Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Diagnosis and surgical correction of strabismus require anatomic knowledge of the extraocular muscles (EOMs) and their relationships with other orbital tissues. This chapter summarizes this anatomy and some surgical applications.
The historically understood six EOMs are the effector arm of the ocular motor system. The EOMs are loosely organized as “yoked pairs” of agonists and antagonists, although exceptions to reciprocal activity have recently been identified for vergence eye movements (see below). These EOMs are highly specialized, and each EOM consists of two kinds of compartments. The global layer (GL) controls oculorotary tension, while the orbital layer (OL) controls the pulling direction by regulating the position of the connective tissue pulleys. The GL and OL contain fiber types with different metabolic and structural properties. A second kind of compartmentalization divides the GL of each transversely according to specialized motor nerve arborizations that enables differential contraction. The pulleys, connective tissue EOM encirclements that are actively controlled by the OLs of the rectus EOMs, mechanically regulate most aspects of ocular kinematics, including Listing's Law (LL) of torsion. Exceptions to LL are implemented actively by the oblique EOM OLs. However, transverse compartmentalization allows for fine tuning EOM pulling direction, and is critical to ocular motor control during vergences, and to interactions with vestibulo-ocular reflexes (VORs). It has been recently recognized that the optic nerve itself is a mechanical load on the EOMs, since the nerve is so short that it tethers the globe in adduction exceeding about 26 degrees ; beyond this angle, the EOMs must stretch the optic nerve and roll the eye about a point eccentric to its center. In large adduction, MR force not only rotates the eye, but distorts the optic disc and peripapillary region in normal subjects, but retracts the globe in people with primary open angle glaucoma.
The six classical oculorotary EOMs pair as antagonists. The medial rectus (MR) adducts and the lateral rectus (LR) abducts. The superior rectus (SR) and inferior rectus (IR) muscles form a vertical antagonist pair, with the SR supraducting and the IR infraducting. However, vertical rectus EOMs have additional actions. The superior oblique (SO) and inferior oblique (IO) muscles form a torsional antagonist pair: SO incycloducts, while IO excycloducts, but their additional actions are not strictly antagonistic.
The oculorotary EOMs consist of two layers subserving distinct functions ( Fig. 75.1 ). The GL, containing approximately 10,000–15,000 fibers in the mid-length, is located adjacent to the globe in rectus EOMs and in the central core of the oblique EOMs. In the rectus EOMs and the SO, the GL anteriorly becomes contiguous with the terminal tendon and inserts on the sclera. In the IO, the GL inserts directly on the sclera. Only the GL of each EOM inserts on the sclera, having passed through its respective pulley. The OL of each rectus EOM contains 40%–60% of its fibers. The OL does not insert on the eyeball at all, but instead inserts on connective tissue pulleys. Neither does the OL insert on the GL; fibers of the OL and GL run approximately in parallel, with little coupling of force from one to another, so as to produce different mechanical effects. The OL is located on the orbital surface of the rectus EOM, but constitutes the concentric outer layer of the SO; fibers in the two layers can act with some degree of independence.
