Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Optimal radiologic investigation of the genitourinary system requires a combination of diverse but complementary examinations that evaluate form and function. This chapter presents an overview of diagnostic tests that are commonly used to evaluate genitourinary disease. First, the pharmacology of iodinated contrast media is reviewed. Adverse effects and an approach to the management of common adverse reactions are also discussed. The chapter then turns to the individual radiologic methodologies and examines the general indications for the tests and guidelines for interpretation. The following sections review the cross-sectional imaging methods—ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI)—as well as angiography and nuclear medicine. Recommended methods for performing these examinations are presented in a series of appendices.
Radiographic contrast media (RCM) were developed to increase differences in the attenuation (absorption) of radiation by soft tissues. As a result, all commercially available radiographic contrast agents are triiodinated derivatives of benzoic acid. The other chemical constituents of the contrast molecule carry the iodine so that it can be delivered in large volumes, in high concentrations, and with as little toxicity as possible. Some older contrast materials are ionic, which means that these agents dissociate into cations and anions in water. The osmolality of a solution is a measure of the number of dissolved particles in each liter of solution. Some of the adverse effects of RCM are related to hyperosmolality, which may be up to six times that of plasma. The density of RCM is related to the number of iodine atoms per milliliter of solution and directly correlates with x-ray attenuation. RCM can be subdivided into three classes, which are based on a ratio between the number of iodine atoms in the molecule and the number of osmotically active particles produced by that molecule in a solution ( Table 1-1 and Fig. 1-1 ). At present, ratio 1.5 (high-osmolar contrast media, HOCM), ratio 3 (low-osmolar contrast media, LOCM), and ratio 6 (iso-isomolar, nonionic dimer, IOCM) agents are in active use.
| Ionicity | Monomer or Dimer | Ratio * | Relative Osmolality † , ‡ | Examples |
|---|---|---|---|---|
| Ionic | Monomer | 1.5 | ~5 | Diatrizoate |
| Iodamide | ||||
| Ioglicate | ||||
| Iothalamate | ||||
| Ioxithalamate | ||||
| Metrizoate | ||||
| Ionic | Dimer | 3 | ~2 | Ioxaglate |
| Nonionic | 3 | 1.5-1.8 | Iohexol | |
| Iopamidol | ||||
| Iopentol | ||||
| Iopromide | ||||
| Ioversol | ||||
| Ioxilan | ||||
| Nonionic | Dimer | 6 | 1 | Iodixonal |
| Iotrol |
* Ratio between the number of iodine atoms per molecule and the number of osmotically active particles produced by that molecule in solution.
† Relative osmolality expressed as a multiple of serum osmolality, 278-305 mOsm/kg serum water.
‡ Data from product package inserts, product brochures, or technical information services.

All ionic contrast media are salts of iodinated, organic molecules that dissociate completely in blood. Thus these molecules consist of a positively charged cation and a negatively charged anion. The diagnostically useful contrast molecule itself is the organic anion, which consists of an iodine-substituted benzene ring, with sodium or meglumine serving as the cation. The cation provides no radiographically significant information but contributes half of the osmotic effect of the medium. Diatrizoate and its derivatives are ionic monomeric salts of triiodinated, fully substituted benzoic acids. As ratio 1.5 agents, ionic monomeric salts are also referred to as HOCM because, for every three iodine atoms in solution, there are two osmotically active particles in the solution. The ionic dimeric salts are ratio 3 contrast agents, consisting of an anionic moiety with two triiodinated benzene rings. Ioxaglate is an example of a monoacidic dimeric salt that is intended for intravascular use but not for myelography.
The nonionic compounds were developed to reduce the osmolality of contrast agents while preserving excellent image contrast. The addition of a nonionizing glucose moiety to the carboxyl group transforms the iodinated benzoic acid derivative into a nonionic compound. The nonionic monomer class of RCM consists of ratio 3 agents because, for every three iodine atoms, there is only one particle in solution. Iopamidol and iohexol are two examples of second-generation nonionic monomers. These agents, along with the ionic dimer class of contrast medium, are referred to as LOCM because the osmolality of these compounds is about two times that of serum osmolality. The third class of RCM is made up of nonionic dimers that do not dissociate in solution. Iotrol and iodixanol provide six iodine atoms for every osmotically active molecule. These ratio 6 agents have the lowest osmolalities but also the greatest viscosity of all RCM because of the large molecular size.
