra-abdominal pressure. More invasive measures include escharotomies, percutaneous peritoneal dialysis catheter drainage, and laparotomy.
Serious burns can also be caused by contact with caustic or corrosive chemicals. An increasingly common specific example is burns related to the illicit production of methamphetamine. There has been a dramatic increase in burn injuries from explosions and fires related to methamphetamine production in illegal labs. Victims of these accidents present unique challenges for a variety of reasons. Substances used in methamphetamine production include chemicals that are corrosive and toxic (e.g., anhydrous ammonia, hydrochloric acid, red phosphorous, and ephedrine). Other ingredients are flammable (acetone, alcohol, and gasoline), and explosions can coat the victims with all these chemicals. As a result, in addition to the victim's toxic exposure, contacting incompletely decontaminated victims of these accidents has injured first responders and hospital workers.
In addition to exposures just described, these patients are usually intoxicated with methamphetamine, as demonstrated by positive urine screen, and may have inhaled toxic fumes such as phosphine gas. Santos et al. found the incidence of inhalation injury twice as great in victims of methamphetamine-related burns as in age- and burn-matched controls. Among their patients requiring intubation for inhalation injury, methamphetamine users also required roughly twice as many ventilator days. Clinical studies have consistently observed increased fluid requirements for resuscitation of methamphetamine patients. For example, Santos et al. found that resuscitation volumes were 1.8 times greater for methamphetamine users with burns than for controls. In addition, methamphetamine users with burns experienced more behavioral problems. These patients are more often agitated and require restraints. Santos et al. reported that all their methamphetamine patients required greater than normal doses of sedatives and displayed what they referred to as “withdrawal-type syndrome.” This behavior may be due to withdrawal of methamphetamine from chronic users.
Acute kidney injury (AKI) is a very common and devastating complication of severe burn injuries. The incidence is often estimated at 30% or more and can increase the mortality in these patients to more than 80%. The risk of AKI with burns increases with all the features intuitively associated with poor outcome. Despite improvements in critical care, AKI continues to complicate burn care and increase mortality. There have been some indications of an improvement, however. Jeschke and colleagues have shown a decrease in mortality in pediatric burn patients with ARF to 56% since 1984. Also, Chung and colleagues found that early application of continuous venovenous hemofiltration in patients with evidence of AKI, especially in the presence of inhalation injury, improved survival compared with conventional care that included hemodialysis after more stringent criteria were met.
The pathogenesis of AKI in burn patients is complex and can involve a number of interconnected mechanisms. A difference between AKI that occurs early after injury has been distinguished from injury developing later. Early AKI is associated with reduced renal blood flow resulting from acute burn shock, whereas AKI that occurs later is associated with sepsis and exposure to nephrotoxic drugs. Early AKI carries a worse prognosis than the late form. Continuous evaluation of the patient's response to fluid resuscitation is necessary for the early recognition and correction of under- or overresuscitation to prevent early AKI.
Another mechanism of early AKI is caused by myoglobinemia resulting from rhabdomyolysis due to compartment syndrome or electrical injury. Treatment of this form of AKI is difficult. Brown and colleagues found that rates of renal failure, dialysis, or mortality were not altered by treatment of rhabdomyolysis with bicarbonate and mannitol. Early diagnosis and treatment of compartment syndrome or muscle damage due to electrical injury is, therefore, critical to preventing its development.
The presence of renal failure complicates intraoperative management. It seriously reduces the margin of error for fluid replacement of blood loss. A central venous catheter (CVC) to monitor filling pressure may be more useful in these patients. Electrolyte balance, especially potassium, should be watched closely and extra caution with dosage and rate of administration of nephrotoxic drugs such as antibiotics is needed.
Increased metabolic rate is the hallmark of the metabolic alterations that take place after thermal injury. The magnitude of the hypermetabolism is influenced by the size of the burn wound, how the burn patient is treated, and the ambient temperature of the patient. Within the range of 30–70% TBSA burn injury, the hypermetabolism tends to be proportional to the size of the burn wound. With burns beyond this range, the hypermetabolism appears to plateau and only increases in smaller increments. Septic complication is an important factor that can increase the metabolic response, as does the physiologic stress of pain. It has been observed that modern-day treatment of burn injuries with early excision and closed-wound treatment ameliorates this hypermetabolism. Burn patients increase their metabolic rate in an effort to generate heat according to a new threshold set point for the body temperature that is influenced by the size of the burn (see the section “ Thermoregulation in Burn Patients ”). The recognition of this fact has led to an increased awareness of the importance of the ambient temperature in modulating the hypermetabolism of the burn patient. By indirect calorimetry, patients with major burn injuries treated according to current standards have resting energy expenditures that are 110–150% above values recorded in nonburned subjects.
