Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The small intestine is a specialized tubular structure within the abdominal cavity in continuity with the stomach proximally and the colon distally. The small bowel increases in length from about 250 cm in the term newborn to about 600 to 800 cm in the adult. The caliber of the small intestine gradually diminishes from proximal to distal, and there is a fourfold reduction in surface area from the distal duodenum to the terminal ileum.
The duodenum is the most proximal portion of the small intestine. It begins with the duodenal bulb, travels in the retroperitoneal space around the head of the pancreas, and ends on its return to the peritoneal cavity at the ligament of Treitz. The biliary and pancreatic ducts usually join together 1 to 2 cm from the outer margin of the duodenal wall and drain into the medial wall of the second portion of the duodenum through the ampulla of Vater. In 5% to 10% of individuals, an accessory pancreatic duct, also known as the duct of Santorini , enters separately through the minor papilla 1 to 2 cm proximal to the ampulla of Vater. The remainder of the small intestine is suspended within the peritoneal cavity by a thin broad-based mesentery that is attached to the posterior abdominal wall and allows relatively free but tethered movement of the small intestine within the abdominal cavity. The proximal 40% of the mobile small intestine is the jejunum, which occupies the left upper portion of the abdomen. The remaining 60% of small intestine is the ileum, and it is normally situated in the right side of the abdomen and upper part of the pelvis. There is no distinct anatomic demarcation between the jejunum and ileum, but the jejunum tends to be thicker, is more vascular, and has a greater diameter than the ileum.
The luminal surface of the small intestine has visible mucosal folds called the plicae circularis or folds of Kerckring. They are more numerous in the proximal jejunum, decrease in number distally, and are absent in the terminal ileum. Microscopic aggregates of lymphoid cells are scattered throughout the small intestine and make up the GI-associated lymphoid tissue. Macroscopic lymphoid aggregates, or Peyer patches, are more concentrated in the ileum and can be seen extending through to the serosa. Peyer patches are more prominent in infancy and childhood and regress in size and number with advancing age.
The jejunum and ileum are freely mobile in the abdominal cavity and are attached to the posterior abdominal wall by the intestinal mesentery. The entire length of jejunum and ileum is suspended in this mesentery, except for the distal terminal ileum at the cecum, which is retroperitoneal. The mesentery is formed by a fan-shaped anterior reflection of the posterior peritoneum that extends from the left side of the body toward the right sacroiliac joint. The mesentery envelops a number of important structures, including the jejunum, ileum, jejunal and ileal branches of the superior mesenteric artery (SMA) and superior mesenteric vein (SMV), nerves, lacteals, lymph nodes, and a variable amount of fat.
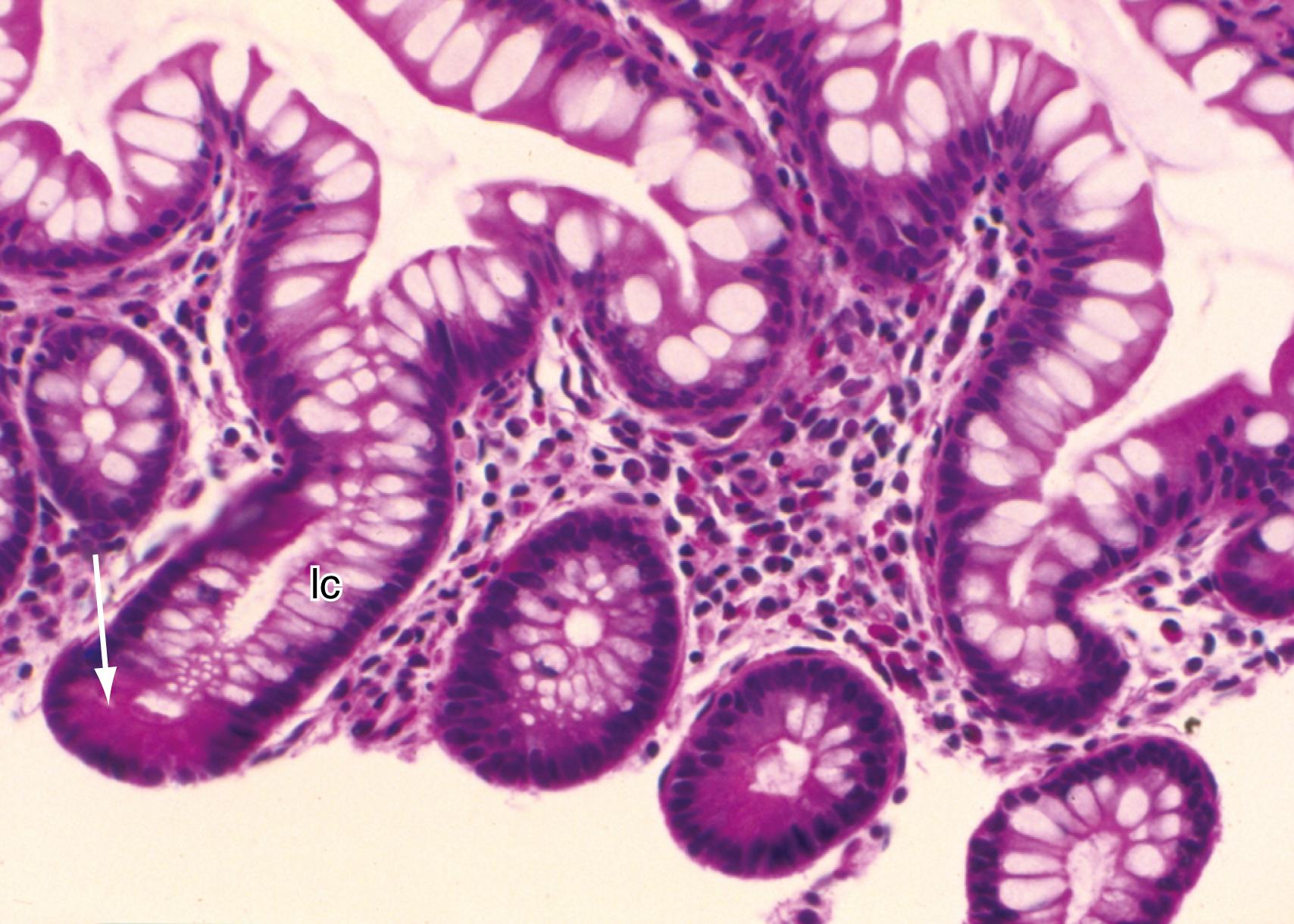
The small bowel transitions to the colon at the ileocecal (IC) valve, which consists of 2 semilunar lips that protrude into the cecum. The IC valve functions like a flutter valve, allowing antegrade flow when a peristaltic wave is strong enough to overcome its resistance but preventing retrograde flow of colonic contents into the small intestine. The angulation between the ileum and cecum, supported by the superior and inferior IC ligaments, is important to the function of the IC valve. The IC valve typically contracts when the cecum is over-distended to prevent ceco-ileal reflux. This explains why during colonoscopy, excessive distention of the cecum with air should be avoided, because this may lead to IC valve contraction, which can then hinder successful intubation of the ileum and also lead to high intracolonic pressure with resultant barotrauma.
The colon is a tubular structure about 30 to 40 cm in length at birth and measuring some 150 cm in the adult, or about one quarter the length of the small intestine. The colon begins at the IC valve and ends distally at the anal verge ( Fig. 98.1 ). It consists of 4 segments: cecum and vermiform appendix, colon (ascending, transverse, and descending portions), rectum, and anal canal. The diameter of the colon is greatest in the cecum (7.5 cm) and narrowest in the sigmoid (2.5 cm) until it balloons out in the rectum just proximal to the anal canal.
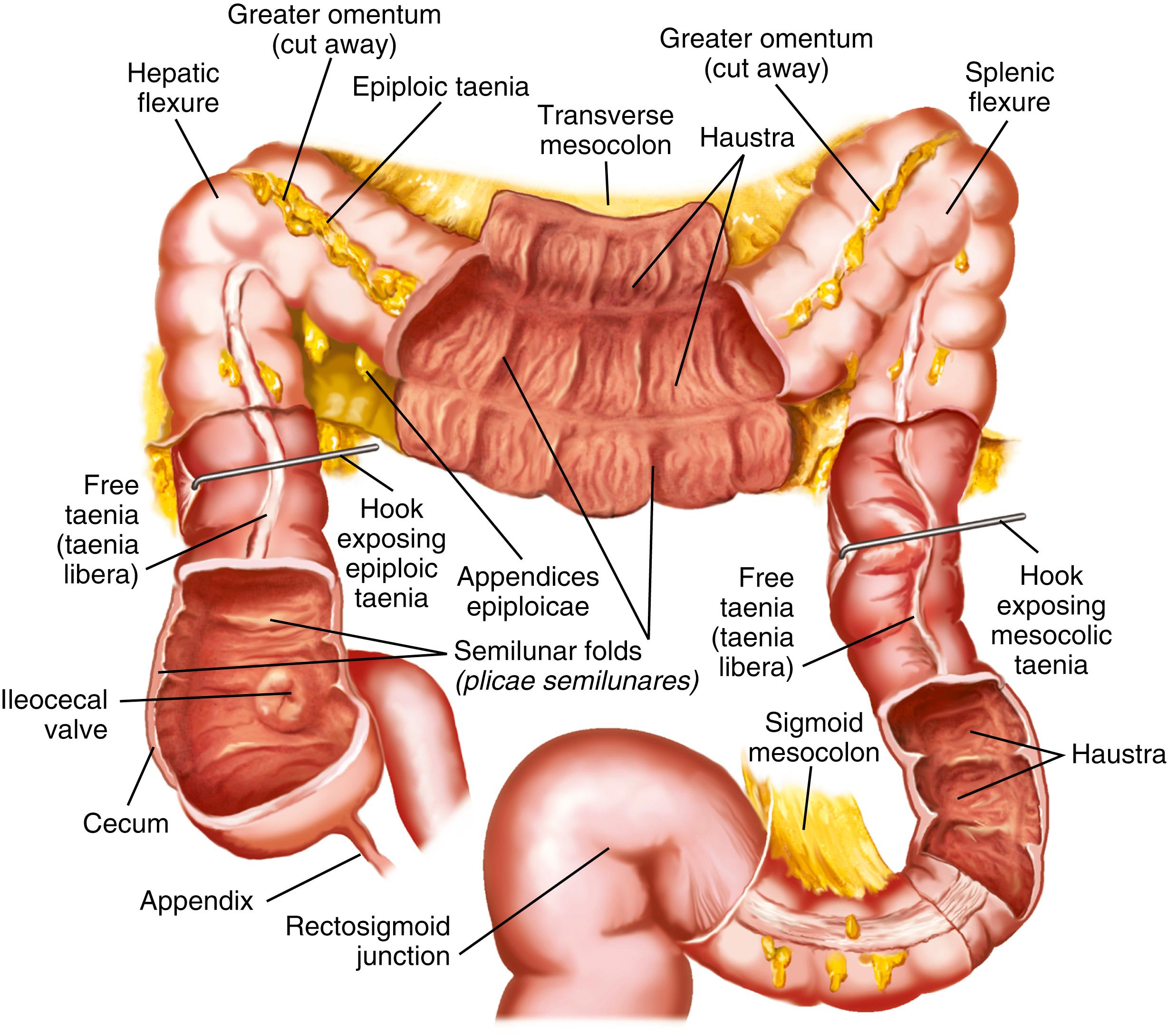
The colon is distinguished from the small intestine by several features. It is larger in caliber, mostly fixed in position, and has outer longitudinal muscle fibers that coalesce into 3 discrete bands called taeniae : the taenia liberis (free tenia), taenia omentalis (omental tenia), and taenia mesocolica (mesenteric tenia). Taeniae are located at 120-degree intervals around the colonic circumference and extend from the cecum to the proximal rectum. Outpouchings, or haustra, occur between the taeniae, and their mucosal surface is sectioned by semilunar folds to give the serosa a sacculated and puckered appearance. Small sacs of peritoneum filled with adipose tissue, the appendices epiploicae, are found on the external surface of the colon. The mesentery fully suspends the transverse colon and sigmoid colon, while the remainder of the colon has mesentery only on its free anterior surface. The appendix has a short mesentery called the mesoappendix .