All RCM are hydrophilic and have low lipid solubility. Having little affinity for proteins and membrane-bound receptors, RCM also are nearly inert having minimal pharmacologic action. After intravenous administration, the decline in the plasma concentration of contrast material results from diffusion into the extravascular space, vascular mixing, and renal excretion. The kidneys normally excrete more than 99% of the intravenous dose of contrast media. Less than 1% is excreted through nonrenal routes, including the hepatobiliary system, sweat, tears, and saliva. All of the currently available contrast media are excreted through the kidney by glomerular filtration with no significant tubular excretion or resorption. HOCM causes significant osmotic diuresis, which secondarily decreases the tubular concentration of contrast medium. By comparison, LOCM and IOCM cause less osmotic diuresis, and as a result, the concentration of ratio 3 and 6 contrast media in the urine is significantly higher.
Contrast-associated nephropathy (CN) is defined as an acute impairment of renal function after exposure to a RCM. Although there are various specific definitions, a common definition of renal impairment is a rise in the serum creatinine level of at least 1.0 mg/dL within 2 to 5 days of exposure to RCM. Creatinine levels usually return to normal by 7 to 12 days. Contrast nephropathy is typically reversible, but rare cases of permanent nephrotoxicity, which is more common when renal failure is oliguric, have necessitated dialysis or transplantation. A delayed, persistent nephrogram at 24 hours has been observed in the majority of patients with CN, but this finding is not specific for CN. Various mechanisms have been suggested to explain the adverse effect of contrast media on renal function. Some putative mechanisms include prerenal effects from dehydration or hypotension; direct effects on intrarenal hemodynamics; direct nephrotoxic effect on tubular cells; intratubular obstruction from proteinuria and uricosuria; and indirect nephrotoxic effects from an altered immunologic response.
The most important risk factor for the development of CN is pre-existing renal insufficiency, practically defined as a serum creatinine level greater than 1.5 mg/dL or estimated glomerular filtration rate (GFR) less than 45. Unsuspected azotemia caused by hypertensive nephropathy or vascular disease is especially prevalent in the elderly patient group. Pre-exposure dehydration, whether inadvertent or intentional, may exacerbate nephrotoxicity, particularly in patients with azotemia. Patients with insulin-dependent diabetes mellitus and secondary renal disease are at a particularly high risk for development of CN; the frequency of CN is 50% to 100% when serum creatinine is more than 3.5 mg/dL in these patients. However, patients with diabetes mellitus or multiple myeloma and normal renal function do not appear to be at an increased risk. Repeated administration of contrast material over a short period (within 24 hours) increases the risk of developing CN. In general, a total iodine dose of 80 g in a 24-hour period is safe. With a total iodine dose of more than 100 g in a 24-hour period, the patient is at increased risk of renal failure.
Compared with ionic counterparts, nonionic monomers cause fewer changes in the GFR and less tubular damage. However, some studies of patients with normal or slightly decreased renal function report no statistical difference in the prevalence of contrast-induced nephrotoxicity between patients receiving ionic compounds and those receiving nonionic compounds. Other studies suggest that patients who have pre-existing renal insufficiency, defined as serum creatinine levels between 1.4 and 2.4 mg/dL, may be at higher risk for nephrotoxicity with HOCM than with LOCM. In 1993, Barrett and Carlisle concluded from a meta-analysis of 24 trials that the use of LOCM may be beneficial in patients with pre-existing renal failure because the mean postexposure change in the serum creatinine level was 0.2 to 6.2 µmol/L less with LOCM than with HOCM.
The prevention of CN first involves determining whether the requested examination is appropriate for the given clinical question. Directing the work up away from an examination requiring contrast material administration is appropriate when the potential risks of adverse reaction might be serious or life threatening. Careful screening of patients for well-defined high-risk factors, known renal disease, advanced age, treatment with nephrotoxic drugs, renal insufficiency, and diabetes mellitus is mandatory. If any of these high-risk factors is present, an assessment of renal function is prudent. In patients with none of these risk factors, the likelihood of suffering permanent renal damage from CN is so remote that routine measurement of renal function is unnecessary. Preparation protocols that involve intentional dehydration or catharsis should be avoided. If multiple examinations requiring contrast material are indicated, they should be performed over an extended period—for example, longer than 72 hours.
As with other drugs, RCMs are associated with untoward reactions attributable to their physicochemical structure, direct toxic effects on sensitive organs, and allergylike reactions (anaphylactoid, idiosyncratic, or pseudoallergic). These adverse side effects occur after administration of 5% to 8% of all intravenous injections with ionic HOCM and in 1% to 3% of injections with nonionic or LOCM. Fortunately, most of these adverse reactions are minor in severity; they include sensations of body warmth, pruritus, urticaria, nausea, and vomiting.
A practical way to classify untoward reactions to RCM is to group them by nature and clinical severity.
