Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Climate change represents a defining challenge of our times, and tackling climate change could be the greatest global health opportunity of this century. Climate change poses clear environmental, technologic, and economic challenges that affect food, water, and habitation, and therefore the health of the world’s population. Table 49.1 lists health issues related to climate change.
| Climate Change | Health Risks |
|---|---|
| Increased frequency and intensity of extreme heat conditions | Increased risk of morbidity and mortality during heat waves, especially in urban settings and for manual workers outdoors |
| Increased variability in precipitation, increasing temperatures | Climate-induced reduction in local crop yields; food insecurity and undernutrition, especially in children Water- and vector-borne disease (e.g., malaria, dengue) more likely |
| Increased variability in precipitation; increased flood, fire, and drought risk | Greater risk of injury, disease, death Likely increase in diarrheal disease, with greater impact in children |
| Increased air pollution, reduced air quality | Increased risk of respiratory illnesses, cardiovascular disease |
Although health care systems have responded to the growing health care needs and the catastrophes posed by climate change, health care itself is polluting and contributes to global warming. Health care is a large, socioeconomically vital sector that contributes significantly to national greenhouse gas (GHG) emissions, both directly from health care facilities and to a much greater extent indirectly from the production of energy, medical goods, and pharmaceuticals that support these facilities. A 2009 report estimated that U.S. health care contributed to 8% of the national GHG emissions. Although many industries have taken actions to reduce emission of GHGs, for example, by producing less-polluting motor vehicles and using less-polluting fuels to produce heat and electricity, health care has been slow to respond. In 2013 an economic input-output life cycle assessment model showed a worsening trend and estimated that 10% of U.S. GHG emissions were attributable to health care. Other national-level studies have shown significant contributions of health-care-sector GHG emissions compared with total GHG emissions: 7% in Australia, 5% in the United Kingdom, and 4% in Canada.
The United States is the second-largest emitter of GHG globally, with 13% of global GHG emissions. China contributes the most, at 26% of global GHG emissions, and the European Union contributes the third most, at 7.5% of global GHG emissions. If the U.S. health care sector were itself a country, it would rank 13th in the world for GHG emissions, ahead of the entire United Kingdom. Careful attention should be given to the current practice of health care, as it offers significant opportunities for environmental efficiency improvements that could decrease cost and resource utilization without compromising patient care.
Fifty percent of incoming solar energy is absorbed by the earth; the remainder is absorbed by the atmosphere and clouds or is reflected to space. GHGs, defined as gases that trap heat in the atmosphere, 11 contribute to global warming by absorbing the reflected heat and radiating it back to the earth’s surface. Anesthesia gases produce 5% of hospital GHG emissions. The commonly used volatile anesthetics sevoflurane, desflurane, and isoflurane and the nonvolatile gas nitrous oxide (N 2 O) are potent GHGs with high global warming potential (GWP). GWP is a measure of how much a given mass of GHG contributes to global warming over a specified time (typically 20 or 100 years, i.e., GWP 20 or GWP 100 ); by definition, carbon dioxide (CO 2 ) has a GWP of 1.
The GWP of a GHG is primarily determined by two factors: (1) its atmospheric lifetime and (2) its radiative efficiency. Because most halogenated anesthetic compounds other than halothane have similar radiative efficiencies, their GWPs depend primarily on their atmospheric lifetimes, which depends on how rapidly molecular bonds can be broken down by OH − radicals. Table 49.2 lists the atmospheric lifetimes for inhaled anesthetics, in addition to their GWP 100 , and a comparison to the GHG emission of driving a car.
| Gas | Lifetime , (years) | GWP 100 , , c | Driving Equivalent (miles/hr) , , d at Fresh Gas Flow | |||
|---|---|---|---|---|---|---|
| 0.5 L/min a | 1.0 L/min a | 2 L/min a | 5 L/min a | |||
| N 2 O | 114–121 | 265–298 | 29 | 57 | 112 | 282 |
| Sevoflurane | 1.1–2.2 | 130–216 | – | 4 | 8 | 19 |
| Desflurane | 10.8–14 | 1790–2540 | 93 | 190 | 378 | 939 |
| Isoflurane | 3.2–3.5 | 491–510 | 4 | 8 | 15 | 38 |
| Halothane b | 1.0 | 41 |
a 0.6 MAC-hour for N 2 O and 1 MAC-hour for sevoflurane, desflurane, and isoflurane.
b Halothane is no longer commercially available in the United States but is available globally.
c GWP 100 is the global warming potential of a greenhouse gas over a 100-year period compared with CO 2 . Desflurane has a GWP 100 of 2540, indicating that 1 kg of desflurane has the same global warming effect as 2540 kg of CO 2 . The technical groups routinely reevaluate gases of concern in the context of changing atmospheric chemistry, a process that can take up to 5 years.
d The driving equivalent analogy provides a practical comparison to driving a typical passenger automobile, which emits approximately 400 g of CO 2 per mile. For example, administering desflurane at fresh gas flow of 1 L/min for 1 hour at 1 MAC produces the same GHG emission as driving a car for 190 miles.