The cecum is the most proximal portion of the colon. It is about 6 to 8 cm in length and breadth and lies in the right iliac fossa, projecting downward as a blind pouch below the entrance of the ileum. The large diameter of the cecum makes it susceptible to rupture with distal obstruction and permits tumors to grow to substantial size before producing symptoms of obstruction. The cecum is normally nonmobile because it is fixed in position by a small mesocecum; anomalous fixation, however, occurs in 10% to 20% of the population, predominantly women, predisposing them to cecal volvulus.
The IC valve passes perpendicularly through the posteromedial wall of the cecum and consists of a superior and inferior fold arranged in an elliptical manner at the IC orifice. The appendiceal orifice is roughly 2.5 cm inferior to the IC valve, and the vermiform appendix is a blind outpouching extending from the cecum in a direction that varies from person to person. Appendiceal anatomy is discussed further in Chapter 120 .
The ascending colon is narrower than the cecum and extends about 12 to 20 cm from the level of the IC valve to the inferior surface of the posterior lobe of the liver, where it angulates left and forward, forming the hepatic flexure. The ascending colon is covered with peritoneum in about 75% of individuals and thus is usually considered to reside in the retroperitoneum.
At the hepatic flexure, the colon turns medially and anteriorly to emerge into the peritoneal cavity as the transverse colon, fully enveloped in mesentery. The transverse is the longest (40 to 50 cm) and most mobile segment of the colon. It lies between the hepatic and splenic flexures and drapes itself across the anterior abdomen and anterior to the stomach. The phrenocolic ligament anchors the colon at the splenic flexure, but the transverse colon is so mobile that in the upright position it may actually dip down into the pelvis. Abdominal or pelvic surgery that results in adhesion formation can fix the position of the normally mobile transverse colon.
The descending colon is about 25 to 45 cm in length and travels posteriorly and then inferiorly in the retroperitoneal compartment to the pelvic brim. It emerges from the retroperitoneum into the peritoneal cavity as the sigmoid colon, an S-shaped redundant segment of variable length, tortuosity, and mobility. The mobility of the sigmoid colon renders it susceptible to volvulus, and because it is the narrowest part of the colon, tumors and strictures of this region typically cause obstructive symptoms early in the course of disease.
The rectum is 10 to 12 cm in length and begins at the peritoneal reflection, follows the curve of the sacrum passing down and posteriorly, and ends at the anal canal. The rectum narrows at its junction with the sigmoid, expanding proximal to the anus. The rectum lies entirely below the peritoneum in close relationship with the structure of the pelvis. The anorectal junction is 2 to 3 cm anterior to the tip of the coccyx. The rectum does not have sacculation, appendices epiploicae, or mesentery. The outer rectal wall is progressively thickened with prominent and anterior bands of muscle as it descends toward the anus. The luminal surface of the rectum has 3 transverse folds called the valves of Houston.
The anal canal is 2 cm long in the infant and 4.5 to 5 cm long in the adult. It occupies the ischiorectal fossa, passing inferiorly and outward toward the anal opening. The anorectal junction is situated within the pelvic diaphragm and made up of the levator ani, coccygeus, and puborectalis muscles which encircle it; contraction of these muscles allows the anorectum to retain stool, and relaxation allows for defecation. The internal anal sphincter is made up of the circular smooth muscular layer of the intestine, which surrounds the upper three quarters of the canal. The external sphincter is made up of striated muscle; it surrounds the anal canal, and its fibers blend with those of the levator ani muscle to attach posteriorly to the coccyx and anteriorly to the perineal body. Distally, the anal verge represents the transition of anoderm to true skin. The mucosa of the distal 3 cm of the rectum and anal canal contains 6 to 12 redundant longitudinal folds called the columns of Morgagni, which terminate in the anal papillae. These columns are joined together by mucosal folds called the anal valves, which are situated at the dentate line. The zona alba is a white zone that demarcates the transition to typical squamous epithelium. The anatomy and function of these muscles are described in more detail in Chapter 129 .
The proximal duodenum receives arterial blood from the right gastric artery, supraduodenal artery, right gastroepiploic artery, and superior and inferior pancreaticoduodenal arteries. Venous drainage is via the SMV and the splenic and portal veins. The SMA delivers oxygenated blood to the distal duodenum, jejunum and ileum, ascending colon, and proximal two thirds of the transverse colon. Branches of the inferior mesenteric artery supply the remainder of the colon. The arterial supply of the anal area is from the superior, middle, and inferior hemorrhoidal arteries, which are branches of the inferior mesenteric, hypogastric, and internal pudendal arteries, respectively. Venous drainage of the anus is by both the systemic and portal systems. The internal hemorrhoidal plexus drains into the superior rectal veins and then into the inferior mesenteric vein, which, with the SMV, joins the splenic vein to form the portal vein. The vascularity of the distal anus drains by the external hemorrhoidal plexus through the middle rectal and pudendal veins into the internal iliac vein. (See Chapter 118 for discussion of the intestinal blood supply and its disorders.)
Lymphatic drainage courses through the mesentery from villus lacteals and lymphatic follicles and converges at preaortic lymph nodes around the SMA and celiac artery. The lymphatic drainage of both the small intestine and colon follows their respective blood supplies to lymph nodes in the celiac, superior preaortic, and inferior preaortic regions. Lymphatic drainage proceeds to the cisterna chyli and then via the thoracic duct into the left subclavian vein. Proximal to the dentate line, lymphatic drainage is to the inferior mesenteric and periaortic nodes, whereas distal to the dentate line it flows to the inguinal lymph nodes. Therefore, inflammatory and malignant disease of the lower anal canal can manifest with inguinal lymphadenopathy.
The autonomic nervous system—sympathetic, parasympathetic, and enteric—innervates the GI tract. The sympathetic and parasympathetic nerves constitute the extrinsic nerve supply and connect with the intrinsic nerve supply, which is composed of ganglion cells and nerve fibers within the intestinal wall. Innervation of the small intestine and colon is discussed in detail in Chapters 99 and 100, respectively.
The small and large intestine share certain histologic characteristics. The wall of the small intestine and colon is composed of 4 layers: mucosa (or mucous membrane), submucosa, muscularis (or muscularis propria), and serosa ( Fig. 98.2 ).
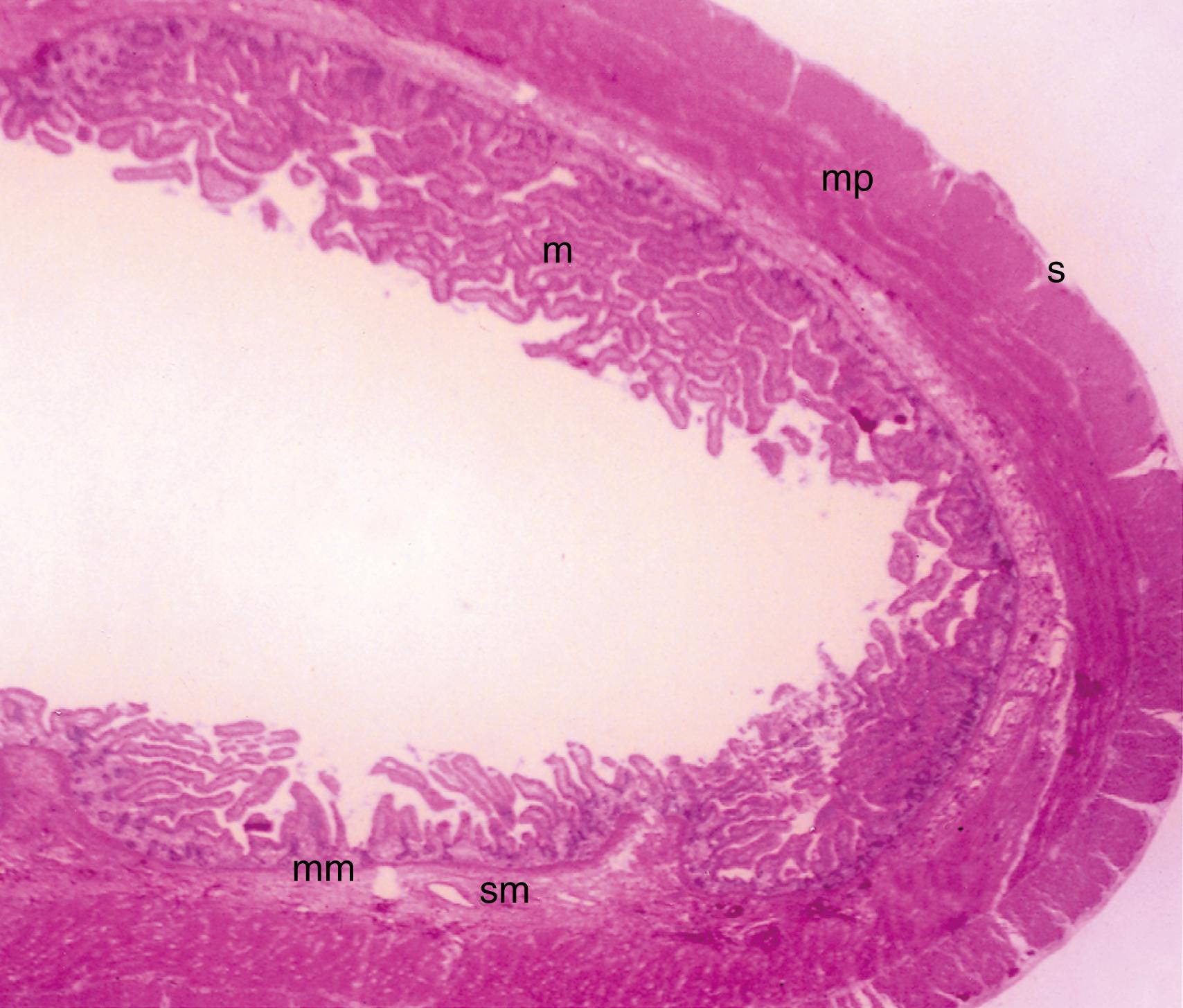
The mucosa consists of the glandular epithelium, lamina propria, and muscularis mucosae ( Fig. 98.3 A and B ). The mucosa is thick and highly vascularized, although less so in distal portions. It has concentric folds ( plicae circulares ) that are also referred to as the valves of Kerckring. The surfaces of the mucosal folds are studded with villus projections, and these features combine to produce a 400- to 500-fold increase in mucosal surface area. An intestinal villus will typically project 0.5 to 1.5 mm into the lumen, and the height of the villus decreases from proximal to distal small intestine. Villi are wider and more leaf-shaped in the duodenal bulb and proximal duodenum, becoming more finger-like in the distal duodenum, proximal jejunum, and remainder of intestine. The villi are covered with mature absorbing enterocytes interspersed with mucus-secreting goblet cells. Each villus contains an artery, vein, and central lacteal. A capillary bed forms along the epithelium, allowing for rapid clearance of absorbed nutrients, fluids, and electrolytes into the systemic circulation. To facilitate the absorptive process, capillary walls are fenestrated with diaphragmatic covers. The core of the villus also contains nerve fibers, plasma cells, macrophages, eosinophils, and fibroblasts. The villi are surrounded by cylindrical structures called the crypts of Lieberkühn, which extend through the lamina propria down to the muscularis mucosae. The crypts are lined with more immature epithelium that primarily functions as a secretory rather than an absorptive epithelium.