Mild contrast reactions include pruritus, hives, nausea, warmth, altered gustatory sensations, swelling of the face, conjunctival injection, and vomiting. In the majority of patients, no treatment beyond reassurance is necessary. However, like all untoward effects, these mild reactions require close observation because they rarely do progress or are prodromal to more serious reactions.
About 1% to 2% of patients receiving conventional HOCM have a non-life-threatening, moderate reaction. Examples of these types of reactions include bradycardia or tachycardia (especially when associated with acute changes in blood pressure), dyspnea, laryngospasm, and bronchospasm. Patients with moderate reactions require close monitoring and often require treatment.
Any reaction may be classified as severe when it is potentially life threatening. Often, the patient loses consciousness or has clinically significant dysrhythmia. Patients with severe reactions not only must be treated promptly, but almost always require hospitalization for optimal treatment. Severe, life-threatening reactions occur after 0.05% to 0.10% of injections with HOCM. Reported fatalities attributable to reactions caused by contrast media are estimated to occur in one of every 75,000 administrations.
Most adverse effects are evident immediately after injection, and all life-threatening reactions occur within 15 minutes after injection. Rarely, delayed reactions can occur 24 to 48 hours after exposure. However, these delayed reactions are almost exclusively mild in character and include rash or pruritus and pain near the injection site. There is an increased risk of a delayed reaction to RCM injection in patients who have received interleukin-2 therapy. Iodine mumps refers to delayed parotid swelling caused by trace levels of free iodide in contrast media. Specific management of the more commonly encountered mild and moderate adverse reactions is outlined in Tables 1-2 and 1-3 .
| Adverse Reaction | First Line | Second Line | Third Line |
|---|---|---|---|
| Urticaria (hives) | Reassurance | Diphenhydramine | Epinephrine intramuscularly or subcutaneously |
| Vagal reaction | Elevate legs; consider volume expansion * | Atropine sulfate | — |
| Laryngeal edema | Oxygen | Epinephrine intramuscularly or subcutaneously | Intubation |
| Bronchospasm | Oxygen | Inhaled beta 2 -agonist † | Epinephrine intramuscularly or subcutaneously ‡ |
| Hypotension and tachycardia | Elevate legs; consider volume expansion * | Epinephrine intravenously | — |
* Volume expansion with 0.9% saline or lactated Ringer solution.
† Inhaled beta 2 -agonists, such as metaproterenol, albuterol, or nebulized terbutaline.
‡ Alternatives to subcutaneous epinephrine in the management of bronchospasm include aminophylline drip or terbutaline (subcutaneous or intramuscular).
| Drug | Trade Name | Dose | Route of Administration |
|---|---|---|---|
| Albuterol | Proventil, Ventolin | — | Inhaled |
| Aminophylline drip | — | 6 mg/kg loading dose; 0.5-1.0 mg/kg/h intravenous drip | Intravenous |
| Atropine sulfate | — | 1-mg doses to a total of 2 mg | Intravenous |
| Diphenhydramine | Benadryl | 25-50 mg | Oral/intramuscular/intravenous |
| Epinephrine | — | 1 : 10,000 dilution; 3-mL doses to a total of 10 mL | Intravenous |
| Epinephrine | — | 1 : 1000 dilution; 0.3-mL dose to a total of 1 mL | Intramuscular or subcutaneous |
| Metaproterenol | Alupent, Metaprel | — | Inhaled |
| Terbutaline | — | 0.25-0.5 mg | Subcutaneous/intramuscular |
The frequency and severity of reactions to contrast material may be influenced by the type, dose, route, and rate of delivery. Experimental and clinical data suggest that LOCM produces fewer chemotoxic adverse side effects compared with HOCM. The prevalence of anaphylactoid reactions may also be lower. Multicenter surveillance studies have estimated that the relative risk of any adverse reaction is reduced by a factor of 3 to 8, and the risk of severe reaction is reduced by a factor of 4 to 12 when LOCM are used. The prevalence of most reactions is greater with the intravenous route than with the intra-arterial route. Exposure of mast cell–rich pulmonary capillary beds to relatively higher concentrations of contrast may explain this observation. However, the prevalence of severe reactions is greater after intra-arterial injections. Bolus intravenous injection produces fewer reactions compared with drip infusion.
If the use of nonionic or other LOCM is selective, the prevention of adverse reactions to HOCM depends on identifying those patients at higher risk for these reactions. In these patients, the selective use of LOCM or medical pretreatment, or both is logical and advised. For patients with a history of allergy or asthma, the relative increased risk of any adverse reaction is about two times that for the general population. In any patient who is debilitated or has a history of severe cardiopulmonary disease, the effects of even a moderate reaction may be poorly tolerated. For patients who have a history that includes an adverse reaction after RCM exposure, the re-reaction prevalence is 17% to 35%, or three to eight times the risk for the general population.