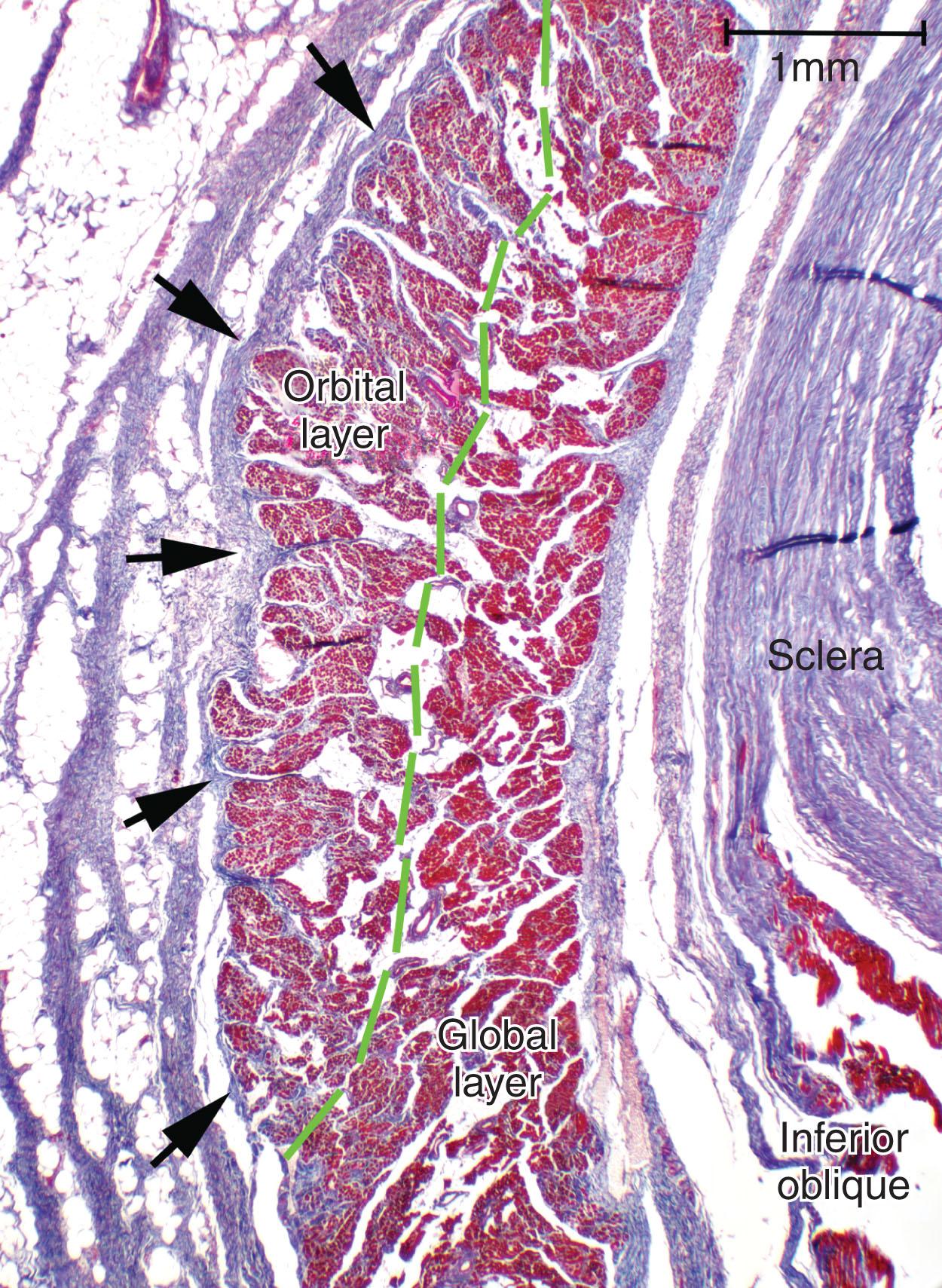
The OL contains two muscle fiber types. About 80% of fibers in the orbital layer of each EOM are fast, twitch-generating, singly innervated fibers (SIFs) resembling mammalian skeletal muscle fibers, while 20% are multiply innervated fibers (MIFs) that either do not conduct action potentials or do so only in their central portions. Orbital SIFs have small diameter and numerous mitochondria. The metabolism and blood supply of orbital SIFs are tailored to their mechanical loading and nearly continuous activity, and thus specialized for intense oxidative metabolism and fatigue resistance. The vascular supply in the OL is higher than in the GL. Orbital SIFs express unique myosin isoforms. The relatively sparse and primitive orbital MIFs may be proprioceptive.
The GL contains one MIF type and three SIF fiber types. The largest SIF is similar to the orbital SIF, while mitochondria are less abundant in the other two SIFs. Global MIFs resemble orbital MIFs. While spindles are absent in the GL, there are palisade endings along the width of each rectus tendon where the GL fibers terminate, perhaps subserving a proprioceptive function (this is controversial ), or perhaps convergence.
Several myosin isoforms occur in EOMs, with the predominant one in orbital SIFs, EOM-specific myosin, occurring uniquely in EOMs. Neonatal and embryonic myosin isoforms persist throughout adult life at the anterior and posterior ends of SIFs. Differences in myosin expression may underlie the susceptibility of the EOMs to disorders such as thyroid ophthalmopathy, as well as their resistance to other disorders such as muscular dystrophy.
Rectus EOMs originate in the orbital apex from the annulus of Zinn. The SO muscle originates from the periorbita of the superonasal orbital wall. The rectus EOMs course anteriorly through loose, areolar lobules of orbital fat until they enter their connective tissue pulleys that ensheath them as they penetrate posterior Tenon's fascia. Contrary to implications of many texts, there is no “muscle cone” of connective tissue among the adjacent rectus EOM bellies in the mid-to-deep orbit. The SO muscle remains tethered to the periorbita via connective tissues as it courses anteriorly, then thins to become continuous with its long, thin tendon. The concentric OL of the SO terminates posterior on a peripherally located sheath; the SO's GL terminates on parallel tendon fibers similar to rectus EOM tendons, but rolled up into a cylinder as it transits the trochlea, a cartilaginous rigid pulley attached to the superonasal orbital wall. After reflection in the trochlea, the SO tendon passes beneath the SR, thins, and flattens as it spreads out to its broad scleral insertion posterolaterally on the globe, while the sheath inserts on the SR pulley. The IO muscle originates from the inferonasal orbital rim, and runs laterally to enter its connective tissue pulley immediately inferior to the IR at the point the IO penetrates Tenon's fascia.
The axes of the bony orbits are angled about 23 degrees laterally from the midsagittal plane. The array of the rectus EOMs and the SO is conical. As they continue anteriorly, the rectus EOMs thin to become strap-like bands about 10 mm in width, and ultimately their GLs become continuous with tendons that insert on the globe. The SO would do the same, except that its tendon rolls up within the trochlea and then unrolls anteriorly.