As a result of this hypermetabolic response, the acutely burned patient has an increased O 2 consumption along with an increased CO 2 production that demands a higher respiratory effort. The anesthetic care of the acute burned patient has to accommodate these changes, and frequently this has to be done in patients with compromised pulmonary function due to burn injury.
The hypermetabolic pattern also increases caloric needs. Numerous studies have shown that optimized nutritional care not only can ameliorate the burn-associated state of catabolism and immune suppression, but also can improve wound healing. Oral or enteral feeding is recognized as the optimal feeding route of the burned patient. Frequently the acute burn patient is fed continuously over extended time periods. If standard guidelines for perioperative fasting are implemented, recurrent operative procedures can significantly impinge on the nutritional needs of the patient and ultimately cause a caloric deficit. Each surgical procedure requiring general anesthesia theoretically necessitates a 10-hour interruption of enteral nutritional support (fasting for 8 hours preoperatively and 2 hours postoperatively). To avoid this interruption of nutritional support, continuation of feeding via a post-pyloric feeding tube has been used. One study indicates that this practice provides a favorable gut oxygen balance. Varon et al. reviewed records of 17 patients fed intraoperatively through post-pyloric feeding tubes and 16 patients fasted for surgery. These patients had an average of seven surgical procedures each. There were no clinical adverse effects with intraoperative feeding, and these patients met nutritional goals sooner than patients fasted for surgery. Larger studies are necessary to establish the safety of intraoperative feeding but many practitioners consider this safe in the presence of a cuffed tracheal tube.
Severe insulin resistance with hyperglycemia and concurrent hyperinsulinemia is a key feature of the metabolic alterations of burn injury. Critical care of burn patients often involves parenteral nutritional support and may also include insulin infusions. It is important to recognize these interventions during the preoperative evaluation of the burn patient in the ICU. Oxygen consumption and glucose balance are altered when general anesthesia, muscle relaxation, and mechanical ventilation are employed. Increased sympathetic tone due to the stress of surgical trauma can also alter glucose production and insulin resistance. These changes often result in significant changes in blood glucose levels that require treatment.
Maintenance of proper body temperature is an important factor in the care of severely burned patients. The thermoregulatory system is controlled by three major components. These include the afferent system that senses changes in core body temperature and transmits this information to the brain, the central regulatory mechanisms located primarily in the hypothalamus that process afferent input and initiate responses, and the efferent limb that mediates specific biological and behavioral responses to changes in core body temperature ( Fig. 13.6 ). Temperature is sensed by Aδ and C fibers present in peripheral tissues such as skin and muscle, as well as in core tissues such as brain, deep abdominal tissues, and thoracic viscera. The vast majority of afferent input arises from the core tissues. Because the skin is in direct contact with the environment, it senses immediate changes in environmental temperature. However the overall afferent input of the skin and other peripheral tissues is estimated to be only 5–20% of total afferent thermoregulatory input. Therefore loss of skin following a burn injury is not likely to markedly alter overall afferent input. Wallace and colleagues have shown that burn patients perceive changes in ambient temperature as effectively as normal controls. This is likely due to the retained ability of burn patients to sense changes in core temperature and transmit this information to the central nervous system. Central control of temperature is a complicated system that is not well understood. The hypothalamus plays an important role in temperature regulation, but the complete mechanism of temperature control is likely to be multifaceted and is an area of intense research. Regardless of the ultimate control mechanisms, temperature control can be divided into three main functions: threshold, gain, and maximum response intensity.
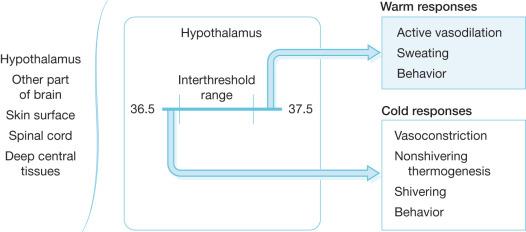
Threshold encompasses a set point at which responses to temperature change are initiated. In normal individuals the threshold range is generally near 36.5–37.5°C. In burn patients, the threshold set point is higher, and the increase is proportional to the size of the burn. The work of Caldwell and colleagues predicts that the temperature set point will increase by 0.03°C/%TBSA burn. This increase in temperature threshold appears to be due to the hypermetabolic state and the presence of pyrogenic inflammatory mediators such as TNF, IL-1, and IL-6 that are present after thermal injury. The elevated temperature set point can be decreased by administration of indometacin, which suggests that prostaglandins act as final common mediators of this response.