Several studies have concluded that medical pretreatment can reduce the prevalence of adverse side effects in high-risk patients to that observed in the general population. Most of these premedication regimens include a corticosteroid administered alone or together with either an H1- or an H2-antihistamine. Steroids exert a salutary effect through stabilization of membranes and therefore may impede release of critical mediators of anaphylactoid reactions. In a group of patients with a history of adverse reaction to RCM, Lasser and associates concluded that pretreatment with an oral regimen of methyl-prednisolone 32 mg, taken 12 and 2 hours before the intravenous administration of HOCM, decreased the occurrence of all classes of reactions. Other studies delivering a three-dose oral regimen of prednisone 50 mg, taken every 6 hours beginning at least 13 hours before exposure to HOCM, and diphenhydramine 50 mg, administered orally or intramuscularly 1 hour before exposure to HOCM, have also demonstrated reduced reaction prevalence ( Box 1-1 ).
Prednisone 50 mg per os or intravenously; 13 hours, 7 hours, and 1 hour before contrast material injection.
Diphenhydramine 50 mg per os or intravenously; 30-60 minutes before contrast material injection.
CT urography (CTU) is the most accurate and comprehensive urinary tract imaging evaluation. It allows detailed images to be obtained of the vasculature, renal parenchyma, and the urothelium along the entire length of the urinary tract. The advent of multidetector CT made CTU possible. There is no significant motion artifact, studies can be completed during a single breath hold, and low dose multiphase imaging can be obtained to cover the entire urinary tract. Indications for CTU are broad, but the main indication is usually hematuria. Other common indications include screening for urinary tract neoplasms in patients with a history of cancer, or for surveillance following cancer treatment, evaluation of congenital urinary tract anomalies, evaluation of ureteral obstruction, evaluation before or after urinary tract surgery, and for patients with suspected urinary tract injuries. CTU allows optimal stone detection, excellent renal mass detection and characterization, diagnosis and staging examination for renal tumors, vascular anatomy assessment, and evaluation of the urothelium with a single examination.
There are three essential phases in a CT urogram. These three phases are a noncontrast CT, a nephrogram phase CT, and an excretory phase CT ( Fig. 1-2 ). Each of these phases is important for a comprehensive study of the urinary tract. Noncontrast CT is used to detect stones, calcifications in renal masses, to get baseline measurements to determine mass enhancement, and for detection of small amounts of fat in a renal mass. The CT nephrogram phase is essential for the detection of renal and urothelial masses ( Fig. 1-3 ), mass characterization including enhancement measurement, and determination of the Bosniak classification for cystic renal masses. The excretory phase study is important to detect abnormalities of papillary necrosis ( Fig. 1-4 ), and for filling defects ( Fig. 1-5 ) and wall thickening in the urothelium. In order to obtain these three essential elements of a CTU, there are various approaches, but two main approaches have evolved and are used for most CTUs. A three scan CTU encompasses three separate scans through the entire urinary tract ( Appendix A ): one scan during the noncontrast phase, one scan during the nephrogram phase, and the final scan during the excretory phase. The two scan technique, also known as a split bolus CTU, includes a precontrast CT of the urinary tract followed by a second scan which combines both the nephrogram phase and the excretory phase. For both protocols all scans should be obtained using 2.5 mm (or thinner) collimation. Multiplanar reconstructions should be obtained using the thinnest reconstructions available for high-resolution reformations.




For the three scan CTU, a precontrast CT of the abdomen and pelvis from just above the kidneys to just below the bladder base is performed. This is followed by nephrogram phase scans. The nephrogram phase scan is obtained following power injection of at least 125 mL of contrast material at a rate of 4 mL/second. The scan delay is optimally between 85 and 120 seconds after the beginning of contrast injection. Excretory phase CT scans should be obtained 10 to 15 minutes following the initiation of intravenous contrast material injection. Furosemide (Lasix) has been shown to substantially improve urinary tract distention during this phase and therefore 10 mg of furosemide may be injected intravenously 3 to 5 minutes before the excretory phase scans. Low-dose CT can be utilized during the noncontrast phase and excretory phase scans to reduce radiation dose for this CTU protocol. Abnormalities detected during these phases tend to be high contrast abnormalities that can be readily detected even with low-dose CT technique.