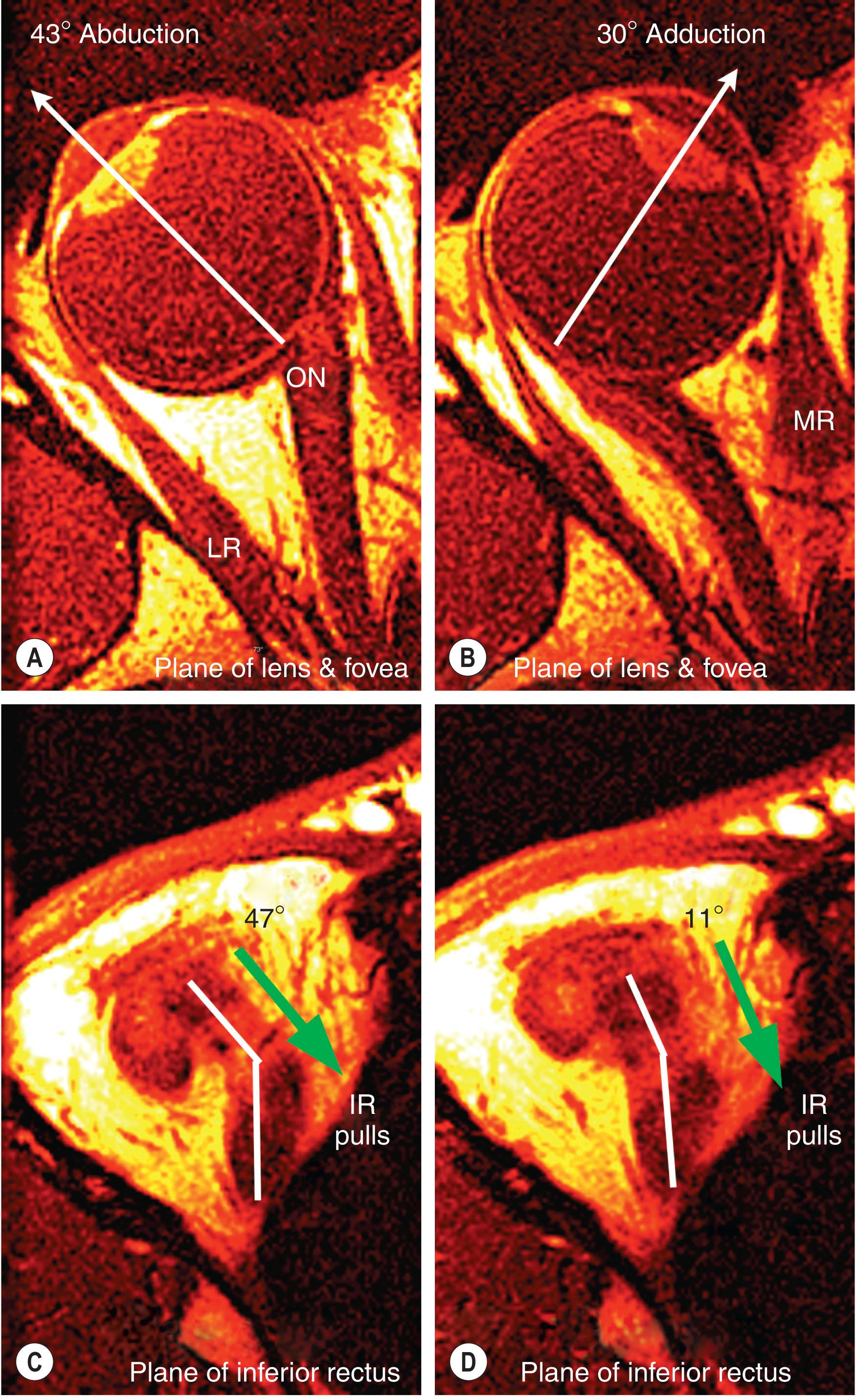
The rectus EOMs do not follow straight-line paths from their origins to their scleral insertions, nor do they wrap over the globe to form an “arc of contact” that is mechanically important. In eccentric gazes, rectus paths are inflected sharply in the anterior orbit ( Fig. 75.2 ). The inflections, due to the pulleys, cause the anterior paths of rectus EOMs, and thus their pulling directions, to change with eye position. Figure 75.2 is a set of axial magnetic resonance images (MRI) illustrating that the anterior IR path changes by half the change in duction angle. All four rectus EOMs behave in this manner.