Gain describes the intensity of response to alterations in temperature. In most cases the gain of thermoregulatory responses is very high, with response intensity increasing from 10% to 90% with only a few tenths of a degree change in core temperature. This response is maintained in most burn patients, resulting in a further increase in metabolic rate. Burn patients respond with a brisk increase in heat generation and metabolic rate in response to changes in core body temperature. However work by Shiozaki and colleagues has shown that burn patients who are slow to respond to postoperative hypothermia are at increased risk of mortality. The decreased responsiveness may be due, in part, to tissue catabolism, poor nutrition, or sepsis. In addition, the response to relative hypothermia is characterized by increased catecholamine release, tissue catabolism, and hypermetabolism. These responses further stress burn patients and decrease their ability to respond to their primary injury.
The most important efferent responses to hypothermia are behavioral responses such as gaining shelter, covering up, and seeking a more desirable ambient temperature. In the acute postburn setting, most of these behaviors are impeded by positioning, sedation, and inability to seek a more favorable environment. Therefore caregivers must be attentive to the patient's temperature and perception of cold so that measures can be undertaken to optimize the patient's temperature. Cutaneous vasoconstriction is another important mechanism for preserving heat and core body temperature. In unburned persons, a temperature gradient of 2–4°C exists between skin and core tissues. This gradient is maintained by cutaneous vasoconstriction. Without cutaneous vasoconstriction, heat is redistributed from the core compartment to the periphery. This heat is ultimately lost to the environment. Peripheral vasoconstriction minimizes temperature redistribution and acts to maintain core body temperature. This mechanism of heat preservation is lost with the loss of large areas of skin, particularly if cutaneous tissues are excised down to the fascial level. The loss of skin facilitates the loss of core body heat into the environment and places the burn patient at risk for core hypothermia. Another mechanism of heat loss in burn patients is evaporation. Burn patients can lose as much as 4000 mL/m 2 burned per day of fluids through evaporative losses. Mechanisms of nonshivering heat production and shivering remain intact in burn patients. However, shivering increases metabolic requirements and is likely deleterious.
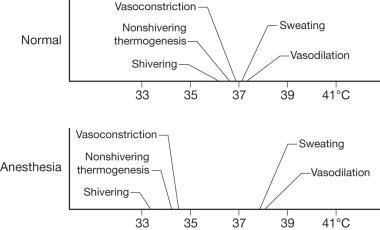
The induction of anesthesia results in relative ablation of thermoregulatory mechanisms and puts the patient at further risk for developing hypothermia. Patients under general anesthesia exhibit a markedly decreased threshold for responding to hypothermia ( Fig. 13.7 ). This is particularly important in burn patients, given their high temperature set point and the deleterious effects of further stress responses and hypermetabolism. Most anesthetics decrease nonbehavioral responses to hypothermia such as vasoconstriction, nonshivering thermogenesis, and shivering. Of course, behavioral responses are ablated during general anesthesia. Therefore, it is the responsibility of the intraoperative caregivers to monitor and maintain patient temperature.
Actions such as maintaining higher ambient air temperature, covering extremities and head, applying warm blankets, utilizing radiant heaters and forced air warming devices, warming fluids and blood, and warming gases are usually effective in maintaining core temperature if applied aggressively. Ideally hypothermia should be corrected prior to transport to the operating room. Hypothermia revealed in the preoperative evaluation may be due to inadequate resuscitation or metabolic instability. Either situation may predispose burn patients to intolerance of anesthetic drugs or the stress of surgery.
Burn injury and its treatment result in physiological changes that may profoundly alter the response to drugs. These changes alter both pharmacokinetic and pharmacodynamic determinants of drug response. Altered drug response in burned patients may require deviation from usual dosages to avoid toxicity or decreased efficacy. The complex nature of the pathophysiological changes and interpatient variation in the nature and extent of burn injuries, as well as the dynamic nature of these changes during healing and recovery make it difficult to formulate precise dosage guidelines for burn patients. However an understanding of the systemic response to large burn injuries can help predict when an altered drug response can be expected and how to compensate.
The two distinct phases of cardiovascular response to thermal injury can affect pharmacokinetic parameters in different ways. During the acute or resuscitation phase the rapid loss of fluid from the vascular space due to edema formation results in decreases of cardiac output and tissue perfusion. Volume resuscitation during this phase dilutes plasma proteins and expands the extracellular fluid space especially, but not exclusively, around the burn injury itself. Decreased renal and hepatic blood flow during the resuscitation phase reduces drug elimination by these organs. Also, decreased cardiac output will accelerate the rate of alveolar accumulation of inhalation agents, which may result in an exaggerated hypotensive response during induction of general anesthesia.
After approximately 48 hours, the hypermetabolic and hyperdynamic circulatory phase is established with increased cardiac output, oxygen consumption and core temperature. During this phase, increased blood flow to the kidneys and liver may increase clearance of some drugs to the point where increased doses are required.