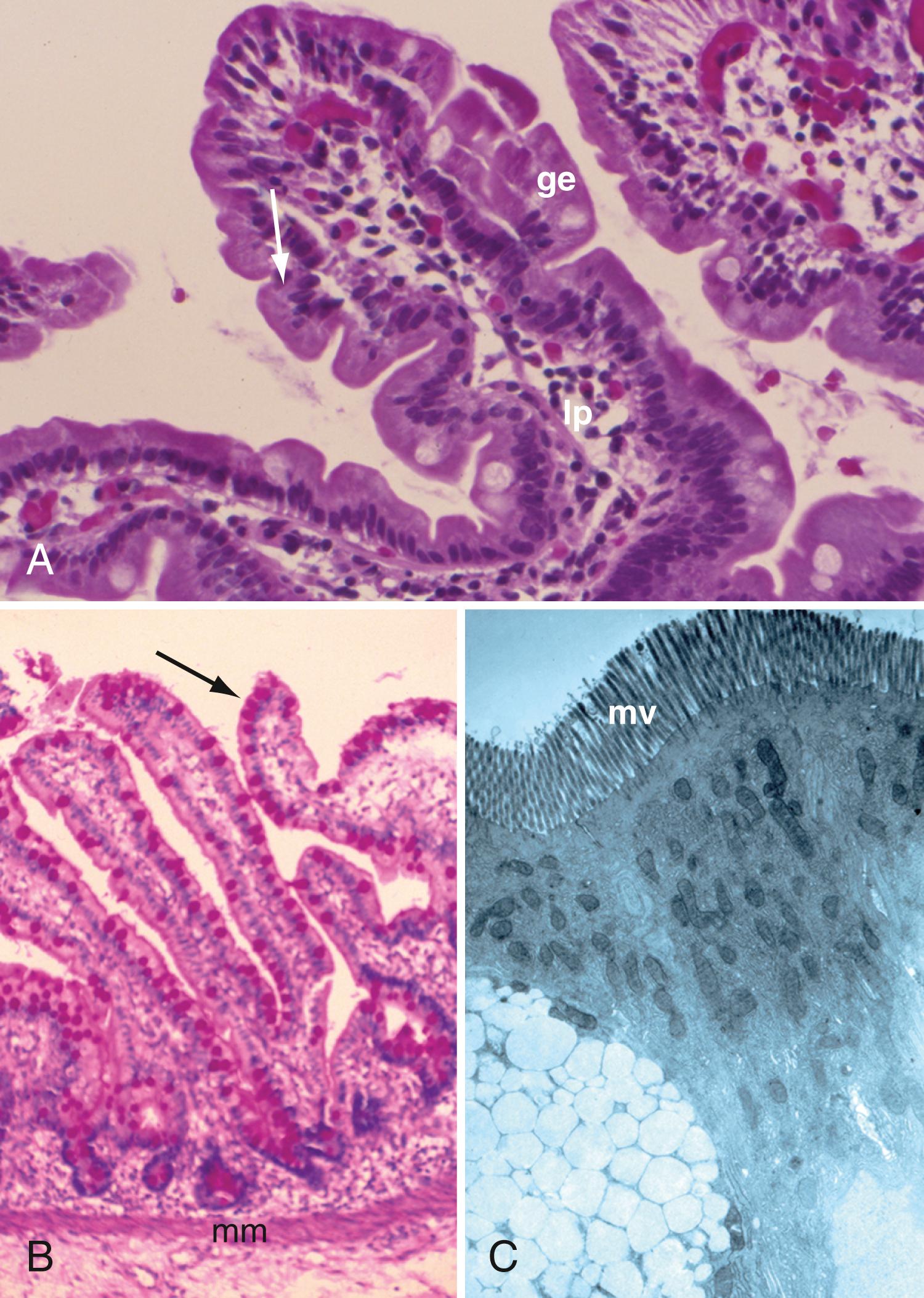
The epithelium of the small intestine is composed of various cell types: absorptive cells (columnar cells), secretory cells (goblet cells), undifferentiated cells, tuft cells, M cells, cup-like cells, and enteroendocrine cells. Crypts contain a similar cell population as the villi, with the addition of Paneth cells and stem cells.
The lamina propria is a layer of reticular connective tissue that provides the structural support for the mucosa, but it also contains many cellular elements important for absorption and immunity. The lamina propria is rich in arterioles, venules lacteals, nerve fibrils, and fibroblasts, lymphocytes, macrophages, neutrophils, eosinophils, and mast cells. The muscularis mucosae consists of a thin layer of smooth muscle only 3 to 10 cells thick at the boundary of the mucosa and submucosa.
Stem cells are pluripotential cells located at the bases of the intestinal crypts. With intense mitotic activity, stem cells give rise to all types of mature intestinal epithelial cells and at the same time replenish themselves through self-renewal. Mucosal epithelial cells turn over every 5 to 7 days. Intestinal epithelial cells are mature by the time they reach the upper third of the villus. Paneth cells are the only cells that do not migrate. Undifferentiated cells have fewer intracellular organelles and microvilli than absorptive cells. The absorptive cells (see Fig. 98.3 A ) are high columnar cells with oval basal nuclei, eosinophilic cytoplasm, and a periodic acid–Schiff (PAS)-positive free surface, the brush border (see Fig. 98.3 B ). On electron microscopic examination, the brush border is seen to be composed of microvilli (see Fig. 98.3 C ), which are more numerous in the small intestinal than in the colonic epithelium. Enterocyte microvilli are estimated to increase the luminal surface area of the cell 14- to 40-fold.
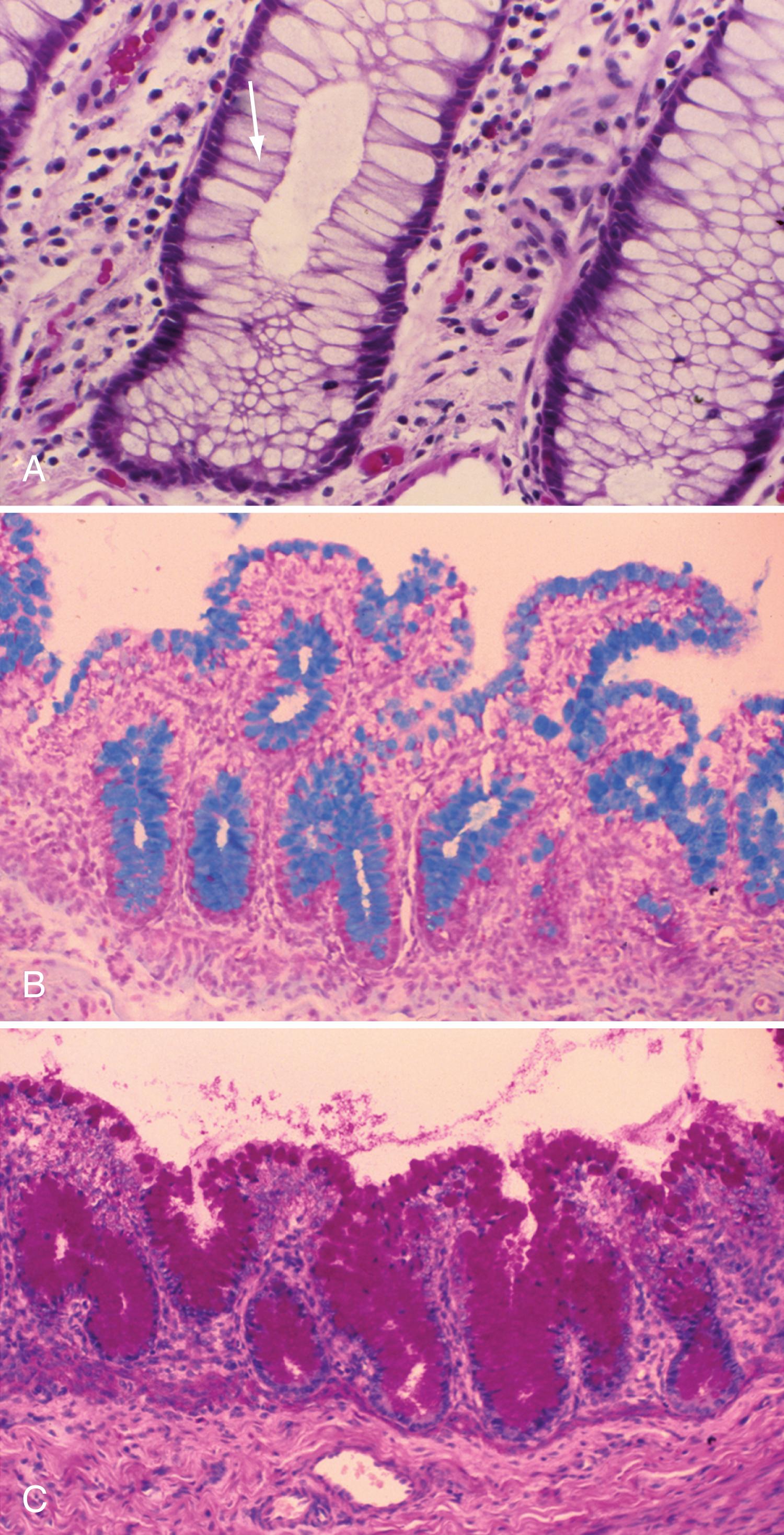
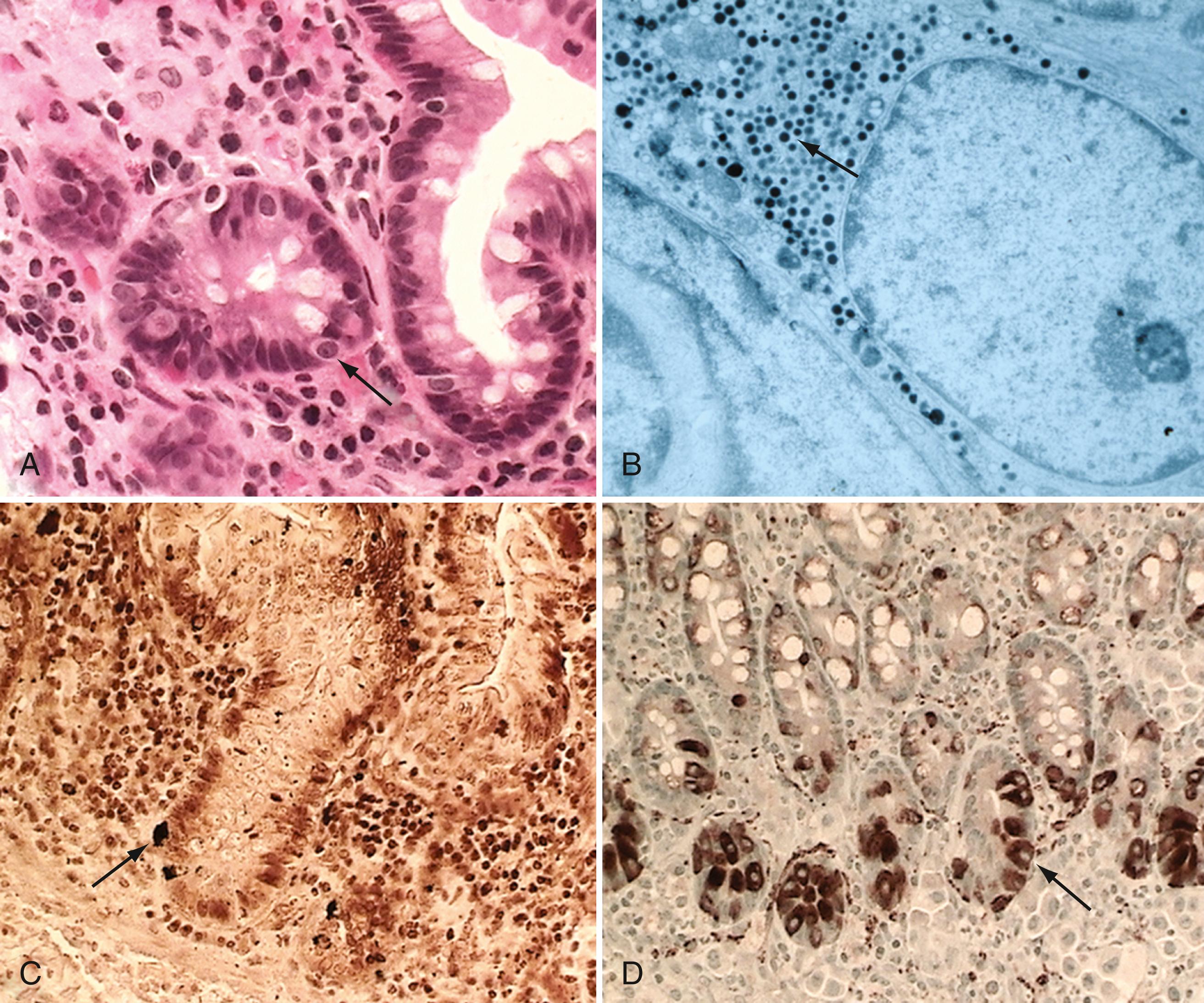
Goblet cells are mucin-producing cells that are scattered among intestinal villi but are more common in the distal ileum and large intestine. Goblet cells are oval or round with flattened basal nuclei ( Fig. 98.4 A ); their cytoplasm is basophilic, metachromatic (see Fig. 98.4 B ), and PAS-positive (see Fig. 98.4 C ) and consists mostly of mucin-secreting granules. Mucin is secreted by 2 pathways: in a neutrally mediated continuous manner, and by the active exocytosis of granules in response to extracellular stimuli.