The rectus EOM inflections in the anterior orbit constitute the functional pulleys. Anterior to the pulleys, rectus EOM paths directly track to the scleral insertions. The pulleys thus act as mechanical origins of the rectus EOMs. Pulleys consist of rings of dense collagen about 2 mm in length, co-axial with less substantial collagenous sleeves around the EOMs ( Fig. 75.3 ). Anteriorly, these sleeves thin to form slings convex to the orbital wall and, more posteriorly, the sleeves thin to form slings convex toward the orbital center. The anterior pulley slings have also been called the “intermuscular septum,” a time-honored but relatively vague term. Fibrils of pulley collagen in the pulleys have a crisscrossed configuration suited to high internal rigidity. Elastic fibers in and around pulleys provide reversible extensibility, particularly in bands that connect the pulleys to bony anchors on the orbital rim, suspending them under elastic tension that draws them anteriorly. Smooth muscle is present in pulley suspensions, particularly in a distribution called the inframedial peribulbar muscle between the MR and IR pulleys.
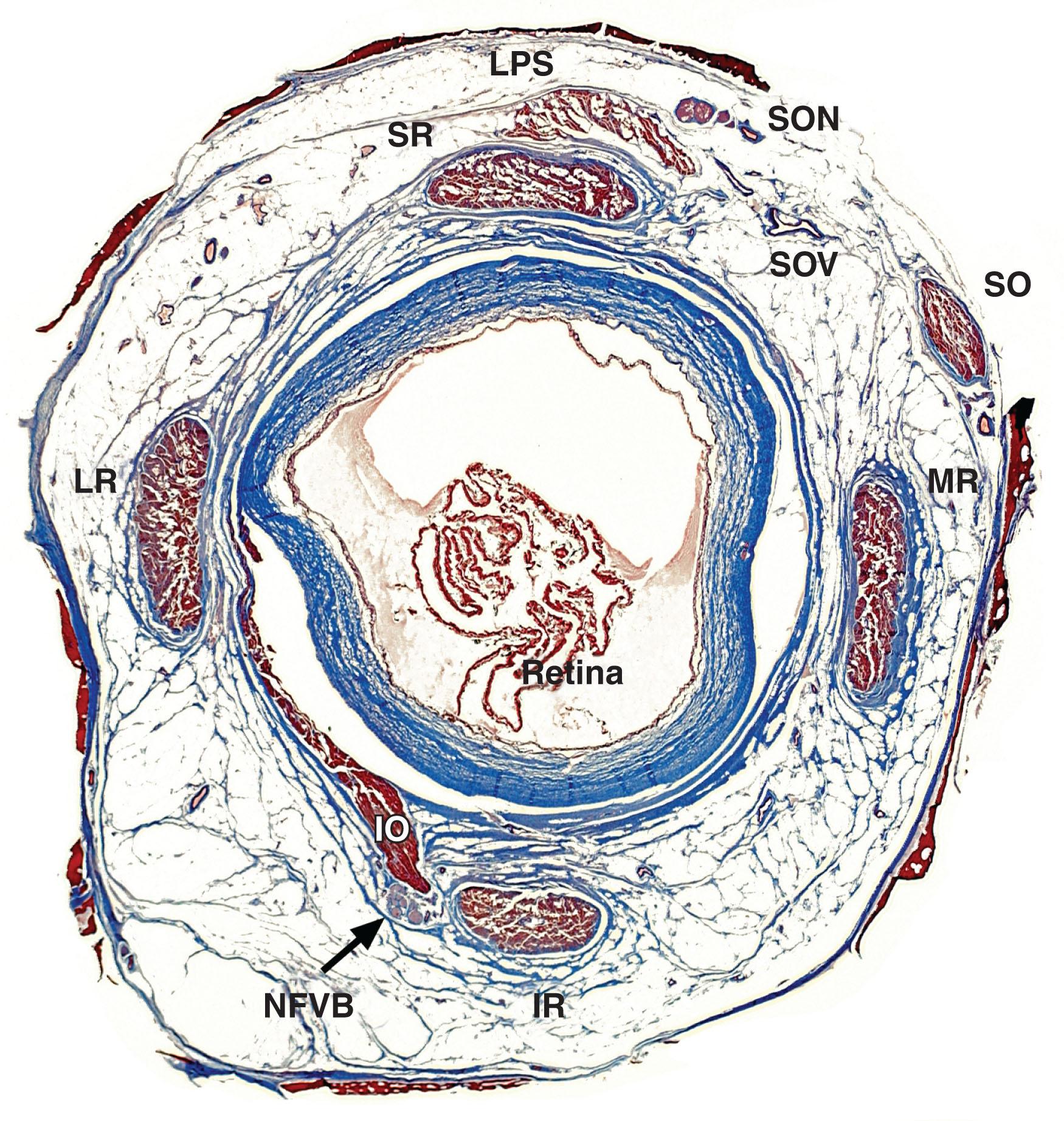
The IR pulley is intimately coupled with the IO pulley in a bond that forms part of Lockwood's ligament where the IR and IO pulleys share a common collagenous sheath stiffened by a heavy elastin deposit at their point of crossing. The IR's OL inserts on its pulley. The IO's OL inserts partly on the conjoined IO–IR pulleys, partly on the IO's connective tissue sheath, and partly on the inferior aspect of the LR pulley. IO contraction displaces the conjoint IR–IO pulley nasally and the LR pulley inferiorly. The overall arrangement of the entire pulley system is illustrated in Fig. 75.4 .