Many drugs are highly protein bound. Drug effects and elimination are often related to the unbound fraction of the drug that is available for receptor interaction, glomerular filtration, or enzymatic metabolism. The two major drug-binding proteins have a disparate response to burn injury. Albumin binds mostly acidic and neutral drugs (diazepam or thiopental) and is decreased in burn patients. Basic drugs (pKa >8, propranolol, lidocaine, or imipramine) bind to α-acid glycoprotein (AAG). AAG is considered an acute-phase protein and its concentration may double after burns. Since these drug-binding proteins respond in opposite ways to thermal injury it can be expected that changes in drug binding and function will depend on which of these proteins has the highest affinity for the drug in question. Martyn et al. observed decreased plasma albumin concentration and increased plasma AAG concentration in burn patients. These observations were associated with an increased unbound fraction for diazepam (bound by albumin) and a decreased unbound fraction for imipramine (bound by AAG).
Volume of distribution (V d ) is changed by alterations to either extracellular fluid volume or protein binding. Large changes in both of these variables occur with thermal injuries. Drugs with high protein binding and/or a V d in the range of the extracellular fluid volume may be associated with clinically significant alterations of V d in burned patients. V d is the most important determinant of drug response following a rapid loading dose. However adjustments in dose to compensate for altered V d are indicated only when V d for the drug is small (<30 L) because with larger V d only a small fraction of the drug is present in the plasma.
Clearance is the most important factor determining the maintenance dose of drugs and can influence the response to drugs given by infusion or repeated bolus during anesthesia. Drug clearance is influenced by four factors: metabolism, protein binding, renal excretion, and novel excretion pathways. The characteristic hepatic extraction of a particular drug influences changes in its clearance that occur after thermal injury. Drugs vary greatly in their extraction by the liver. Hepatic clearance of drugs highly extracted by the liver depends primarily on hepatic blood flow and is insensitive to alterations in protein binding. Clearance of these drugs may increase during the hyperdynamic phase when hepatic blood flow is increased. In contrast, clearance of drugs that have a low hepatic extraction coefficient is not affected by changes in hepatic blood flow but is sensitive to alterations in plasma protein levels. For these drugs it is the unbound fraction of the drug that is metabolized. As noted, changes in unbound fraction depend on whether the drug is bound by albumin or AAG. Changes in protein levels produce clinically significant pharmacokinetic changes only for drugs that are highly bound (>80%).
During resuscitation, renal blood flow may be reduced and renal excretion of drugs may be impaired. Later, during the hypermetabolic phase, renal blood flow is increased as a result of the elevated cardiac output. During this period excretion of certain drugs can be increased to the point that the dose may need to be increased. Loirat et al. reported increased glomerular filtration rates and reduced half-life of tobramycin in burn patients. However, this was age-dependent and patients over 30 years of age did not have increased glomerular filtration or reduced half-life.
Burn patients may also experience altered drug clearance due to novel excretion pathways. Glew et al. found that 20% of a daily gentamicin dose was eliminated in the exudates lost to wound dressings. In addition, rapid blood loss during surgery may speed the elimination of drugs when blood loss and transfusion amount, essentially, to an exchange transfusion.
Hepatic clearance of drugs with low extraction coefficients is also sensitive to alterations of hepatic capacity (enzyme activity). There is evidence of impairment of hepatic enzyme activity in burn patients. Phase I reactions (oxidation, reduction, or hydroxylation by the cytochrome P-450 system) are impaired in burn patients, whereas phase II reactions (conjugation) seem to be relatively preserved. However, these generalizations do not always produce predictable alterations in pharmacokinetic parameters. For example, contradictory observations of morphine clearance in burn patients have been reported. Morphine metabolism is by glucuronidation. This is a phase II reaction that is normally retained in thermally injured patients. Morphine clearance in burn patients has been reported unchanged or decreased. With so many variables involved, such as hepatic blood flow, V d , plasma proteins, multiple drug exposure, and variation in burn injury, this inconsistency is not surprising. The key to effective drug therapy in burn patients is to monitor drug effects and carefully titrate the dose to the desired effect.
In terms of anesthetic management, the most profound and clinically significant effect of burn injuries on drug response relates to muscle relaxants. Burn injuries of more than 25% TBSA influence responses to both succinylcholine and the nondepolarizing muscle relaxants. In burned patients, sensitization to the muscle-relaxant effects of succinylcholine can produce exaggerated hyperkalemic responses severe enough to induce cardiac arrest. In contrast, burned patients are resistant to the effects of nondepolarizing muscle relaxants. These changes are explained by up-regulation of skeletal muscle acetylcholine receptors.