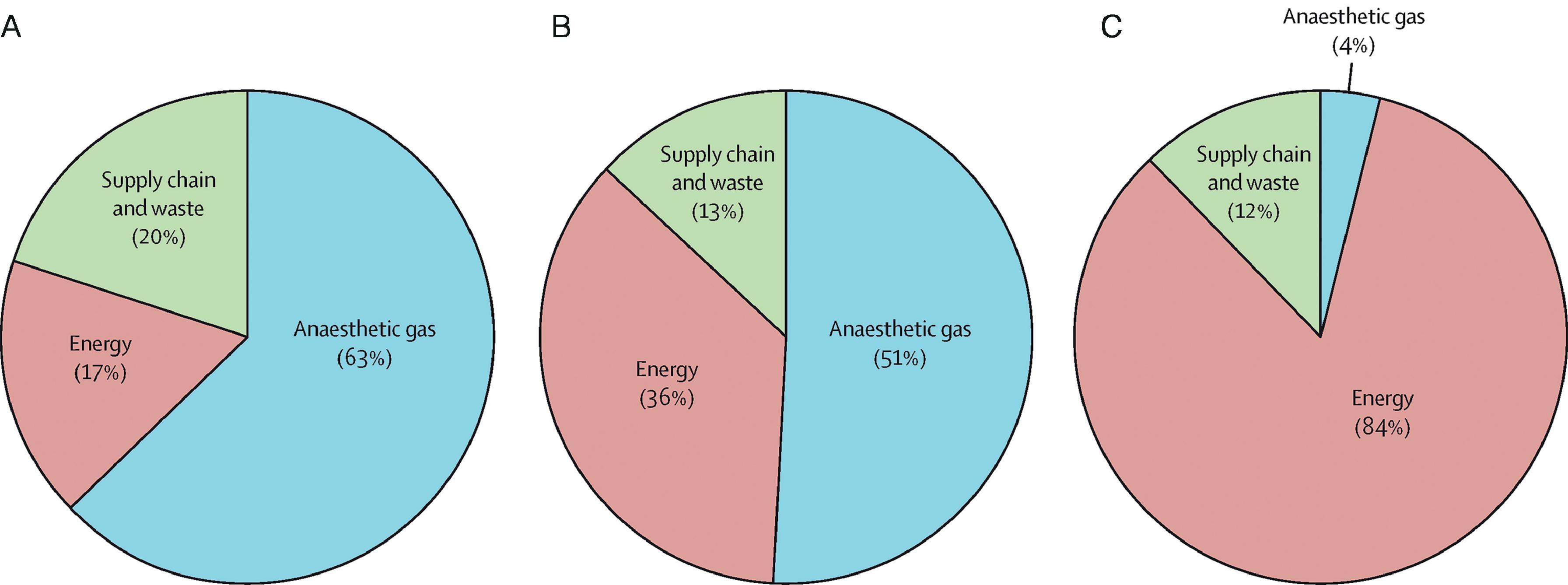
A 2017 study analyzed the carbon footprint of the operating room (OR) across three health care systems in three different countries. Anesthetic gases and energy use were the largest sources of GHG emissions. In addition, preferential use of desflurane accounted for a 10-fold difference in carbon emissions from anesthesia gases between these hospitals ( Fig. 49.1 ).
N 2 O is the third most significant GHG after CO 2 and methane. It has the longest atmospheric lifetime of all inhaled anesthetics and a relatively high GWP 100 . Atmospheric N 2 O originates primarily from agriculture, fuel combustion, wastewater management, and industrial processes. Anesthetic use is estimated to be responsible for 3% of global N 2 O emissions. However, this relatively small contribution should not be ignored, as it remains a significant source of ozone depletion caused by anesthesia. , Inhaled N 2 O is still highly popular for labor analgesia outside the United States because it is inexpensive, easy to administer, and has a favorable safety profile for mother and child (also see Chapter 33 ).