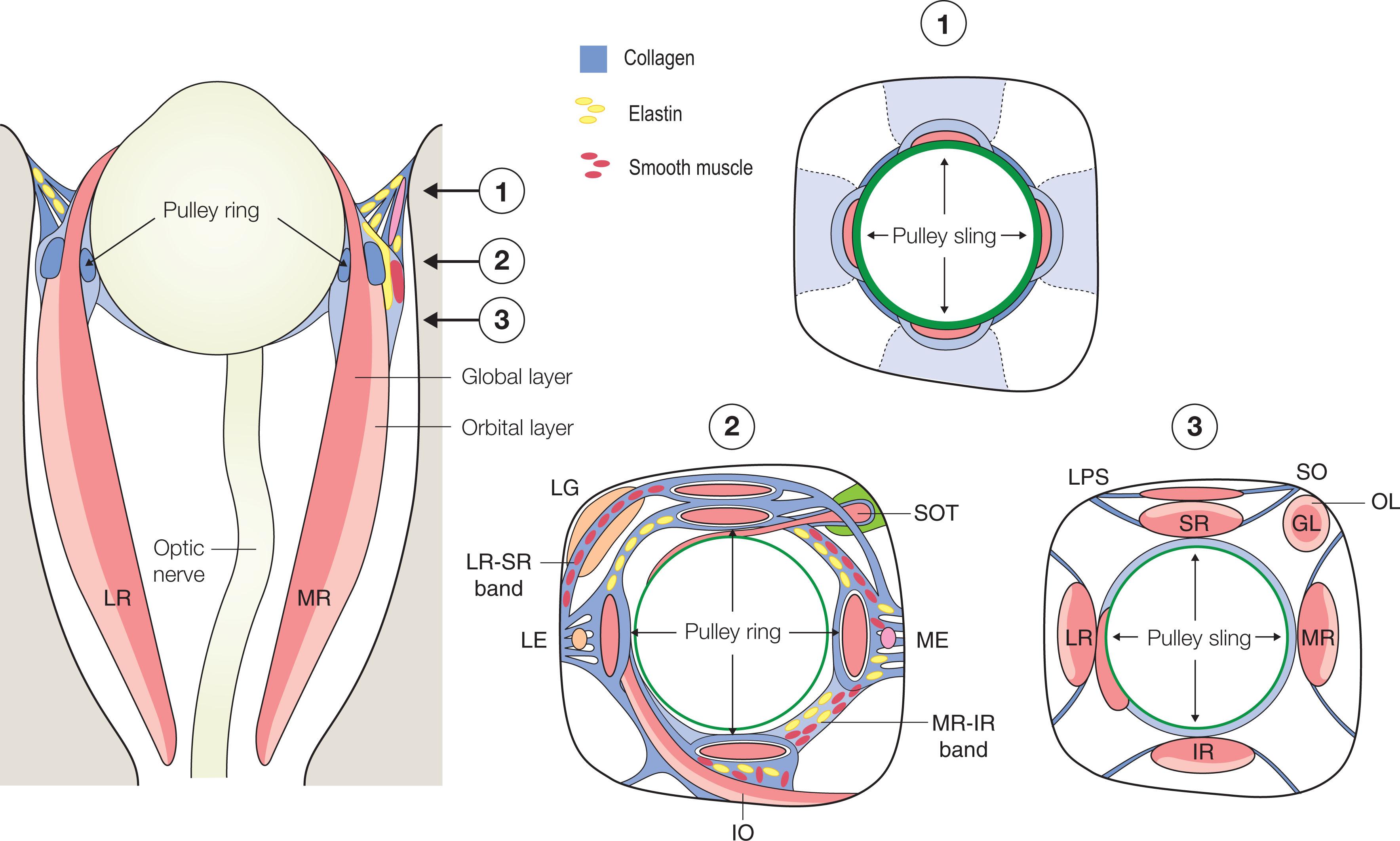
Although the rigid SO pulley – the trochlea – has been known since antiquity, the SO's OL inserts via the SO sheath on the medial aspect of the SR pulley. The SO pulling direction changes half as much as ocular duction despite an immobile pulley, because of the uniquely broad, thin insertion of the SO tendon as it wraps over the globe.
The foregoing anatomical relationships can be demonstrated in surgical exposures. Following engagement of a rectus EOM on a surgical hook, the white anterior pulley slings are visible. These tissues play little role in constraining EOM paths. The anterior pulley slings can usually be displaced posteriorly by blunt dissection, after which fine fibrous bands constituting the insertion of the OL of the EOM on the glistening white pulley suspension can be visualized ( Fig. 75.5 ). This insertion is anterior to the pulley ring, which is obscured by the overlying white tissue of Tenon's capsule. After transposition of a rectus tendon for the treatment of, for example, LR palsy, the path of the transposed EOM continues to be toward the original pulley (see Fig. 75.5 ). The clinical effect of rectus transposition can be improved by suture fixation from a posterior point on the transposed EOM belly to the sclera adjacent the palsied EOM. This operation displaces the pulley further towards the transposed direction.