The technique for split bolus CTU is significantly different. Initially a noncontrast CT of the entire urinary tract is obtained using thin collimation as described above. After the scan 10 mg of furosemide is injected intravenously. Immediately following this injection 50 mL of intravenous contrast material is injected. Following a 6-minute delay, a second bolus of 100 mL of contrast material is injected at a rate of 4 mL/second. The entire urinary tract is scanned using a 100 second delay. The second scan in this protocol will yield combined nephrogram and excretory phase images.
There are significant benefits of utilizing the three scan CTU protocol. It is very important to detect lesions that show urothelial enhancement. These may be impossible to detect using the split bolus technique. The detection of urothelial tumors is greatly improved when enhancement within the lesions can be visualized. This may be obscured on the combined excretory and nephrogram phase split bolus scans. For the detection of urothelial carcinomas, a challenge for radiologists, it has been shown that there is a higher sensitivity using the nephrogram phase than the excretory phase. A radiologist is more likely to miss flat bladder ( Fig. 1-6 ), and pelvocalyceal cancers utilizing the split bolus technique than with the three scan CTU protocol. The authors of this chapter recommend utilizing the three scan protocol for most patients, and limiting use of the split bolus technique to those patients who have very low risk of urothelial carcinoma, predominately for very young patients, such as kidney transplant donor candidates.

The precontrast CT images should be evaluated for the presence of urinary tract calculi, fat containing renal masses, and to determine baseline measurements of any masses detected on later scans. Nephrogram phase scans should be evaluated in great detail to detect renal parenchymal tumors and enhancing urothelial lesions. A substantial number of renal masses will go undetected during earlier corticomedullary phase scans. The nephrogram phase, 85 to 120 seconds following the initiation of contrast material injection, is the optimal phase for detecting renal tumors. It is also crucial to carefully evaluate the entire length of the urothelium, including the ureters and bladder, during the nephrogram phase. Urothelial carcinomas are best, and sometimes only, detected during this phase of the CTU. Urothelial carcinomas almost always enhance very avidly and therefore are usually readily detectable during this phase. Most urothelial carcinomas will appear as focal areas of enhancement during this phase. Other causes of increased enhancement include inflammation and infection. In most of these benign situations the enhancement covers a long segment of the urothelium without enhancing focal masses. Excretory phase images should be evaluated carefully for intraluminal filling defects and for changes of papillary necrosis. Images in this phase of scanning should be evaluated with various window and level settings. Standard soft tissue setting should be used to evaluate for wall thickening, and bone window and level settings can be utilized to detect intraluminal filling defects that may be obscured by the excreted contrast material.
CTU is a very sensitive test for the detection of urothelial carcinomas, but it is not highly specific for smaller masses. Approximately 80% of urothelial masses larger than 5 mm in diameter are malignant. This percentage is increased to 92% if urine cytology is suspicious or positive for urothelial carcinoma. Approximately 50% of cases of urothelial thickening represent carcinoma. This percentage is increased to 90% if there is positive or suspicious urine cytology in the patient. Alternatively, masses smaller than 5 mm are not usually malignant. These abnormalities typically represent inflammatory lesions or imaging artifacts. When only small masses are detected, imaging follow up or ureteroscopy is advisable before initiation of treatment. As urothelial carcinomas are often multifocal, once a mass is detected, careful scrutiny of the remainder of the urothelium is advised. While cystoscopy is often performed in patients with hematuria, careful evaluation of the bladder during the nephrogram phase is recommended. The detection of bladder carcinomas with CTU is nearly as accurate as cystoscopy.
In summary, CTU is the best imaging test for hematuria evaluation and as a comprehensive evaluation of the urinary tract. Key components of performance and interpretation for CTUs have been described. The three scan CTU is preferable in most instances since there is improved sensitivity for detection of urothelial carcinomas. A nephrogram phase scan at approximately 100 seconds following the injection of contrast material should be utilized to optimize detection of renal parenchymal tumors and urothelial masses. Thoroughly evaluate the entirety of the urinary track during the nephrogram phase to detect small enhancing masses. Cancers will be best, or perhaps only, detected during the nephrogram phase. Furosemide should be used whenever possible to improve ureteral distention during the excretory phase scans. Excretory phase scans should be viewed with various window and level settings to detect wall thickening, changes of papillary necrosis, and inflammatory filling defects.
Intravenous urography (IVU), once the test of choice as a screening examination of the upper and lower urinary tracts, is rarely used today, having been superseded by CTU, MRI, and ultrasound examinations. This test is primarily used to investigate a suspected or known congenital anomaly of the urinary tract, or a limited IVU for suspected ureteral obstruction during pregnancy when other tests are unavailable or inconclusive.