Paneth cells are flask shaped with an eosinophilic granular cytoplasm and a broad base that is positioned against the basement membrane ( Fig. 98.5 ). In the small intestine, Paneth cells are located exclusively in the crypts of Lieberkühn and secrete α-defensins, antimicrobial proteins, lysozyme, and phospholipase A, thought to be important in protection from infectious pathogens and function to maintain enteric homeostasis.
Cup cells and tuft cells are 2 intestinal epithelial cell types with unidentified functions. Cup cells are present in villi and crypts largely limited to the ileum. Tuft cells are marked by a tuft of long microvilli projecting from the apical surface of the cell.
The mucosa also contains specialized cells called enteroendocrine or neuroendocrine cells ( Fig. 98.6 A ) with specific endocrine functions. Intestinal endocrine cells are sparsely distributed and consist of 11 different cell types ( Table 98.1 ). These are tall columnar cells present in both the crypts and villi and contain prominent secretory granules. The neuroendocrine cells have been divided histologically into argentaffin (i.e., their granules are able to reduce silver nitrate) or enterochromaffin cells and argyrophilic cells (i.e., granules reduce silver nitrate only in the presence of a chemical reducer). These chemical properties subdivide the cell types, but a unifying concept derived from their common origin and functional capacity has led to the term APUD cells. The a mine p recursor, u ptake, and d ecarboxylation concept characterizes the cells as having a common embryonic origin from the neural crest and displaying similar cytochemical and electron microscopic features.
| Vesicle Markers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell Type | Cell Product | LDCV | SLMV | Duod | Jej | Ileum | App | Colon | Rec |
| P/D1 | Ghrelin | CgA, VMAT2 | f | f | f | ||||
| EC | 5-HT | CgA, VMAT1 | Syn | + | + | + | + | + | + |
| D | Somatostatin | CgA | Syn | + | + | f | f | f | f |
| L | GLI/PYY | SgII > CgA | Syn | f | + | + | + | + | + |
| PP | PP | CgA, SgII, VMAT2 | Syn | e | |||||
| G | Gastrin | CgA | Syn | + | |||||
| CCK | Cholecystokinin | + | + | f | |||||
| S | Secretin, 5-HT | CgA | + | + | |||||
| GIP | GIP/Xenin | CgA | + | + | f | ||||
| M | Motilin | + | + | f | |||||
| N | Neurotensin | CgA | f | + | + | ||||
Ultrastructurally, enteroendocrine cells contain membrane-bound granules with variably sized electrodense cores (see Fig. 98.6 B ) that consist of large dense-core vesicles and smaller synaptic-type microvesicles. Neurosecretory granules can be demonstrated as dark granules with nonspecific agents (e.g., Grimelius stain [see Fig. 98.6 C ]), or more specific immunohistochemical stains can be used (e.g., neuron-specific enolase, chromogranin, synaptophysin). Chromogranin enables identification of the large dense-core vesicles, and synaptophysin targets the small synaptic-like microvesicles (see Fig. 98.6 D ). With specific immunohistochemical staining agents, it is possible to identify the individual chemical and protein components of neuroendocrine cells. The differential expression of certain proteins also makes it possible to subdivide neuroendocrine cell populations. For example, vesicular monoamine transporter (VMAT) has 2 isoforms: VMAT1 is restricted to serotonin-producing enterochromaffin cells, and VMAT2 is expressed by histamine-producing cells, enterochromaffin-like cells, and pancreatic islet cells.
The hormone products of these cells are discharged into the extracellular space on the basal and basolateral surfaces and have paracrine effects on absorption, secretion, motility, mucosal cell proliferation, possibly immunobarrier control, and even some endocrine effects upon systemic absorption.
The preferred designation of neuroendocrine cells is by their stored peptide. Serotonin-producing enterochromaffin cells, vasoactive intestinal polypeptide cells, and somatostatin D cells are distributed throughout the small and large intestine. Cells that produce gastrin, ghrelin, gastric inhibitory peptide, secretin, and CCK are found predominantly in the stomach and proximal small intestine. Cells that secrete peptide YY, glucagon-like peptide-1, glucagon-like peptide-2, and neurotensin are found in the ileum.
M cells are specialized epithelial cells overlying lymphoid follicles and Peyer patches in the small intestine and colon. M cells are an important site of luminal antigen sampling for immune processing by the mucosal lymphoid system. This process of sampling plays an important role in the development and maintenance of immune tolerance, host defense against pathogens, and intestinal homeostasis.
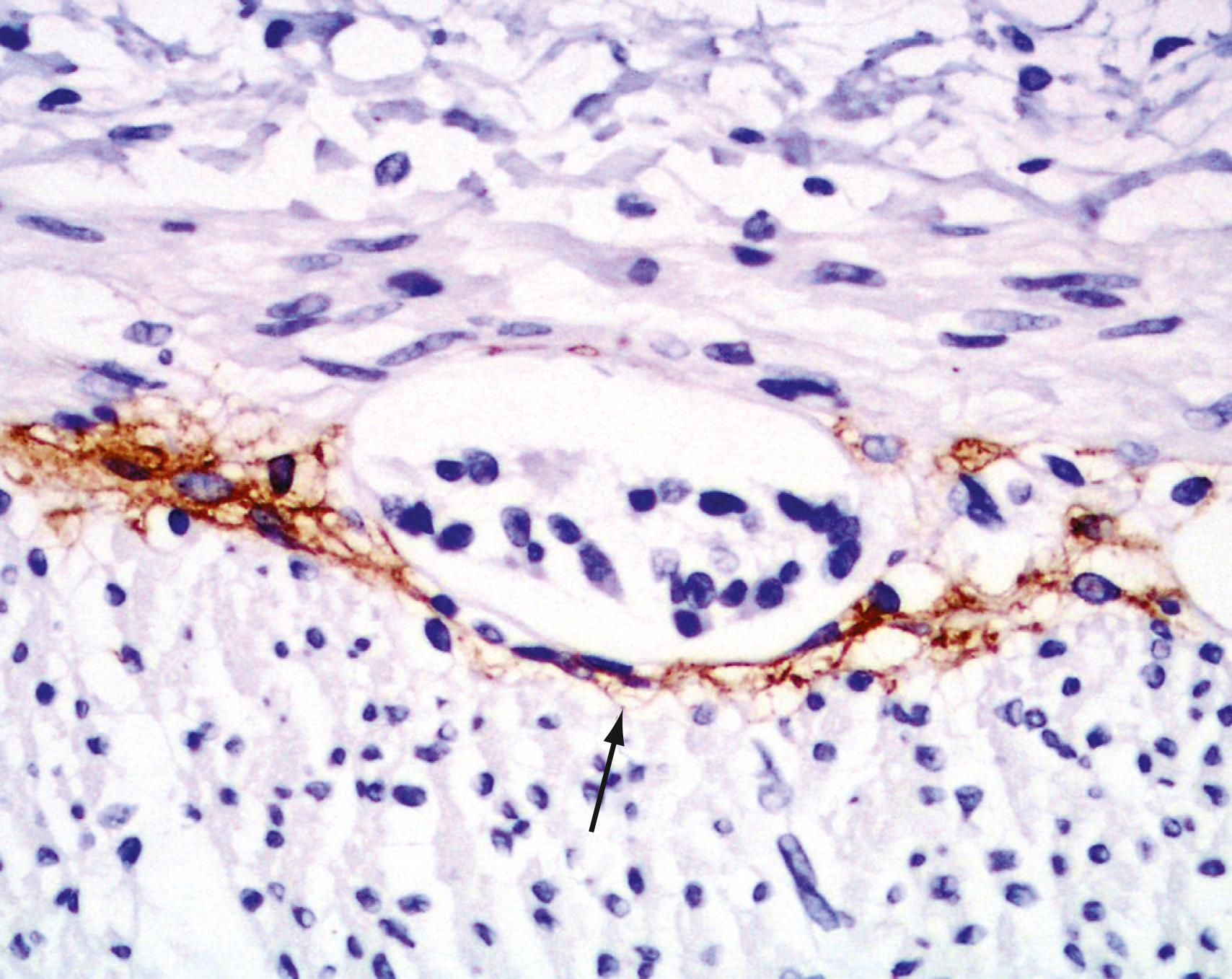
The interstitial cells of Cajal (ICC) are found in both the small intestine and colon and are located in the myenteric plexuses within the muscularis propria and the submucosa ( Fig. 98.7 [see Chapters 99 and 100 Chapter 100Chapter 99]). The ICC are important in the regulation of intestinal peristalsis and function as the pacemaker cells of the intestine. They influence the frequency of smooth muscle contraction, amplify neuronal signals, mediate neurotransmission from enteric motor neurons to smooth muscle cells, and set the smooth muscle membrane potential gradient. The ICC are spindle-shaped or stellate cells with long, ramified processes and express c-kit (CD117), a tyrosine kinase receptor critical for their survival.
The submucosa is a fibrous connective tissue layer that lies between the muscularis mucosae and the muscularis propria. It contains lymphocytes, fibroblasts, mast cells, blood and lymphatic vessels, and a nerve fiber plexus—Meissner plexus—composed of non-myelinated postganglionic sympathetic fibers and parasympathetic ganglion cells. The submucosa supports the mucosa in specialized functions of nutrient, fluid, and electrolyte absorption by conveying a rich network of blood vessels, lymphatics, and nerves that ensure efficient handling of absorbates.
Brunner glands are submucosal glands (see Fig. 98.9 B ) found primarily in the first portion of the duodenum and in decreased numbers in the distal duodenum; in children, these glands may also be present in the proximal jejunum.