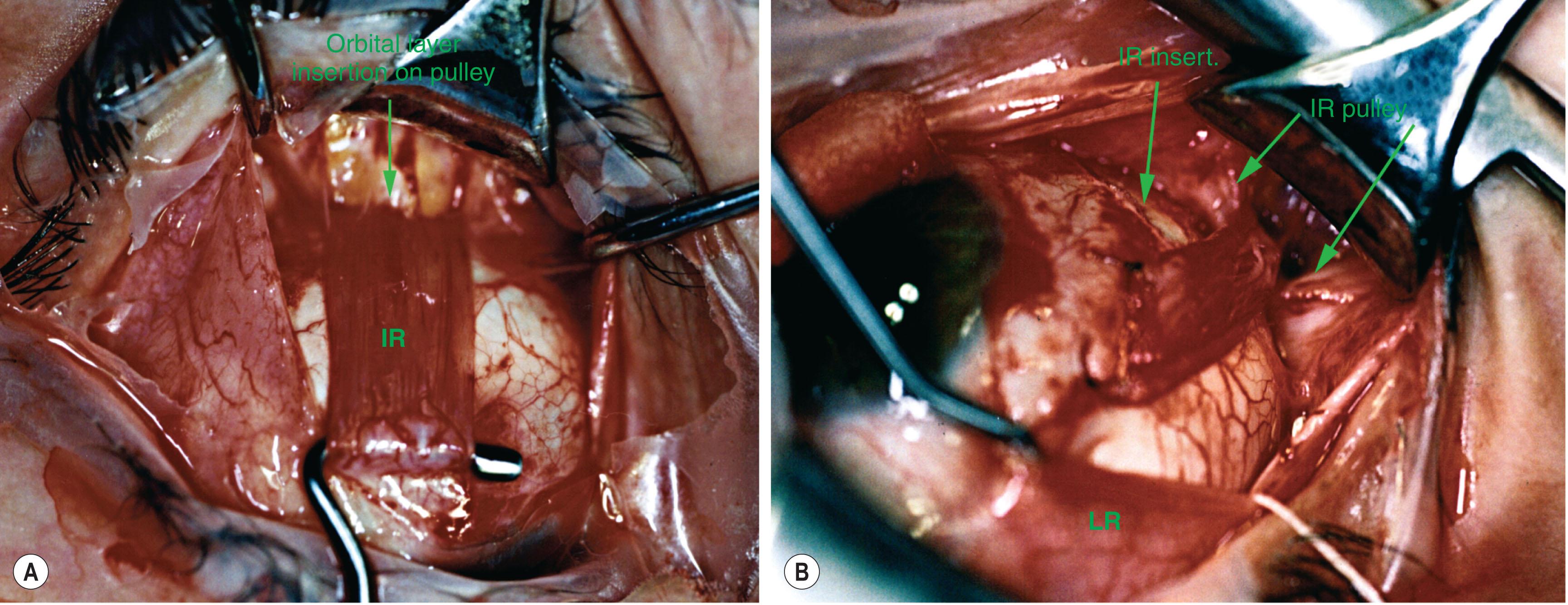
The insertion of each rectus EOM's OL on its corresponding pulley translates (linearly moves) that pulley posteriorly during EOM contraction. This may be seen in the axial, contrast-enhanced MRI scans in Fig. 75.6 . Pulley tissues move in coordination with the insertion and sclera, although histological examinations show the absence of connections between these tissues. Coronal MRI scans show changes in the anteroposterior position of the inflections in rectus EOM paths in tertiary gaze positions. This behavior has been confirmed for all four rectus pulleys.