Even though the global amount of GHGs released each year from inhaled anesthetic administration is comparatively small (approximately 0.01% of total GHG emissions from fossil fuel combustion), direct release of inhaled anesthetics is a major contributor to the environmental impact of anesthesia. For individual anesthesia providers, the most effective climate change mitigation strategies are to avoid desflurane and N 2 O, adopt low-flow and closed-circuit anesthesia, and familiarize themselves with techniques using regional and total intravenous anesthesia (TIVA).
In addition to their environmental effects, inhaled anesthetic agents cause OR pollution and can increase health risks for providers in the OR. During induction of general anesthesia and before tracheal intubation, high fresh gas flow (FGF) rates are often used. This period represents the highest risk for spillage of waste anesthetic gases directly into the OR instead of the anesthesia workstation scavenging system (also see Chapter 15 ).
The potential health hazards of occupational exposure to waste anesthetic gases, including increased risk of spontaneous abortion, genetic damage, and cancer, were first noted in survey-based studies from the 1970s, an era in which scavenging of inhaled anesthetics was poor by today’s standards. In 1977 the National Institute for Occupational Safety and Health (NIOSH) issued recommended exposure limits for both N 2 O and halogenated agents, in addition to guidance on scavenging systems for waste anesthetic gases and ventilation system capacity in ORs and recovery rooms. After implementation of waste anesthetic gas regulations from NIOSH (and similar regulations in other countries), more recent studies have found little to no increase in adverse effects associated with waste anesthesia gases when they are scavenged effectively. However, these risks may remain for individuals chronically exposed to workplaces that do not meet recommended guidelines for waste anesthetic gas exposure.
Because inhaled anesthetics undergo very little in vivo metabolism, they are exhaled, scavenged, and vented into the atmosphere, largely unchanged, as medical waste gases. Thus the ecologic impact is largely determined by the gas used and the FGF rate at which it is administered. Although there is no universal definition, low-flow anesthesia is most commonly defined as FGF <1 L/min and minimal flow anesthesia as FGF <0.5 L/min. , Essentially any technique in which the FGF is less than alveolar ventilation can be considered low-flow anesthesia.
Table 49.3 lists advantages and disadvantages of low-flow anesthesia. The clinical use of low-flow anesthesia has been facilitated by newer anesthesia workstations, gas analyzers (for inspired/exhaled O 2 , CO 2 , and anesthetic agent), and CO 2 absorbents that do not produce compound A (also see Chapter 15 ).
| Advantages | Disadvantages |
|---|---|
| Environmental | Slower inhaled anesthesia induction |
| Decreased anesthetic gas consumption | Slower emergence from inhaled anesthesia |
| Decrease in waste anesthetic gas (greenhouse gases) | Reduced ability to rapidly change inspired anesthetic concentration |
| Decrease in operating room pollution | |
| Economic | Increased CO 2 absorbent consumption |
| Decreased cost of volatile anesthetic agents | Risk of hypercarbia (with exhausted CO 2 absorbent) |
| Physiologic | |
| Reduced respiratory heat loss | Requires ongoing adjustment of inspired oxygen concentration to prevent hypoxic gas mixture |
| Preservation of humidity of inspired gas, (helps to maintain mucociliary function) |
The maintenance phase of anesthesia presents the best opportunity to reduce flows, but attention should also be given to the induction and emergence phases to minimize environmental contamination and decrease waste. Recommendations for low FGF management for all three phases of anesthesia are listed in Box 49.1 .
1.Set FGF close to minute ventilation during mask ventilation
2.Increase FGF if measured Fi O 2 and gas concentration lower than set level
3.During intubation, turn off the FGF, leave the vaporizer at its set point
4.Set FGF to half of the minute ventilation after intubation
5.Then watch measured anesthetic concentration after intubation
Reduce FGF progressively based on gas concentration
If needed, increase/decrease vaporizer setting to maintain desired concentration
6.Move to anesthesia maintenance management if there is no significant difference between exhaled and inspired anesthetic gas concentration
1.First, set total oxygen flow (mL/min):
Patient’s estimated oxygen consumption (5 mL/kg/min)
Add 200 mL/min if sidestream gas analyzer sample gas does not return to the circuit
Add 100 mL/min for potential leaks
2.Decrease total oxygen flow in 50-mL/min increments until Fi O 2 begins to decrease
3.Monitor exhaled anesthetic gas concentration to maintain desired MAC level
4.Monitor Fi O 2
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here