Martyn and Richtsfeld have recently reviewed the mechanisms of exaggerated hyperkalemic responses to succinylcholine. There are several disease states, including burns, denervation, and immobilization, that are associated with potentially lethal hyperkalemic responses to succinylcholine. The molecular mechanism appears to be both quantitative and qualitative changes in skeletal muscle postsynaptic nicotinic acetylcholine receptors. Animal and human studies consistently demonstrate an association of increased numbers of skeletal muscle acetylcholine receptors with resistance to nondepolarizing muscle relaxants and increased sensitivity to succinylcholine. In addition, the distribution of the new receptors is altered. Nicotinic receptors are normally restricted to the neuromuscular synaptic cleft, but, in these disease states, new receptors are distributed across the surface of the skeletal muscle membrane. The new receptors are also a distinctly different isoform (α7AChR) that has been referred to as an immature, extrajunctional, or fetal receptor. The immature receptors are more easily depolarized by succinylcholine, and their ion channel stays open longer. The immature receptors are also strongly and persistently depolarized by the metabolite of acetylcholine and succinylcholine, choline. It has been suggested that the hyperkalemic response to succinylcholine after burn or denervation injury results when potassium is released from receptor-associated ion channels across the entire muscle cell membrane rather than just the junctional receptors. Depolarization persists because the channels stay open longer and the breakdown product of succinylcholine, choline, is also a strong agonist for the immature receptors.
Cardiac arrest in burned patients after succinylcholine administration was first reported in 1958. It was not until 1967, however, that an exaggerated hyperkalemic effect was identified as the cause of this phenomenon. However, considerable individual variability exists, and only a few patients in these series developed dangerously high potassium levels. The size of the increase was greatest about 3–4 weeks after injury. The earliest exaggerated hyperkalemic response described occurred 9 days after injury, and normal responses were observed in the remaining patients in this series for up to 14–20 days. The shortest post-burn interval of an association of succinylcholine with cardiac arrest was 21 days when a 4-year-old patient experienced a fatal cardiac arrest during a fourth anesthetic induction and intubation with succinylcholine. Controversy has surrounded recommendations regarding the safe use of succinylcholine after burn injury. Various authors recommend avoidance of succinylcholine at intervals ranging from 24 hours to 21 days after burn injury. A series of letters from experts in this area to the editor of Anesthesiology illustrates the controversy. It was pointed out by Martyn that, at the time when the mechanism of the cardiac arrest after succinylcholine was elucidated, surgical treatment of burns was delayed for approximately 2 weeks until the eschar spontaneously separated. As a result, there are few clinical data regarding potassium changes during this early period. On the basis of indirect evidence from experimental data, Martyn recommended avoidance of succinylcholine starting 48 hours after injury. This seems rational and prudent. Brown and Bell described the super-sensitivity of burned pediatric patients to the relaxant effect of succinylcholine. They observed more than 90% depression of muscle activity with 0.2 mg/kg succinylcholine without dangerous hyperkalemia. Despite these observations Brown and Bell state that it is generally advisable not to use succinylcholine in patients with large burns. The question remains: in the presence of life-threatening laryngospasm in a burn patient, is it acceptable to give a small dose of succinylcholine (e.g., 0.1 mg/kg) to relieve laryngospasm without full paralysis and accept a theoretical risk of treatable hyperkalemia in order to treat a real and immediate risk of asphyxia? Administration of a large dose of a nondepolarizing relaxant requires more time for onset than succinylcholine and produces total paralysis. There is not enough clinical evidence to answer this question conclusively, and, at present, it remains a matter of clinical judgment.
In some cases another clinical choice is now available since the U.S. Food and Drug Administration (FDA) approval of sugammadex in December 2015. Sugammadex is a reversal agent with a novel and unique mechanism of action. It is a cyclodextrin that irreversibly chelates aminosteroid muscle relaxants by forming a 1 : 1 tight complex (encapsulation) with the relaxant molecule, which reduces the concentration of the free drug in plasma below the minimum necessary for muscle relaxation. A Cochrane review found sugammadex to be an effective reversal agent without evidence of increased frequency of adverse effects when compared to neostigmine. In fact, sugammadex has been found to provide more rapid recovery of more than 90% twitch strength than either spontaneous recovery with succinylcholine or reversal with neostigmine. Sugammadex has a lower affinity for pancuronium, and higher doses are required for this agent than for rocuronium or vecuronium. Although the frequency of adverse effects due to sugammadex has been very low, there have been some serious complications associated with its administration, including anaphylaxis and severe bradycardia requiring chest compressions. Another more common issue relates to the potential for sugammadex to inactivate hormonal contraceptives. Female patients treated with contraceptives should be advised to use an alternative form of contraception for a period after receiving sugammadex.