Because the appearance of contrast medium in the renal tubules depends on glomerular filtration, renal visualization may be suboptimal in patients with moderate and severe renal failure. In general, urography is unlikely to be useful in patients with serum creatinine levels above 3.5 to 4.0 mg/dL. In addition, the risk of contrast nephropathy is increased with serum creatinine levels above 1.5 mg/dL.
A careful evaluation of the preliminary or scout film is mandatory. This preliminary film is not only important for the subsequent interpretation of the IVU, but it may provide important ancillary information about the axial skeleton, abnormal calcifications, visceral enlargement, soft tissue masses, and bowel gas pattern (i.e., “bones, stones, mass, gas”). Renal shadows and the pubic symphysis must be included on the preliminary film.
At 60 to 90 seconds after the bolus administration of contrast medium, a cortical nephrogram can be seen. The nephrogram represents contrast material within the tubules and depends on the plasma concentration of contrast and the GFR. The peak nephrogram density after bolus administration of contrast occurs earlier and is somewhat greater, but it decreases more rapidly than when contrast medium is given by intravenous drip infusion. The lower limit of normal renal length can be approximated by the distance between the superior endplate of L1 and the inferior endplate of L3. Renal length should not exceed the span of the first four lumbar vertebrae. Peak opacification of the intrarenal collecting system and renal pelvis occurs approximately 5 minutes after contrast material administration ( Fig. 1-7 ). Ureteral filling with contrast begins at about this time, and peak opacification occurs 5 to 10 minutes after intravenous contrast material is given. As contrast medium slowly appears in the bladder, it preferentially collects against the dependent posterior wall in the patient positioned supine. In the patient positioned prone, contrast is seen along the anterior bladder wall, which is in a relatively more cephalad position than the posterior wall. The mucosal pattern of the bladder is best assessed on the film after voiding because dense contrast in the filled bladder may obscure a lesion ( Fig. 1-8 ). Furthermore, a radiograph obtained after voiding that shows complete emptying suggests normal bladder function. The converse is not true, however, because a moderate amount of residual urine may be explained by causes other than dysfunctional micturition.


Retrograde cystography is the radiologic evaluation of the bladder after instillation of contrast material by catheter, either transurethral or suprapubic, or by needle puncture. Voiding cystourethrography (VCUG) is contrast radiography of the urinary bladder and urethra during spontaneous micturition ( Appendix B ). Dynamic retrograde urethrography is radiography of the urethra while it is being distended by instillation of contrast through a catheter ( Appendix C ).
The main indications for cystography are the evaluation of acquired disorders of micturition, vesicoureteral reflux, and traumatic injury of the bladder. Injury to the bladder should be suspected in a patient with difficulty voiding, pelvic fracture, gross hematuria after trauma, or iatrogenic injury during surgery or instrumentation. Radiologic evaluation of the bladder frequently is performed before renal transplantation and in patients with spinal cord injury. Cystography has been used to distinguish a mechanical obstructive cause of micturition dysfunction from a neurogenic cause. VCUG in children is used to determine whether vesicoureteral reflux or a congenital anomaly of the urinary tract is responsible for urinary tract infection or collecting-system dilatation. In adults, reflux should be suspected in the patient with an upper urinary tract infection when no other cause is plausible or when reflux nephropathy is noted on urograms. In women, cystourethrography frequently is used to evaluate stress incontinence or suspected urethral diverticulum. In men, benign prostatic hyperplasia and urethral stricture are common reasons why cystourethrography is performed.
In boys or men, the main indication for dynamic retrograde urethrography is suspected injury or stricture of the anterior urethra. If no trauma to the urethra is documented by urethrography, the catheter can be advanced safely into the bladder. As necessary, cystography may follow. Urethrography with a double-balloon catheter is performed in women primarily when a urethral diverticulum is suspected but cannot be confirmed by VCUG or MRI.
The wall of the distended bladder is smooth and thin. In men, the height (vertical dimension) of the bladder may be greater than the width (horizontal dimension); the opposite is often true in women. The base of the bladder is normally at or just below the level of the superior pubic ramus. The base is slightly convex in the supine position, but it is funnel-shaped when the patient voids in an upright position.
The male urethra consists of the anterior and posterior segments ( Fig. 1-9 ). The posterior urethra is divided into the prostatic and membranous parts and extends from the internal sphincter (at the bladder neck) to the external sphincter (at the urogenital diaphragm). The prostatic part is normally wide and passes through the transitional zone of the prostate gland. The verumontanum (urethral crest) is an elongated, oval filling defect on the posterior wall of the prostatic urethra; the prostatic urethra ends at the distal end of the verumontanum. The external sphincter is distal to the verumontanum and creates a narrowing on the retrograde urethrogram; this is the membranous part of the posterior urethra. The anterior urethra is divided into the bulbar and penile parts. The bulbous part of the anterior urethra extends from the external sphincter to the penoscrotal junction, where the penile urethra is angled by the suspensory ligament of the penis. Cowper glands are embedded in the muscle of the urogenital diaphragm, and its ducts enter the floor of the bulbar urethra. The penile or pendulous urethra is the most distal part of the anterior urethra and ends at the external meatus. Just proximal to the external meatus, there may be a slight widening of the penile urethra, the fossa navicularis.