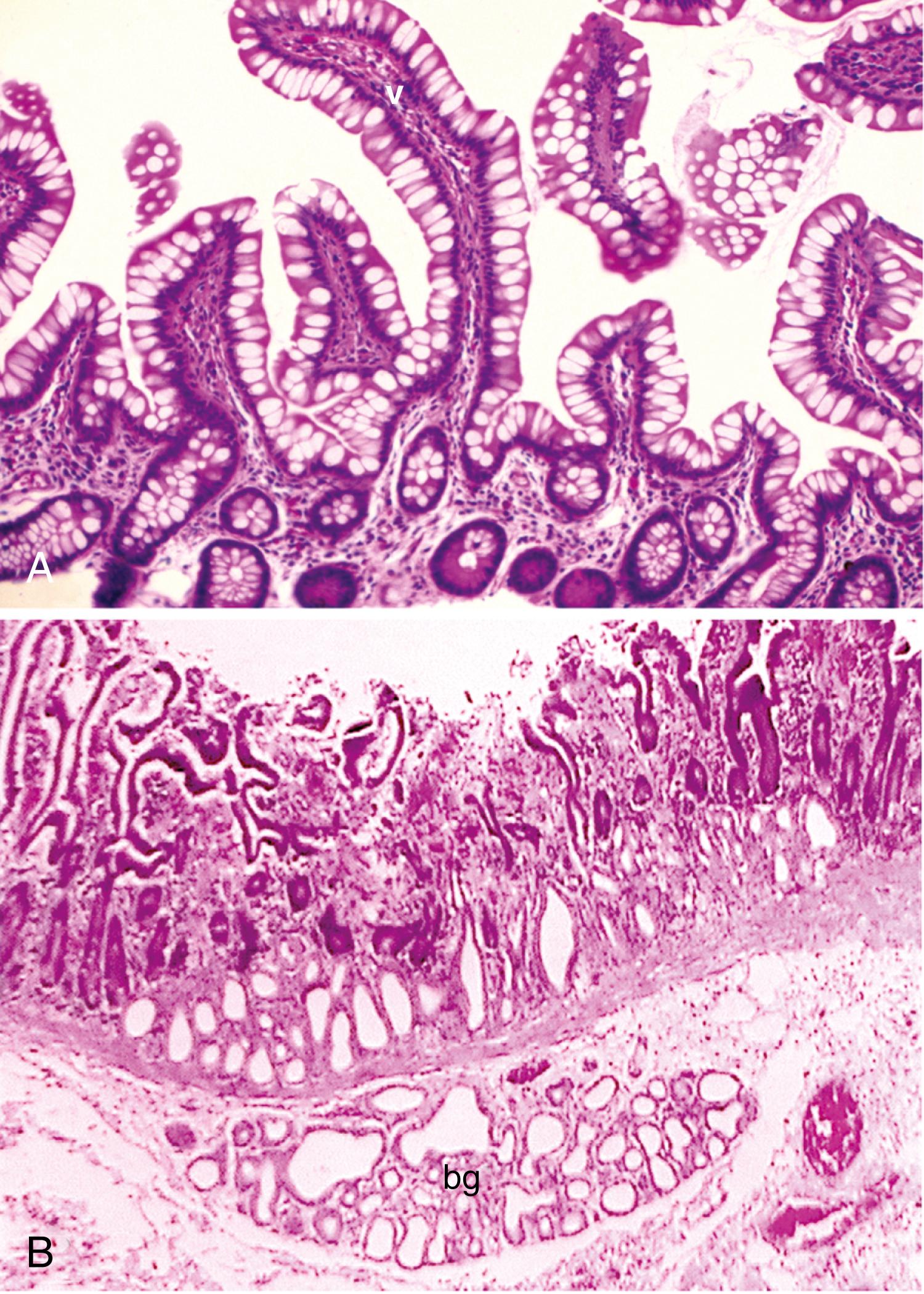
The function of Brunner glands is to secrete a bicarbonate-rich alkaline secretion that helps neutralize gastric chyme; a mucinous secretion that helps lubricate the mucosa; EGF; a variety of trefoil peptides, bactericidal factors, proteinase inhibitors, and surface-active lipids. The secretions that drain into the base of the duodenal crypts contribute to increased luminal pH by promoting pancreatic secretion and gallbladder contraction. The mucous layer protects the epithelial surface from peptic digestion; this protection is thought to be due to glycoprotein class III mucin glycoproteins.
The muscularis propria is mainly responsible for contractility and peristaltic movement of luminal contents through the GI tract. It consists of 2 layers of smooth muscle: an inner circular coat and an outer longitudinal coat arranged in a helicoidal pattern. A prominent nerve fiber plexus called the myenteric or Auerbach plexus is located in the plane between these 2 muscle layers ( Fig. 98.8 ). The ganglia in the myenteric plexus are more prominent than their submucosal counterpart. Parasympathetic and postganglionic sympathetic fibers terminate in parasympathetic ganglion cells, and postganglionic parasympathetic fibers terminate in smooth muscle.
The serosa is the outermost layer of the intestinal wall and is composed of a thin layer of mesothelial cells, representing an extension of the visceral peritoneum and mesentery as it envelops the intestine.
The mucosa of the small intestine is characterized by folds ( plicae circulares , or valves of Kerckring) and villi. The mucosal folds actually comprise mucosa and submucosa. Villi are mucosal folds that decrease in size from the proximal to distal small intestine and are of different shapes in the various segments of the small intestine. They may be broad, short, or leaf-like in the duodenum, tongue-like in the jejunum, and finger-like more distally ( Fig. 98.9A ). The villous pattern may vary in different ethnic groups; biopsy specimens from Africans, Indians, South Vietnamese, and Haitians have shorter and thicker villi, an increased number of leaf-shaped villi, and more mononuclear cells in comparison with specimens from North Americans. The implications of these changes with regard to symptoms and subclinical GI infection are discussed in Chapter 108 .
The height of the normal villus is 0.5 to 1.5 mm; villus height should be more than half the total thickness of the mucosa and 3 to 5 times the length of the crypts. Villi are lined by enterocytes, goblet cells, and enteroendocrine cells.
Enterocytes are tall columnar cells, each with a basally located, clear, oval-shaped nucleus and several nucleoli. The cells are tightly cemented to the basal lamina and adjoined to adjacent enterocytes at the apical pole by intracellular tight junctions. The luminal surface has microvilli that contain necessary enzymes for nutrient absorption; a central core cytoskeleton is made of actin, villin, fimbrin, brush border myosin, and spectrin. The apical surface of the epithelium carries brush border transporters, Na + /H + exchangers, and anion exchangers (see Chapter 101 ). The junction complexes are made up of 3 components: the proximal tight junction ( zonula occludens ), the intermediate junction ( zonula adherens ), and the deep junction, which includes the spot desmosome and the macular adherens zone (see Chapter 101 ). Movement through junctions is by paracellular transport and is the dominant pathway for passive ion and fluid flow. Tight junctions consist of claudins, occludens, and junctional adhesion molecules that bind and prevent passage of molecules between them in a regulated manner. They are leakier and have a lower resistance in the proximal small intestine, and tighter in the distal intestine. The zonula adherens is less adherent and involved in cell signaling. Spot desmosomes are thought to augment transmembrane linkages spanning the intercellular gap and are involved in cell wall communications. The basolateral membrane is responsible for carriers to facilitate diffusion of organic solutes not coupled to ion movements. Gap junctions allow for communication and intercellular passage of ions and low molecular weight nutrients and intracellular messengers such as cyclic adenosine monophosphate.
Two types of glands are present in the small intestine: Brunner glands (see previously) and crypts of Lieberkühn (intestinal crypts). The crypts of Lieberkühn are tubular glands that extend to the muscularis mucosae (see Fig. 98.5 ); they are occupied mainly by undifferentiated cells and Paneth cells. Cells are generated at the crypt base and migrate up the villus. During this migration, these cells mature and differentiate into a secretory lineage (goblet cells, enteroendocrine cells) and enterocytes. The commitment of the stem cells to differentiate is acquired in the upper third of the crypt where cells lose their ability to divide. The constant renewal of enterocytes is regulated by human acyl-coenzyme A synthetase.
Paneth and columnar cells predominate in the base of the crypt. Above the base are absorptive cells and oligomucin cells; the latter originate from undifferentiated cells and differentiate into goblet cells. Goblet cells predominate in the upper half of the crypt. Enteroendocrine cells are admixed with goblet cells. A certain number of CD3 + intraepithelial T lymphocytes (up to 30 per 100 epithelial cells) are normally present in the villi. Smooth muscle is found in the lamina propria of the small intestinal villus, extending vertically up from the muscularis mucosae. Plasma cells containing primarily immunoglobulin A, and mast cells are also present. Lymphoid tissue is prominent in the lamina propria as both solitary nodules and confluent masses—Peyer patches—and is seen in the submucosa. Peyer patches are distributed along the anti-mesenteric border and are most numerous in the terminal ileum; their numbers decrease with age.
Most types of enteroendocrine cells are present in the duodenum. Cells that produce ghrelin, gastrin, CCK, motilin, neurotensin, gastric inhibitory peptide, and secretin are restricted to the small intestine.
The proportions of cells differ in the villi and crypts as well as in different segments of the intestine. Ninety percent of the villus epithelial cells are absorptive cells intermingled with goblet and enteroendocrine cells. The proportion of goblet to absorptive cells is increased in the ileum. The ICC are more abundant in the myenteric plexus of the small intestine than in the colon.
The colonic walls are similar to those of the small intestine. The outer layer forms the taeniae coli , which run in parallel to the long axis of the colon throughout its entire length. The width of the taeniae extends from 6 to 12 mm, and thickness gradually increases from the cecum to the sigmoid colon. The epithelial layer is smooth with crescentic folds corresponding to external sacculations. The surface epithelium is simple columnar type and is interspersed with vascular cells and goblet cells. The epithelial surface and upper third of the crypts are mostly lined with tall, slender absorptive columnar cells called principal cells. Goblet cells are the second most abundant cells on the surface of the colonic epithelium; they produce mucin, which aids in the passage of feces. Colonic epithelial cells are generated from stem cells at the base of the crypts and migrate toward the intestinal lumen 3 to 5 days after initiation of apoptosis. Most epithelial cells undergo apoptosis when they lose contact with the extracellular matrix and are shed into the lumen through caspase (cysteine-aspartic protease) activation. Caspase activation is responsible for the cleavage of essential intracellular proteins that leads to apoptosis and, therefore, loss of anchorage.
The mucosa of the large intestine is characterized by the crypts of Lieberkühn, which dip to the muscularis mucosae and contain goblet cells, absorptive and enteroendocrine cells, and undifferentiated cells that are restricted to the lower third of the crypts. Glucagon-like immunoreactant pancreatic polypeptide-like peptide (PYY) with N -terminal tyrosine amide–producing L-cells predominate in the large intestine. Paneth cells are scarce and normally are noted only in the proximal colon. The lamina propria of the large intestine contains solitary lymphoid follicles that extend into the submucosa. Lymphoid follicles are more developed in the rectum and decrease in number with age. Confluent lymphoid tissue is present in the appendix. Macrophages (muciphages) predominate in the subepithelial portion of the lamina propria, are weakly PAS positive, and are associated with stainable lipids.