As of this writing (January 2017), sugammadex has not yet received FDA approval for pediatric patients, and most reported pediatric data are in the form of case reports and small studies. The clinical experience with sugammadex in children has been reviewed by Tobias. Sugammadex has been found to be an effective rescue agent in situations where pediatric patients cannot be intubated or ventilated. It has also proved useful in pediatric patients with neuromuscular diseases such as Duchenne muscular dystrophy and myotonic dystrophy. In some situations rocuronium might be an additional choice to relieve laryngospasm in an acute burn patient when there is a risk of intubation or ventilation impossibility if sugammadex is available since it can reverse relaxation with rocuronium faster that spontaneous recovery from succinylcholine. Still, the onset of action for rocuronium is slower than for succinylcholine.
Responses to nondepolarizing relaxants are also altered by burn injury. Three- to fivefold greater doses are required to achieve adequate relaxation. Resistance is apparent by 7 days after injury and peaks by approximately 40 days. Sensitivity returns to normal after approximately 70 days. Two reports described slight but measurable resistance to nondepolarizing relaxants persisting for more than a year after complete healing of the wounds. The mechanism of the altered response appears to involve pharmacodynamic rather than pharmacokinetic changes. Up-regulated immature receptors are less sensitive to nondepolarizing relaxants. Burns of greater than 25% TBSA require higher total dose and greater plasma concentrations of nondepolarizing blockers to achieve a given level of twitch depression.
Proliferation of acetylcholine receptors across the muscle membrane has been used to explain both resistance to nondepolarizing muscle relaxants and the exaggerated hyperkalemic response to succinylcholine. The observation of resistance of a patient to metocurine for up to 463 days after the burn has been used to suggest that hyperkalemic responses to succinylcholine also could persist for more than a year. However, no pathologic hyperkalemic responses to succinylcholine in burned patients have been reported more than 66 days after burns.
In contrast to other nondepolarizing neuromuscular blockers, mivacurium dosage requirements in pediatric patients appear to be unchanged by the burn injury. The time to onset of drug action, the degree of paralysis achieved by a specific dose, and the rate of infusion required to maintain a given level of relaxation were all the same in burn patients as values reported for nonburned control patients. Plasma cholinesterase activity is reduced in burn patients. In a study by Martyn, the observation of an inverse relationship between plasma cholinesterase activity and recovery time of 25–75% twitch tension suggests that reduction of metabolic degradation of mivacurium may compensate for other factors that induce resistance to relaxants. This observation suggests that mivacurium can be administered to burn patients in normal doses that would avoid cardiovascular perturbations associated with required larger doses of other relaxants in burn patients. It also illustrates the complex nature of altered drug responses in burned patients.
If injuries do not preclude conventional airway management (i.e., mask fit, jaw lift, and mouth opening), standard induction and intubation procedures are appropriate. Hu et al. reported that gastric emptying was not delayed in patients with severe burns so that a rapid-sequence induction is not necessary. However, attention should be given to gastric residuals during enteric feeding. Development of sepsis can slow gastric emptying, which can result in retained fluids in the stomach and risk of aspiration.
When burns include the face and neck, swelling and distortion may make direct laryngoscopy difficult or impossible. In addition, loss of mandibular mobility may impair airway manipulation and make mask ventilation difficult. Fiberoptic intubation while maintaining spontaneous ventilation is a safe and reliable technique under these conditions. Fiberoptic intubation can be performed in awake adults but pediatric patients are unable to cooperate and must be sedated. Since most anesthetics cause the collapse of pharyngeal tissues and airway obstruction, they are unsuitable for fiberoptic intubation in patients whose airway would be difficult to manage with a mask. Ketamine, however, is unique among anesthetic drugs because it maintains spontaneous ventilation and airway patency.
Ketamine anesthesia has been found safe and effective for airway management in infants with difficult airways caused by congenital airway anomalies. Reports of successful nasotracheal intubation in infants with congenital airway malformations have been made both by manipulations guided by fiberoptic nasopharyngoscopy and the conventional technique of fiberoptic intubation with the endotracheal tube mounted on the fiberscope. In the latter case an ultra-thin bronchoscope (2.7 mm) was required because a larger fiberscope would not fit through the appropriate-sized endotracheal tube. To facilitate intubation under ketamine anesthesia, topical anesthesia of the larynx with lidocaine prior to instrumentation of the larynx is advised. Since the ultra-thin bronchoscope lacks a working channel for administration of topical lidocaine, fiberoptic intubation with the 2.7-mm bronchoscope was preceded by nasopharyngoscopy with a 3.5-mm fiberscope for administration of topical lidocaine. At SBH Galveston we have also found this technique, utilizing two fiberscopes, effective in infants with burn injuries. Wrigley et al. evaluated the use of a 2.2-mm intubating fiberscope during halothane anesthesia in ASA 1 or 2 children aged 6 months to 7 years. In this series of 40 patients, a number of complications were experienced including laryngospasm and failure to achieve intubation with the fiberscope. This experience is in contrast to numerous reports of safe and effective airway management with ketamine.