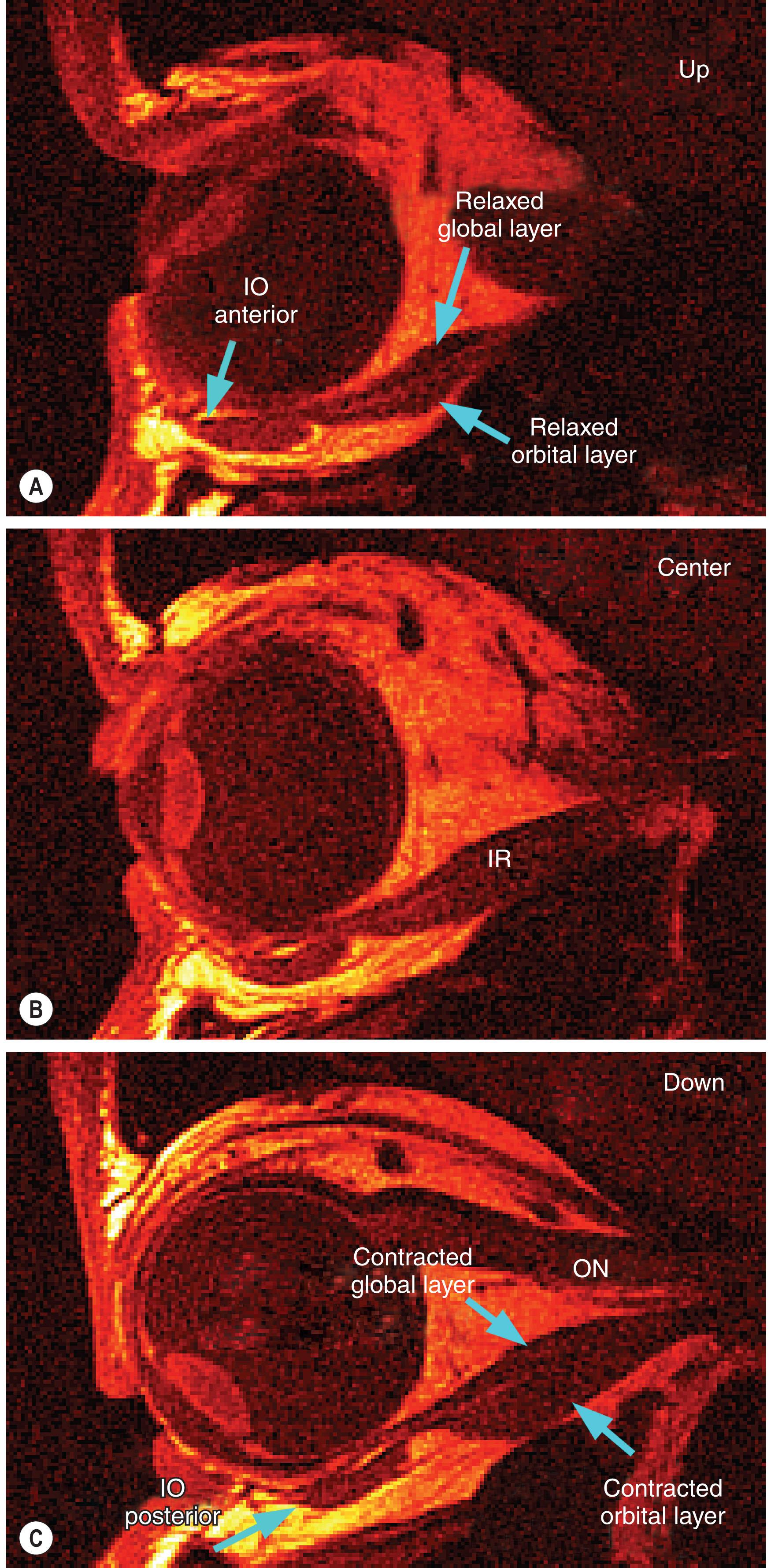
The IO pulley shifts anteriorly in supraduction, and posteriorly in infraduction. This shift is easily appreciated from the obvious change in the IO's path observed by MRI in the quasi-sagittal plane parallel to the long axis of the orbit ( Fig. 75.7 ). As is also evident from Fig. 75.7 , the IO pulley moves by half as much as the IR insertion, as necessary for optimal control of the IO's pulling direction.
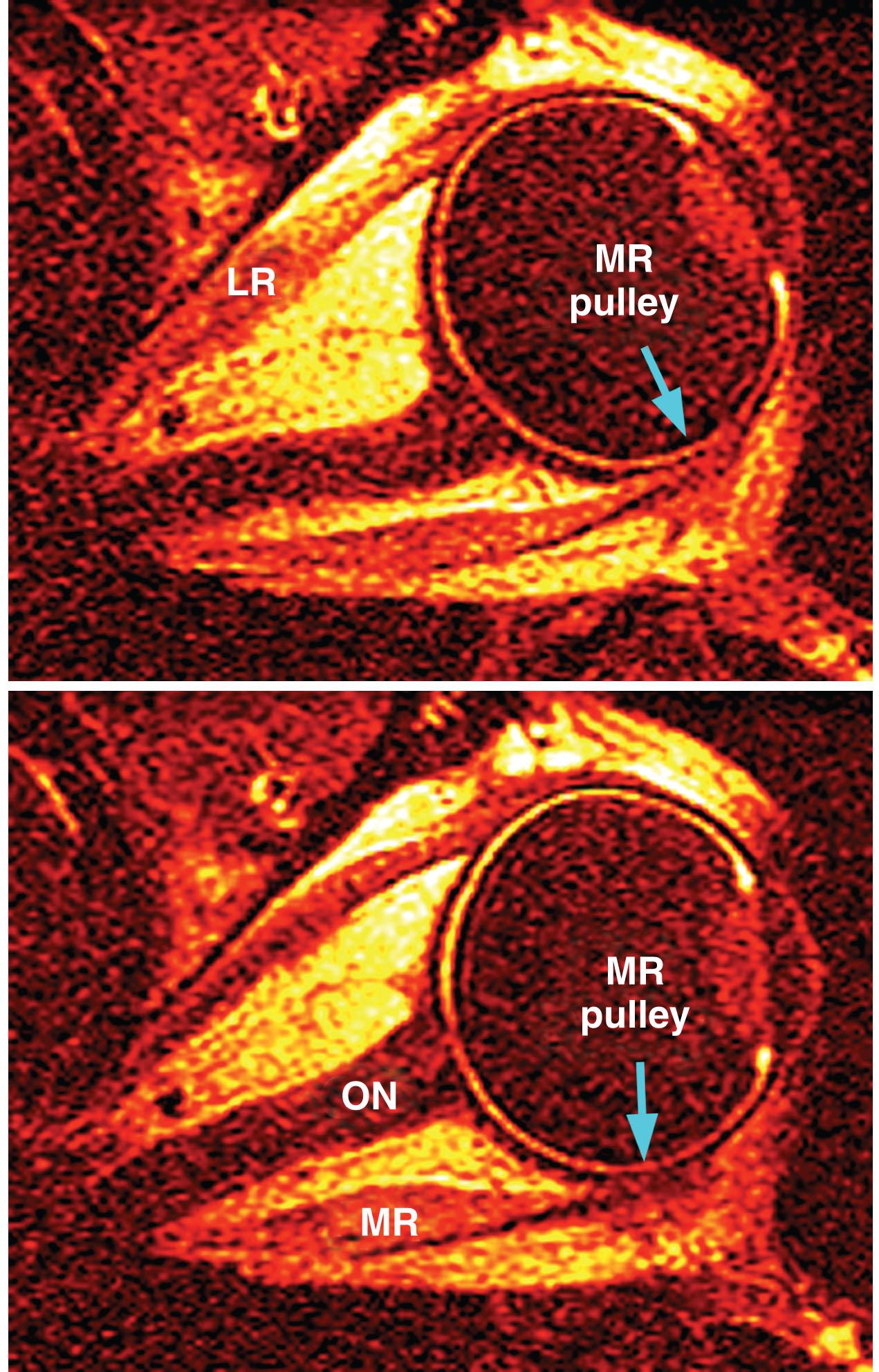
The SR pulley also has discrete mechanical couplings to other pulleys. The most prominent such coupling is a dense band extending from the lateral border of the pulley to the superior border of the LR pulley. This band contains dense collagen and elastin throughout, and divides the orbital lobe of the lacrimal gland.
Although the rectus and IO pulleys are mobile along the lengths of their EOMs, pulleys are located quite stably and stereotypically in the transverse directions. Since EOMs must transit their pulleys, and the pulleys encircle the EOMs, pulley locations may be inferred from EOM paths. Even the anteroposterior pulley locations can be determined by imaging EOM path inflections produced by the pulleys in eccentric gaze positions. In describing pulley location, it is essential that a coordinate reference be defined. Table 75.1 specifies the coordinates of the rectus pulleys of normal young adults in a coordinate system originating in the globe center. Remarkably, the 95% confidence intervals for normal rectus pulley coordinates range over less than ±0.6 mm. Precise pulley placement is important since the pulleys serve as the functional mechanical origins of the EOMs, and since the pulleys are close to globe center. Normal aging causes inferior sagging of the horizontal rectus pulleys by 1–2 mm, and more in the sagging eye syndrome, as described below. Vertical rectus pulley positions change little over the lifespan.