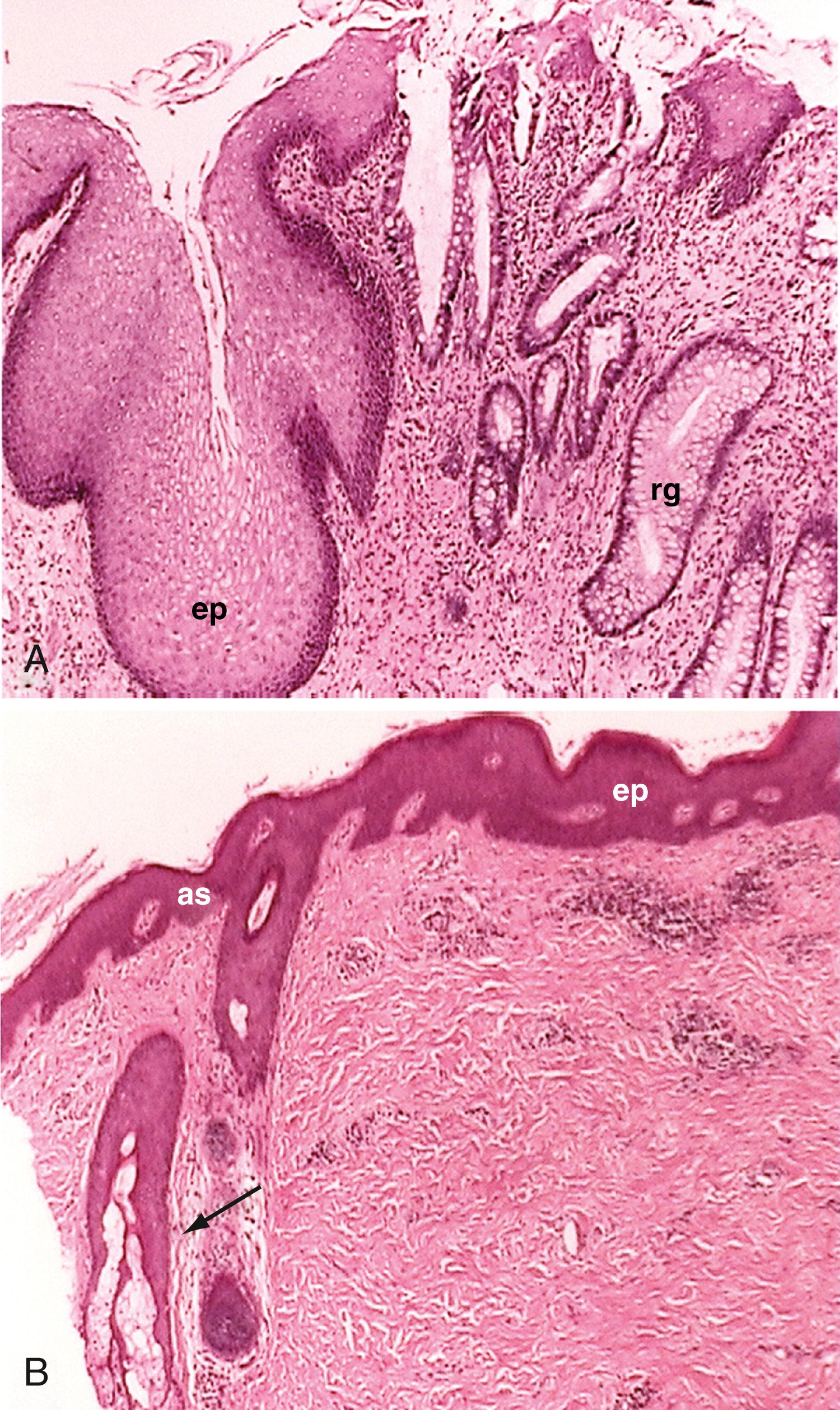
Microscopically, the anal canal is divided into 3 zones: proximal, intermediate or pectinate, and distal or anal skin. The proximal zone is lined by stratified cuboidal epithelium, and the transition with the rectal mucosa, which is lined by high columnar mucus-producing cells, is called the anorectal histologic junction ( Fig. 98.10 A ). The intermediate or pectinate zone is lined by stratified squamous epithelium but without adnexae (e.g., hair, sebaceous glands) and is also referred to as anoderm. Its proximal margin, in contact with the proximal zone, is called the dentate line; its distal margin, in contact with the anal skin, constitutes the pectinate line, also referred to as the mucocutaneous junction (see Fig. 98.10 B ). Some authors use the terms pectinate line and dentate line interchangeably. The anal skin is lined by squamous stratified epithelium and contains hair and sebaceous glands.
Large arterial branches enter the muscularis propria through the serosa and pass to the submucosa, where they branch to form large plexuses. In the small intestine, 2 types of branches arise from the submucosal plexuses: some arteries branch on the inner surface of the muscularis mucosae and break into a capillary network that surrounds the crypts of Lieberkühn; other arteries are destined for villi, each villus receiving 1 or 2 arteries, to set up the anatomic arrangement that allows a countercurrent mechanism, thus aiding absorption. One or several veins originate at the tip of each villus from the superficial capillary plexus, anastomose with the glandular venous plexus, and then enter the submucosa to join the submucosal venous plexus.
In the colon, branches from the submucosal arterial plexus extend to the surface, giving rise to capillaries that supply the submucosa, and there branch to form a capillary meshwork around the crypts of Lieberkühn. From the periglandular capillary meshwork, veins form a venous plexus between the base of the crypts and the muscularis mucosae. From this plexus, branches extend into the submucosa and form another venous plexus from which large veins follow the distribution of the arteries and pass through the muscularis propria into the serosa.
The lymphatics of the small intestine are called lacteals and become filled with milky-white lymph called chyle after eating. Each villus contains 1 central lacteal, except in the duodenum, where 2 or more lacteals per villus may be present. The wall of the lacteal consists of endothelial cells, reticulum fibers, and smooth muscle cells. At the base of the villus, the central lacteals anastomose with the lymphatic capillaries between the crypts of Lieberkühn. They also form a plexus on the inner surface of the muscularis mucosae. Branches of this plexus extend through the muscularis mucosae to form a submucosal plexus. Branches from the submucosal plexus penetrate the muscularis propria, where they receive branches from plexuses between the inner and outer layers. Lymphatic vessels are absent in the colonic mucosa, but the distribution of lymphatics in the remaining colonic layers is similar to that in the small intestine.
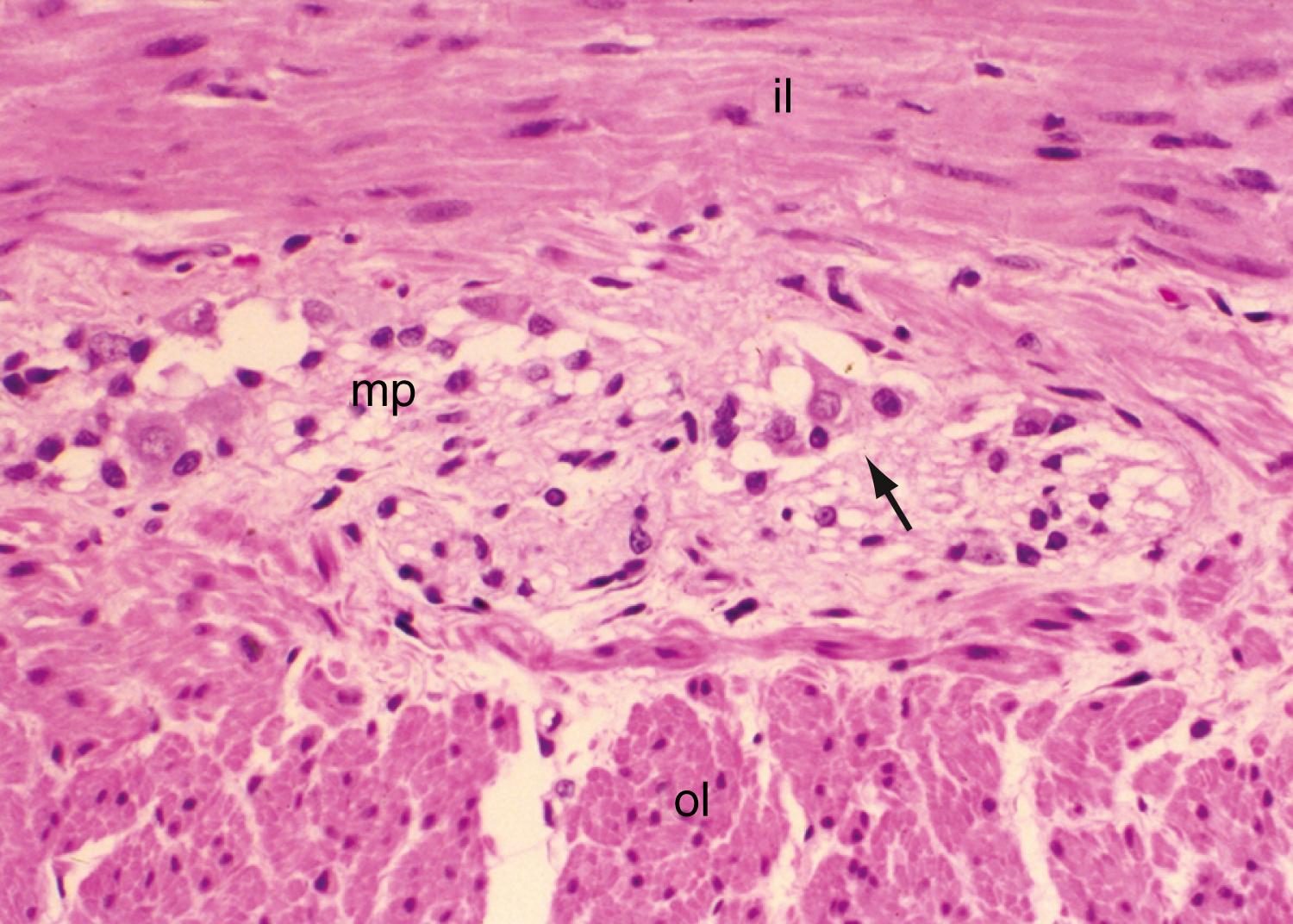
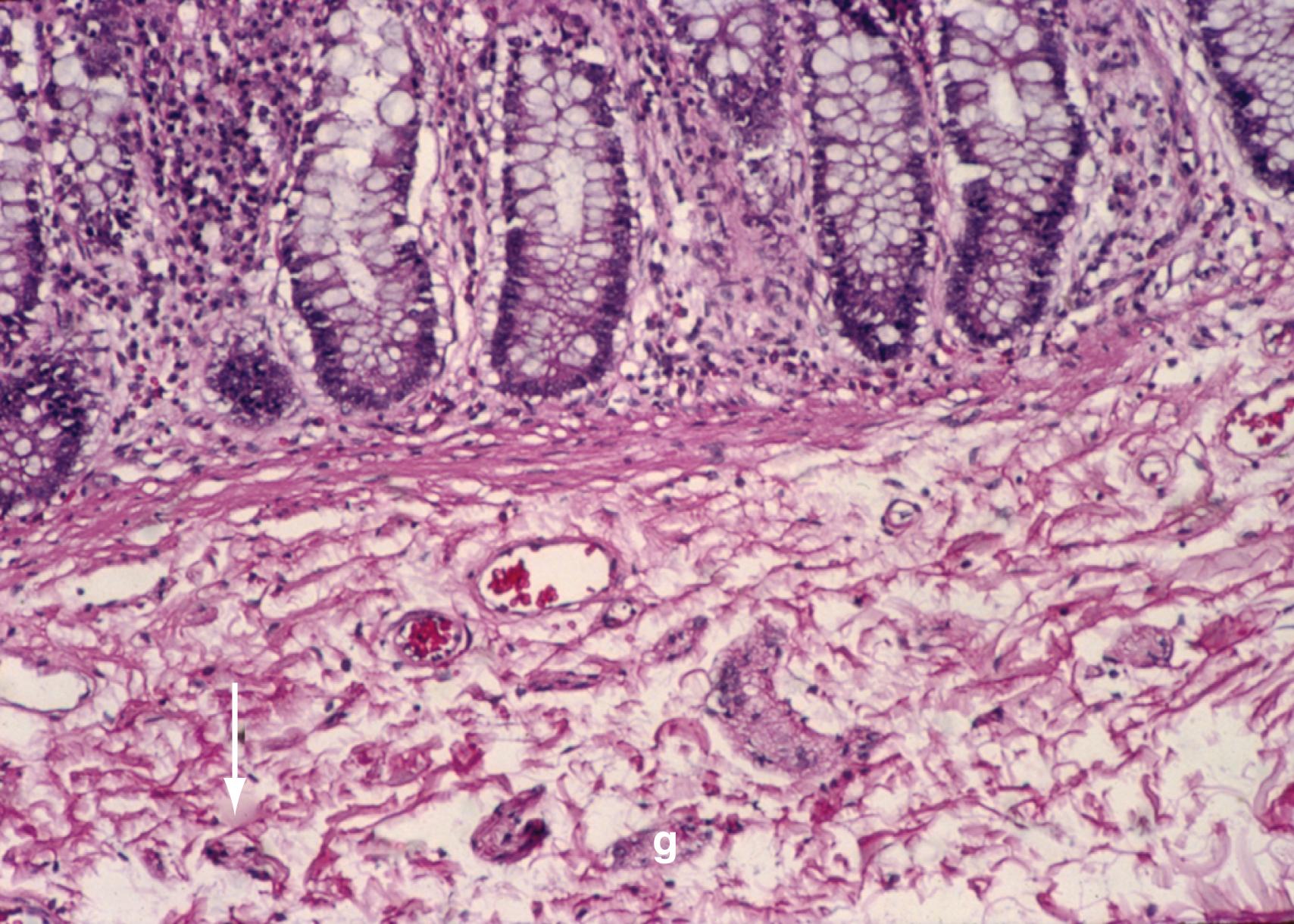
The intrinsic nervous system (enteric nervous system [ENS]) consists of subserosal, muscular, and submucosal plexuses. The subserosal plexus contains a network of thin nerve fibers without ganglia that connects the extrinsic nerves with the intrinsic plexus. The myenteric plexus, or Auerbach plexus, is situated between the outer and inner layers of the muscularis propria (see Fig. 98.8 ); it consists of ganglia and bundles of unmyelinated axons that connect with the ganglia to form a meshwork. These axons originate from processes of the ganglion cells and extrinsic vagus and sympathetic ganglia. The deep muscular plexus, or Schabadasch plexus, is situated on the mucosal aspect of the circular muscular layer of the muscularis propria. It does not contain ganglia; it innervates the muscularis propria and connects with the myenteric plexus. The submucosal plexus, or Meissner plexus, consists of ganglia and nerve bundles. The nerve fibers of this plexus innervate the muscularis mucosae and smooth muscle in the core of the villi. Fibers from this plexus also form a mucosal plexus that is situated in the lamina propria and provides branches to the intestinal crypts and villi. The ganglion cells of the submucosal plexus are distributed in 2 layers; one is adjacent to the circular muscular layer of the muscularis propria, and the other is contiguous to the muscularis mucosae. Ganglion cells are large cells, isolated or grouped in small clusters called ganglia ( Fig. 98.11 ). Ganglion cells have an abundant basophilic cytoplasm, a large vesicular round nucleus, and a prominent nucleolus. Ganglion cells are scarce in the physiologically hypoganglionic segment 1 cm above the anal verge.