Securing an endotracheal tube in a patient with facial burns presents a variety of problems, and numerous techniques have been described. Tape will not adhere to burned skin and ties crossing over burned areas will irritate the wound or dislodge grafts. In addition, if the head is burned, especially if the patient has also required a large fluid resuscitation, then during the first few days after injury, the head is swelling due to edema formation or shrinking as edema resolves. Under these conditions tape surrounding the head will become loose or too tight within hours. A useful technique to avoid these problems involves the use of a nasal septal tie with one-eighth-inch umbilical tape ( Fig. 13.8 ). The umbilical tape is placed around the nasal septum using 8 or 10 French red rubber catheters that are passed through each naris and retrieved from the pharynx by direct laryngoscopy and McGill forceps. A length of umbilical tape is tied to each of the catheters, and, when the catheters are pulled back through the nose, each end of the umbilical tape is pulled out of its respective naris, producing a loop around the nasal septum. Before securing with a knot, care should be taken to assure that the uvula is not captured in the loop. A knot in the nasal septal tie should be snug enough to prevent excessive movement of the endotracheal tube but loose enough to prevent ischemic necrosis of the underlying tissues. Nasal endotracheal tubes are often avoided in ICUs due to concern for sinusitis. However, nasogastric or nasal feeding tubes carry similar risk. Over the past 25 years our hospital has had two patients with suspected sinusitis. Sinus cultures from these patients were negative for bacterial growth (unpublished observations). The nasal endotracheal tube is much more secure than an oral tube, better tolerated by the patient, and the patient cannot occlude the nasal tube by biting it.
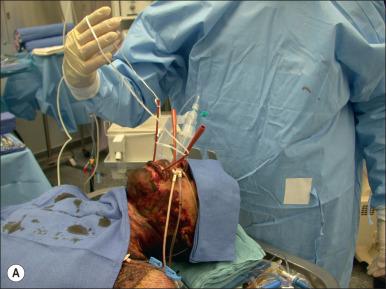
A red rubber catheter may also be used to secure an oral endotracheal tube ( Fig. 13.9 ). A red rubber catheter is placed in a nostril and brought out the mouth by direct laryngoscopy and MacGill forceps. A loop is formed which the endotracheal tube is secured to with umbilical tape. This is very secure and avoids ties around the neck, which can irritate wounds and grafts, and the problem of changing head circumference due to formation and resolution of edema.
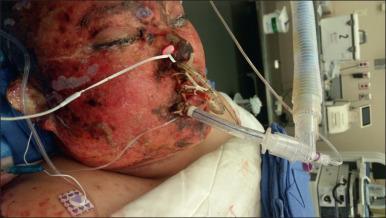
Airway management using a laryngeal mask airway has also been used successfully during burn surgery for children. McCall et al. reported their experience with 141 general anesthetics administered to 88 pediatric burn patients. Nineteen (14.5%) of the procedures were complicated by respiratory events such as unseating, desaturation, and partial laryngospasm that required intervention. Two of these events required intraoperative intubation without sequelae, while all other events resolved with therapy. Interestingly the presence of preoperative respiratory problems or face/neck burns did not predict intraoperative respiratory problems. These authors suggest that, in patients with upper airway mucosal injury, laryngeal mask airway management may help avoid further laryngeal injury that might occur with intubation of the trachea.
Hagberg et al. published a case report describing the successful use of an esophageal tracheal Combitube in a patient undergoing elective surgery for burn scars involving the mouth. The patient had a class IV oral airway by Samsoon and Young's modification of the Mallampati airway classification and limited mouth opening. A translaryngeal endotracheal tube was undesirable because tracheostomy had resulted in subglottic stenosis that could have been exacerbated by an endotracheal tube. After induction with fentanyl and propofol, the Combitube was placed and the patient was relaxed with rocuronium and mechanically ventilated during the 60 min procedure.
As with any critically ill patient suffering from multiorgan system involvement, the choice of monitors in a burned patient will depend on the extent of the patient's injuries, physiological state, and planned surgery. In addition to the preoperative pathophysiology associated with thermal injuries, perioperative monitoring must be adequate to assess rapid changes in blood pressure and tissue perfusion associated with the massive blood loss that can accompany excision of burn wounds. The minimum standards of the American Society of Anesthesiologists require monitoring of circulation, ventilation, and oxygenation. Standard monitors include electrocardiography (EKG), measurement of systemic blood pressure, pulse oximetry, capnography, and inspired oxygen concentration. The ability to measure body temperature should be readily available and is highly recommended for the burn patient.
Standard EKG gel electrodes usually will not adhere to burn patients because the skin is injured or covered with antibiotic ointment. For acute burn surgery, surgical staples and alligator clips are useful. Respiratory rate can be quantitated using bioimpedance from the EKG signal or from the capnogram. Pulse oximetry in burn patients can be difficult when transmission pulse oximetry sites are either burned or within the operative field. Reflectance pulse oximeter probes are now available, and nasal clip probes are much improved ( Fig. 13.10 with oral red rubber).