| Extraocular muscle | Millimeters from globe center | ||
|---|---|---|---|
| Lateral | Superior | Anterior | |
| Medial rectus | −14.2 ± 0.2 | −0.3 ± 0.3 | −3 ± 2 |
| Lateral rectus | 10.1 ± 0.1 | −0.3 ± 0.2 | −9 ± 2 |
| Superior rectus | −1.7 ± 0.3 | 11.8 ± 0.2 | −7 ± 2 |
| Inferior rectus | −4.3 ± 0.2 | −12.9 ± 0.1 | −6 ± 2 |
The globe itself makes translations – linear shifts – during ocular ductions. The globe translates 0.8 mm inferiorly from 22 degrees infraduction to 22 degrees supraduction, for example. The globe also translates in the direction of its rotation, in rolling fashion, for both abduction and adduction. These translations affect EOM pulling directions since the globe center is only 8 mm anterior to the rectus pulleys, and may influence the effect of strabismus surgery in axially elongated globes.
Rectus pulleys make small transverse shifts under physiologic conditions. The MR pulley translates 0.6 mm superiorly from 22 degrees infraduction to 22 degrees supraduction. Due to the insertion upon it of the IR's OL, the LR pulley translates 1.5 mm inferiorly from infraduction to supraduction. The IR pulley, due to the insertion upon it of the IO's OL, is drawn 1.1 mm medially by IO contraction in supraduction, but 1.3 mm temporally during IO relaxation in infraduction. The SR pulley is relatively stable in the mediolateral direction, but moves inferiorly in supraduction as it is posteriorly displaced by the SR OL, and superiorly in infraduction as the SR OL relaxes. The small gaze-related shifts in rectus pulley positions are also highly uniform among people with normal eyes.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here