The female urethra is approximately 4 cm in length and extends from the internal urethral orifice (at the bladder neck) to the external orifice (anterior in the vagina). The urethra is widest at the bladder neck and tapers distally; it has an oblique anterior course ( Fig. 1-10 ).

Further discussion of the anatomy of the male urethra and other examples of normal radiographic examinations of the bladder and urethra can be found in Chapter 6 .
Retrograde pyelography (RP) is radiography of the ureter and renal collecting system after direct injection of contrast material into the ureter. The technique usually requires cystoscopy. A 4- to 7-F ureteral catheter with a round, bulb, or spiral tip is used to cannulate and obstruct the ureteral orifice. Many of the current catheters have open ends that allow passage of a guidewire for further endourologic manipulation. Contrast medium is instilled slowly by syringe or by gravity flow. Alternatively, a flexible catheter can be passed over a guidewire into the upper ureter or renal pelvis, and contrast is instilled through the catheter.
RP is not an adequate screening examination of the urinary tract because the renal parenchyma is not directly opacified. For this reason, and because it is an invasive procedure, RP is usually performed after preliminary urography, sonography, MRI, or CT, which may suggest the presence of a filling defect, mass, or obstruction of the collecting system. Indications for RP include evaluation of the ureter when not adequately visualized by other methods, evaluation of a filling defect in the renal pelvis or ureter, and evaluation of the collecting system in patients with hematuria when no clear cause is found after other imaging investigation. RP is also performed as a preliminary examination before selective collection of urine from each kidney or before ureteral brushing and biopsy in suspected urothelial malignancy.
For a study result to be considered normal, the entirety of the ureters and collecting system must be demonstrated. If present, the entire length of a ureteral filling defect should be shown. The calyces are blunted frequently on retrograde pyelograms because contrast material is injected under positive pressure. Backflow occurs when there is rupture at the calyceal fornices or retrograde opacification of the collecting ducts (pyelotubular backflow). With pyelosinus back-flow, there is opacification of renal sinus tissues around the calyces, renal pelvis, or proximal ureter after forniceal rupture ( Fig. 1-11 ). The arcuate veins, distal collecting tubules, and hilar lymphatic vessels are opacified with pyelovenous, pyelotubular, and pyelolymphatic backflow, respectively.

The most commonly reported complication of RP is perforation of the ureter or renal pelvis. Spasm or edema of the ureter may cause transient obstruction. Contrast medium can be absorbed during RP, and moderate or severe reactions may occur, although they are rare. Sepsis may complicate an injection of contrast material proximal to a site of pelvoureteral obstruction.
Dilatation of the collecting system does not always result from mechanical obstruction. Residual ectasia from previous obstruction, vesicoureteral reflux, congenital malformation, or high urine-flow states are common disorders that can cause dilatation of the collecting system in the absence of obstruction. Evaluation of a dilated collecting system with a renal scan or by measuring contrast material excretion rates after antegrade or RP may not be optimal in patients with poor renal function, a markedly dilated or tortuous ureter, or a urinary bladder diversion. For patients in whom it is unclear whether collecting system dilatation is associated with a pressure gradient between the upper collecting system and bladder, urodynamic antegrade pyelography (ureteral perfusion test or Whitaker pressure/flow examination) is indicated. This study quantifies resistance within the collecting system by measuring the pressure gradient between the kidney and bladder at known rates of flow.
Urodynamic antegrade pyelography is an invasive examination, requiring antegrade pyelography. VCUG is performed first to exclude vesicoureteral reflux. The vesicle catheter is left in place, and the patient is turned to a prone position on the fluoroscopy table. Standard needle puncture of the kidney is performed with a 20- to 22-gauge needle. The antegrade needle and the vesicle catheter are attached to separate manometers, which are positioned at the level of the urinary bladder. The opening or resting pressure of the kidney is recorded. An antegrade pyelogram is performed. Next, dilute iodinated contrast medium is instilled through the antegrade pyelography needle, and an infusion pump is used to regulate the flow. Perfusion of the collecting system at a rate of 10 mL/minute is continued until the entire pyeloureteral system is opacified, usually after approximately 5 minutes. At this time, the infusion pump is stopped, and the pressure within the collecting system and urinary bladder is measured and recorded ( Fig. 1-12 ).