The embryo is a bilaminar germ disk at 3 weeks’ gestation. Through a process called gastrulation, this disk becomes trilaminar and gives rise to the 3 primary germ layers: ectoderm, mesoderm, and endoderm. It also establishes bilateral symmetry, a dorsal-ventral orientation, and an anterior-posterior (A-P) axis.
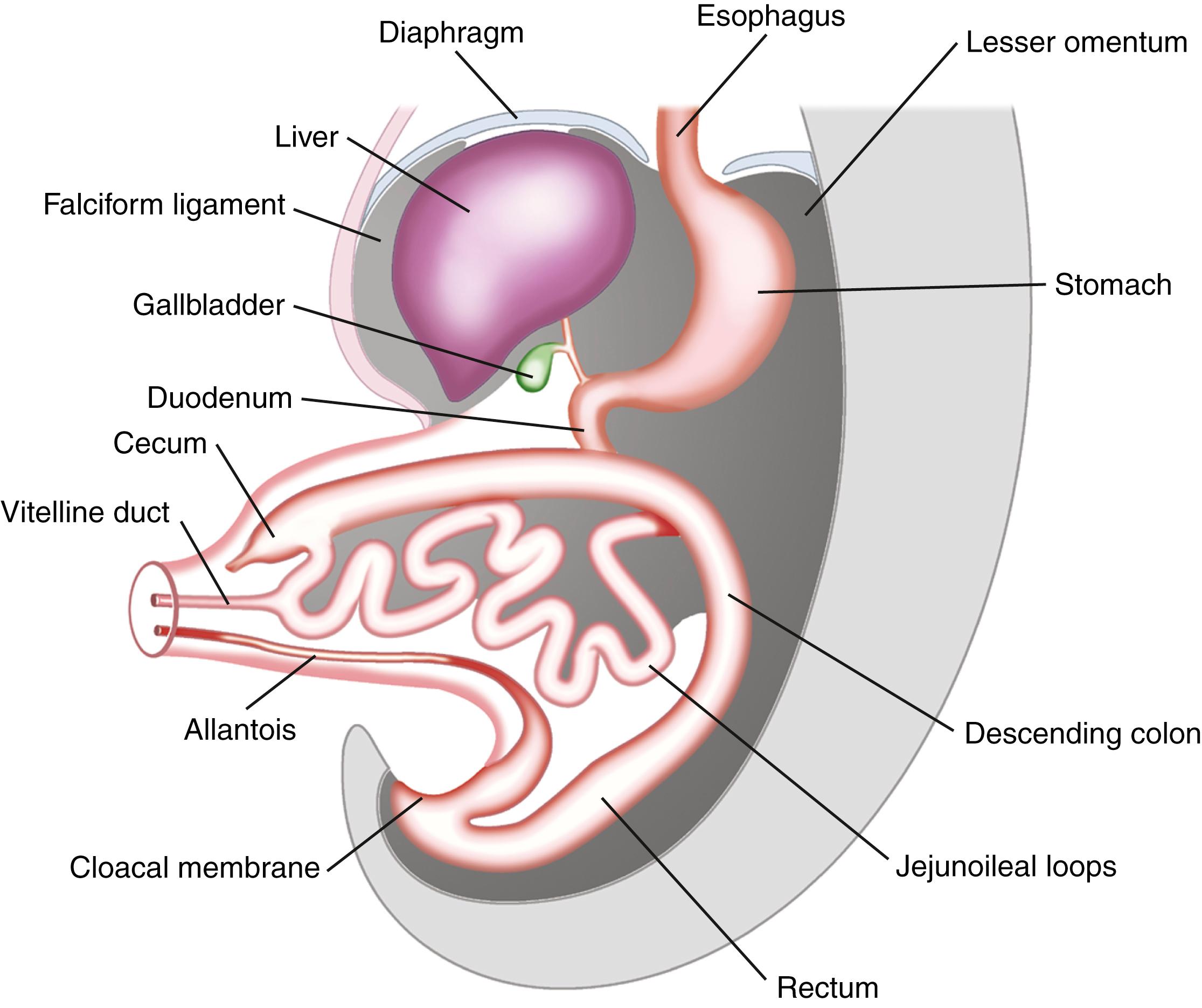
The surface facing the yolk sac becomes endoderm, the surface facing the amniotic sac becomes ectoderm, and the middle layer becomes mesoderm. The oral opening is marked by the buccopharyngeal membrane; the future openings of the urogenital and digestive tracts become identifiable as the cloacal membrane. At 4 weeks’ gestation, the alimentary tract is divided into 3 parts: foregut, midgut, and hindgut, the endoderm connecting with the yolk sac ( Fig. 98.12 ).
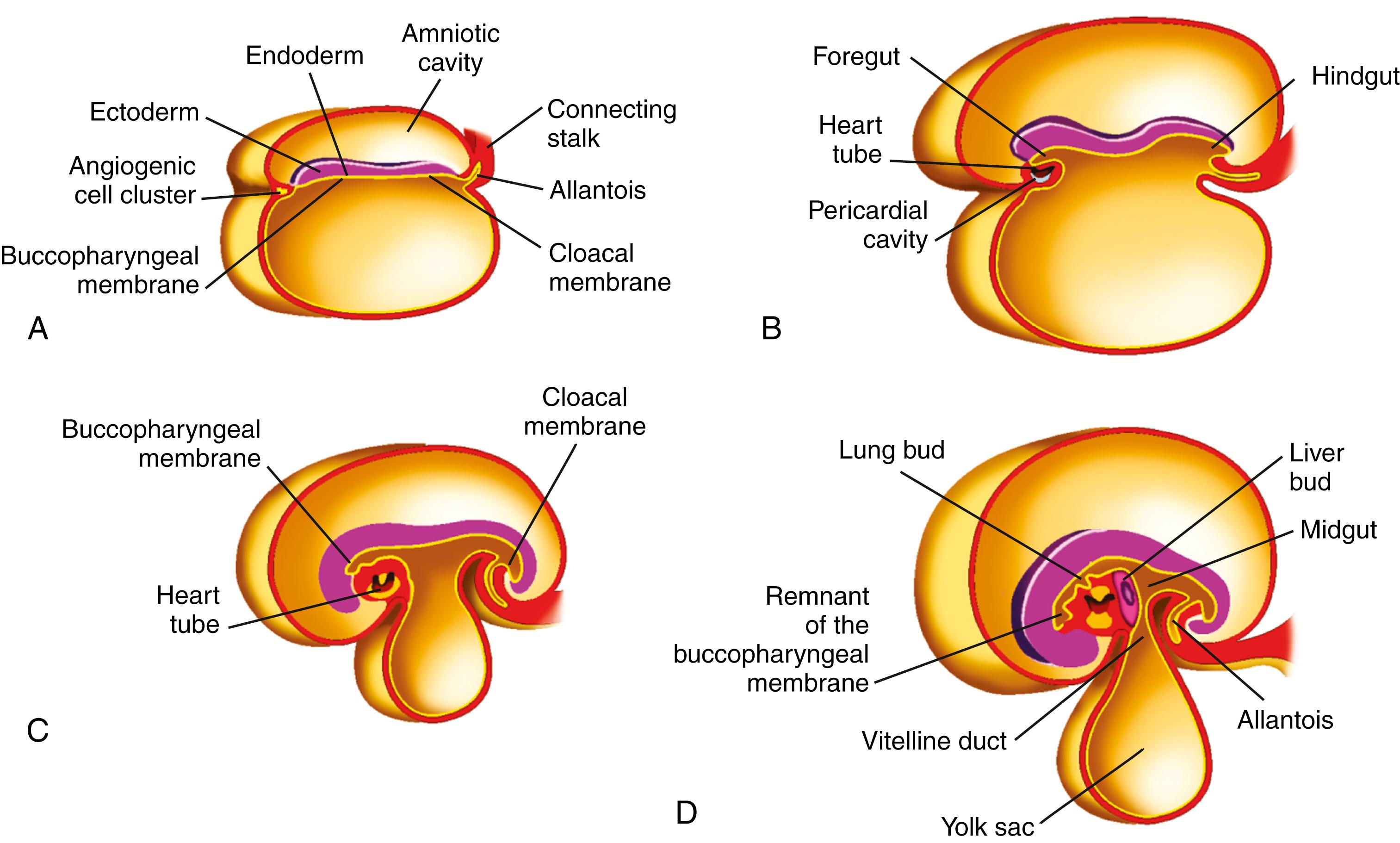
These segments form a tube by growth and folding. The folding process brings together the endodermal, mesodermal, and ectodermal layers with the corresponding layers on the opposite side, converting the flat endodermal layer into the intestinal tube. Initially the foregut and hindgut are blind-ending tubes separated by a midgut that is open to the yolk sac. As the lateral edges of the midgut fuse to become a tube, there is a narrowing of the communication between the yolk sac and endoderm, producing the vitelline duct (see Fig. 98.12 ). With folding of the embryo during week 4 of development, the mesodermal layer splits. The portion that adheres to endoderm forms the visceral peritoneum, whereas the part that adheres to ectoderm forms the parietal peritoneum. The space between the 2 layers becomes the peritoneal cavity.