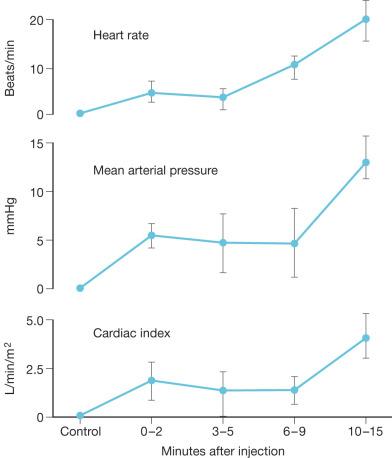
If direct arterial pressure monitoring is not necessary, a blood pressure cuff can provide accurate measurements even if placed over bulky dressings applied to an extremity. Systolic blood pressures obtained from the pressure at which the pulse oximetry signal returns during cuff deflation have also been found to be accurate.
When vasoactive infusion is needed or blood loss is expected to be rapid and extensive, the blood pressure may change more rapidly than the interval between cycles of noninvasive blood pressure measurement. In this case an arterial catheter can provide direct and continuous measurement of blood pressure. This monitor can provide much more information regarding the patient's circulatory status than just systolic and diastolic blood pressure. The arterial pressure wave form is influenced by preload, contractility, and vascular tone. Perioperative variation in the rate of rise of arterial pressure, the area under the pressure wave, position of the dicrotic notch, and beat-to-beat alterations in systolic pressure related to respiration all reflect clinically significant hemodynamic changes. With experience, trends in these variables can help guide volume and vasoactive therapy. Display of the beat-to-beat arterial pressure allows measurement of systolic pressure variation (SPV) and other measures sensitive to variation in stroke volume. These dynamic measures of the interaction between preload and stroke volume have been used to predict the response to fluid volume administration. SPV is the difference between maximum and minimum systolic blood pressure during a single cycle of positive pressure mechanical ventilation. Several studies have correlated SPV with cardiac output response to volume infusion. Tavernier et al. reported that in septic patients on mechanical ventilation, SPV is a better predictor of left ventricular ejection volume response to volume loading than either pulmonary artery occlusion pressure or echocardiographic measurement of left ventricular end diastolic area. When hemorrhage is brisk, the decision to administer volume is not difficult. In other cases during burn wound manipulation, hypotension and other evidence of poor perfusion can occur in the apparent absence of significant blood loss. Inappropriate fluid administration can cause hemodilution, increased cardiac filling pressure, and fluid overload. Measurement of central venous pressure (CVP) can indicate if there is room in the intravascular space for volume administration, and dynamic measures of fluid responsiveness can identify patients who will respond to fluid loading with an increase in cardiac output and tissue perfusion. The use of these dynamic measures of fluid responsiveness, as well as their limitations, have been reviewed recently. However no single physiological variable can be relied on to correct all deficiencies in perfusion during burn wound debridement. Wound manipulation can release inflammatory mediators and bacterial products that alter myocardial compliance and contractility as well as vascular tone. During challenging cases with brisk bleeding volume replacement alone may not correct hemodynamic deficiencies. It is necessary to monitor blood gases, electrolyte changes, and urine output, as well as arterial and central venous pressures. Inadequate tissue perfusion may manifest as metabolic acidosis despite apparently adequate arterial and central venous blood pressures.
Arterial blood sampling for blood gas analysis can also provide valuable information regarding pulmonary function and ventilatory support, acid–base balance, and electrolyte abnormalities. Blood samples from a central vein are not truly mixed venous, but trends in central venous oxygen tension can help identify inadequate tissue perfusion. A CVC sutured into place also provides very secure intravenous access and is an ideal route for administration of vasoactive infusions. A pulmonary artery catheter is usually not required for burn surgery. In some cases, however, the ability to more closely monitor ventricular function and oxygen supply/demand relationships may be helpful in the presence of pre-existing disease or when large doses of inotropes or high PEEP is required.
Urine output is the most useful perioperative monitor of renal function. Urine output of 0.5–1.0 mL/kg per hour is often recommended as an end point for fluid management in acute burn patients. Adequate urine output is one measure of both renal and global perfusion. When intraoperative transfusion is planned, examination of the urine may be the only reliable indicator of a transfusion reaction since signs and symptoms other than hematuria are masked by general anesthesia or hemodynamic changes associated with burn surgery. Myoglobinuria may also occur after burn injury, and, in this case, a Foley catheter is necessary to monitor response to therapy. Diuretic therapy for myoglobinuria or any other indication will negate the usefulness of urine output as an index of global perfusion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here