The primitive intestine results from incorporation of the endoderm-lined yolk sac cavity into the embryo following embryonal cephalocaudal and lateral folding. The endoderm gives rise to the epithelial lining of the GI tract; muscle, connective tissue, and peritoneum originate from the splanchnic mesoderm. During the 9th week of development, the epithelium begins to differentiate from the endoderm, with villus formation and differentiation of epithelial cell types. Organogenesis is complete by 12 weeks’ gestation.
Initially the foregut, midgut, and hindgut are in broad contact with the mesenchyma of the posterior abdominal wall. The intra-embryonic cavity is in open communication with the extra-embryonic cavity. Subsequently the intra-embryonic cavity loses its wide connection with the extra-embryonic cavity. By week 5 of embryonic development, splanchnic mesoderm layers are fused in the midline and form a double-layered membrane, the dorsal mesentery, between the right and left halves of the body cavity. The mesoderm surrounds the intestinal tube and suspends it from the posterior body wall, allowing it to hang into the body cavity. The caudal portions of the foregut, midgut, and most of the hindgut are suspended from the abdominal wall by the dorsal mesentery, which extends from the duodenum to the cloaca. The dorsal mesentery forms the mesoduodenum in the region of the duodenum, the dorsal mesocolon in the region of the colon, and the mesentery proper in the region of the jejunum and ileum.
Molecular regulation of intestine formation is a complex network of carefully orchestrated gene expression, activation of signal transduction pathways, and cell-cell interactions that works in a cooperative manner; the balance of signals often determines the developmental pathways that follow. Only selected molecular elements are presented here, but comprehensive reviews are available.
Development of the intestinal tube requires simultaneous inductive and patterning steps. The transforming growth factor-β superfamily member Nodal is required for the mesoderm and endoderm specification in all vertebrate species and plays a secondary role in anterior-posterior (A-P) patterning. Crosstalk and inductive cues exchanged between the mesoderm and endoderm are thought to play a critical role in gastrulation. The interruption of Fox factors (Fox A2, FoxH1), GATA factors, Sox17, Mixl1, or Smad signaling will result in a failure of tube formation, primarily by altering endoderm development and specification. The Wnt signaling pathway also plays a critical role in intestinal tube formation.
Genes expressed during A-P patterning include Hhex , FoxA2 , and Sox2 in the anterior gut, while Cdx is expressed posteriorly. Hox genes play an important role in patterning of the mesoderm and ectoderm, while Cdx2 is a critical gene in hindgut formation and intestinal specification and patterning, particularly in cecal development. Other genes and factors that play a role in A-P patterning of the endoderm include FGF , Wnt , BMP , and retinoic acid signaling. Intestinal elongation is also controlled by a number of genes. Deletion of Wnt5a results in an 80% reduction in small intestine and a 63% reduction in colonic length. The absence of any one of a family of proteins that interact with Wnt5a (secreted frizzled-related proteins) also adversely affect bowel length.
The endoderm transitions from simple epithelium to columnar epithelium in a rostral-caudal (proximal-distal) manner, including in the colon, which initially has villus-like structures until it undergoes reorganization. The mesenchyme invaginates to form longitudinal ridges that become epithelial folds. These folds evolve into villi, and crypt-shaped structures form as secondary lumina. This reorganization occurs through extensive crosstalk between the endoderm and mesoderm that involves transforming growth factor-β, PDGF, FGF, WNT, and EGF. BMPs also expressed in the mesenchyme influence endoderm-mesoderm interactions and epithelial development. A mutation in the receptor BMPR1a results in epithelial cell hyperproliferation and polyp formation, as seen in juvenile polyposis syndrome.
Other important factors in the formation of the epithelium include the Hedgehog signals (Sonic [Shh] and Indian [Ihh]) and Gli transcription factors (Gli2, GLi3). The protein ezrin, which is required for polarization of the epithelium, and the transcription factor Elf3 interact with Crif1 to regulate epithelial differentiation and villus formation. The transcription factor HNF4α is expressed throughout the intestinal epithelium and, if deleted, causes the epithelium to develop into a colonic phenotype. Finally, beyond genes and transcription factors, global chromatin remodeling also has effects on intestinal epithelial development.
The formation of villi occurs as epithelial cells proliferate and reorganize from a pseudostratified appearance to a simple columnar epithelium. As villi form, distinct epithelial cell types can be identified by morphology and the expression of specific markers. Unlike other aspects of intestinal development, proliferation and differentiation of the epithelium remain important processes that must be maintained throughout adult life. Two major signaling pathways involved in these processes are Wnt/β-catenin and Notch. Wnt/β-catenin is important in crypt formation, for maintaining the stem cell compartment, proliferation and differentiation in the embryonic and adult intestine, and for Paneth cell maturation. Notch proteins are transmembrane receptors that are important in both proliferation and differentiation of the developing intestine. Evidence suggests that Notch activity regulates factors that influence whether undifferentiated cells will become absorptive or secretory epithelial cells. There also are factors downstream of Wnt/β-catenin and Notch that effect specific lineages: neurogenin is required for the formation of enteroendocrine cells; SPDEF directs terminal differentiation of goblet cells; Sox9 regulates Paneth and goblet cell formation; and Klf4 regulates colonic goblet cell differentiation.
The duodenum originates from the terminal portion of the foregut and cephalic part of the midgut. Early during week 4 of gestation, the caudal foregut begins to expand to initiate formation of the stomach. The liver and pancreas arise at the junction of the midgut and foregut. With rotation of the stomach, the duodenum becomes C-shaped and rotates to the right; the fourth portion becomes fixed in the left upper abdominal cavity. The mesoduodenum fuses with the adjacent peritoneum; both layers disappear, and the duodenum becomes fixed in its retroperitoneal location. The lumen of the duodenum is obliterated during the second month of development by proliferation of its cells; this phenomenon is shortly followed by recanalization. Small intestinal villus and crypt formation occurs in a proximal-to-distal progression. The villi appear during week 8 of gestation, along with the microvillus enzymes. At 12 weeks’ gestation, crypts are present and grow between the 10th and 14th week of gestation. At 14 weeks, the intestinal enzymes are at an adult level of activity.
Because the foregut is supplied by the celiac artery and the midgut by the SMA, the duodenum is supplied by both arteries and therefore is relatively protected from ischemic injury.
In a 5-week embryo, the midgut is suspended from the dorsal abdominal wall by a short mesentery and communicates with the yolk sac by way of the vitelline duct. The midgut gives rise to the duodenum distal to the ampulla, the entire small intestine, and the cecum, appendix, ascending colon, and proximal two thirds of the transverse colon. The midgut rapidly elongates with formation of the primary intestinal loop. Rapid growth of the midgut causes it to elongate, rotate, and to begin to form a loop that protrudes into the umbilical cord. The cephalic portion of this loop, which communicates with the yolk sac by the narrow vitelline duct, gives rise to the distal portion of the duodenum, jejunum, and a portion of the ileum; the distal ileum, cecum, appendix, ascending colon, and proximal two thirds of the transverse colon originate from the caudal limb. During week 6 of embryonic development, the primary intestinal loop enters the umbilical cord (physiologic umbilical herniation) ( Fig. 98.13 ). At 7 weeks’ gestation, the small intestine begins to rotate counterclockwise around the axis of the SMA. At 9 weeks, growth of the intestine causes it to herniate further into the umbilical cord, where it continues to rotate 90 degrees before it returns to the abdominal cavity. At 11 weeks’ gestation, the intestine retracts into the abdominal cavity and continues its counterclockwise rotation another 180 degrees to a total of 270 degrees. The jejunum returns first and fills the left half of the abdominal cavity ultimately taking its position in the LUQ. The ileum returns next and fills the right half of the abdominal cavity ultimately assuming its final position in the RLQ. The colon enters last, with fixation of the cecum close to the iliac crest and the ascending and descending colon attaching to the posterior abdominal wall. Elongation of the bowel continues, and the jejunum and ileum form a number of coiled loops within the peritoneal cavity.
The cecum originates as a small dilatation or bud of the caudal limb of the primary intestinal loop by approximately 6 weeks of development. Initially, after returning to the abdominal cavity, it lies in the RUQ, then it descends to the right iliac fossa, placing the ascending colon and hepatic flexure in the right side of the abdominal cavity. The appendix originates from the distal end of the cecal bud. Because the appendix develops during descent of the colon, its final position is frequently retrocecal or retrocolonic ( Fig. 98.14 ).
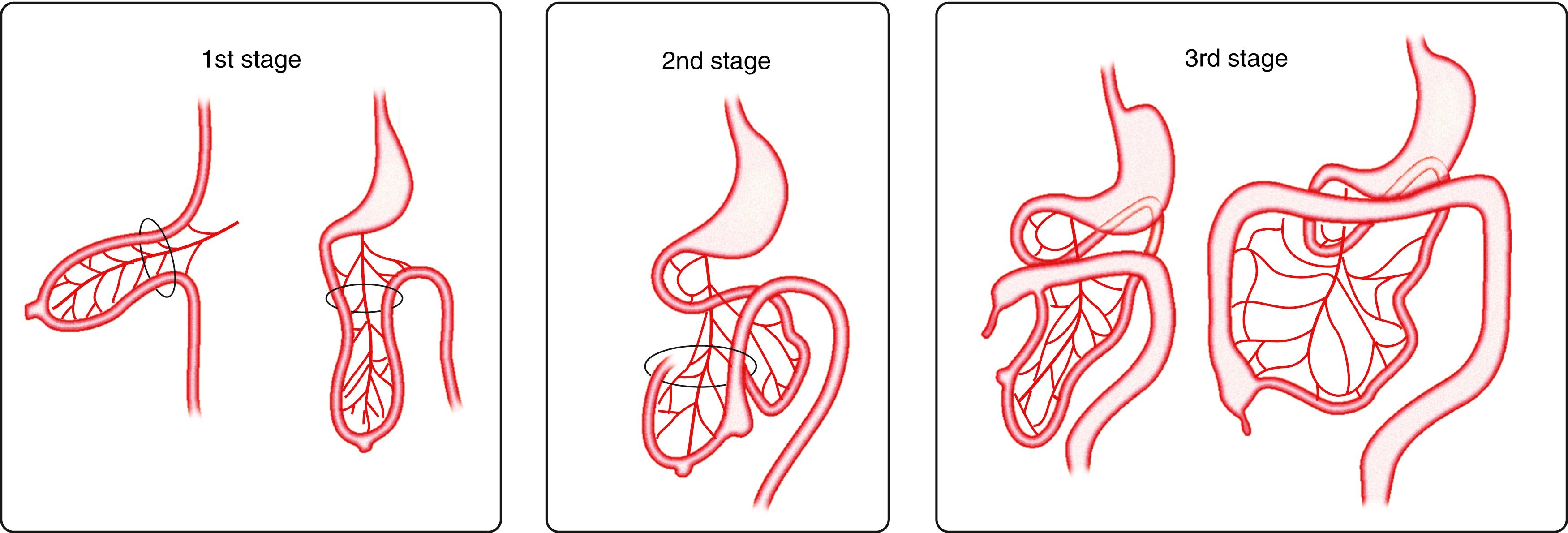
As the caudal limb of the primitive intestine moves to the right side of the abdominal cavity, the dorsal mesentery twists around the origin of the SMA. After the ascending and descending portions of the colon reach their final destinations, their mesenteries fuse with the peritoneum of the posterior abdominal wall, and they become retroperitoneal organs. The appendix, cecum, and descending colon retain their free mesentery. The transverse mesocolon fuses with the posterior wall of the greater omentum. The mesentery of the jejunum and ileum is at first in continuity with the ascending mesocolon; after the ascending colon becomes retroperitoneal, the mesentery only extends from the duodenum to the IC junction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here