With chronic hydronephrosis, elevated intrarenal pressure gradually decreases as renal function decreases and as the compliance of the dilated collecting system increases. The opening pressure of the renal collecting system may be normal or only slightly elevated, particularly in the setting of chronic obstruction. For this reason, the opening intrarenal pressure is significant only when it is substantially elevated (i.e., >50 cm H 2 O). A more meaningful assessment of pyeloureteral resistance is the differential pressure (DP) between the bladder and the kidney. As the bladder fills, intravesical and renal pelvis pressures increase, but the DP decreases. At a perfusion rate of 10 mL/minute, the normal DP is less than 13 cm H 2 O. Mild, moderate, or severe mechanical obstruction is suggested by a DP of 14 to 20, 21 to 34, or greater than 34 cm H 2 O, respectively. When the DP is in the high normal or low abnormal range, the infusion rate can be increased from 10 mL/minute to 15 mL/minute or even 20 mL/minute. At these higher rates of flow, the normal range of values for the DP increases. At an infusion rate of 15 mL/minute in a patient with an empty bladder, the normal upper-limit value for the DP is 18 cm H 2 O, and at 20 mL/minute it is 21 cm H 2 O.
IVU, CTU, radionuclide studies, and sonography are used commonly to evaluate patients with a cutaneous ureteroileostomy. The urogram can be used to evaluate the collecting system and ileal loop, and in the majority of patients it provides the necessary anatomic and functional information. Radionuclide imaging can be used to quantify upper urinary tract function and provides some anatomic information. Sonography is used to evaluate the size of the intrarenal collecting system, particularly in patients with poor renal function or contraindications to urography, but it is not well suited to evaluate the ureters. For rapid, complete opacification of the conduit, ureters, and pelvocalyceal system; or if poor renal function precludes optimal anatomic evaluation of the collecting system by CT or IVU; or if there is a contraindication to performing urography, a retrograde contrast examination of the ileal loop and renal collecting system (ileostoureterogram or ileal loopogram) may be performed ( Appendix D ).
Ileostoureterography is usually performed to evaluate the urothelium for neoplasms, to detect ureteral obstruction, or to delineate the site of obstruction if the patient has progressive hydronephrosis and the cause is not apparent after IVU or radionuclide renography. Causes of mechanical obstruction include urolithiasis, ureteroileal anastomotic strictures, midloop stenosis, and stomal stenosis. Although CTU is usually diagnostic in patients with ureteroileal leak, an ileal loopogram can also confirm this diagnosis.
The ileal conduit should be evaluated for size and shape. The ileal conduit is usually constructed in the right lower quadrant and is 12 to 15 cm in length. It is important to evaluate for segmental narrowings attributable to loop ischemia. These stenoses may cause obstruction.
Free reflux of contrast material through the ureteroileal anastomoses permits evaluation of the collecting system of the upper urinary tract. However, in approximately 10% of patients, reflux of contrast material is not possible and the antegrade flow of urine through the ureteroileal anastomosis is normal. The course and contour of the ureters must be evaluated. The left ureter may opacify later than the right ureter because the left ureter is tunneled beneath the mesentery. Mild dilatation of the ureter and blunting of the calyces is normal because contrast material is instilled under positive pressure ( Fig. 1-13 ). Any filling defect or stenosis should be filmed in multiple projections.

Because the vigorous instillation of contrast medium can result in bacteremia, preprocedure medication with antibiotics is recommended. A rare complication of loopography is the development of autonomic dysreflexia in patients with spinal cord injuries. This complication is thought to result from overdistension of the ileal loop and may result in severe hypertension.
Hysterosalpingography is a radiographic method of examining the endometrial cavity and the lumen of the fallopian tubes after instillation of contrast material into the cervical canal ( Appendix E ). The main indication for hysterosalpingography is the evaluation of patients with primary or secondary infertility. Patients who have had tubal reconstructive surgery may undergo hysterosalpingography to evaluate tubal patency and morphology and to assess the development of paratubal adhesions. Hysterosalpingography is also performed in patients with known or suspected müllerian anomalies, which may manifest as infertility, multiple miscarriages, premature labor, or fetal malpresentation. Hysterosalpingography may also be used to determine the location of a uterine leiomyoma relative to the endometrial cavity. Three contraindications to hysterosalpingography are pregnancy, acute pelvic infection, and active menstruation.
The opacified endometrial and endocervical cavities have the shape of a triangle with an elongated apex. The base of the triangle is the fundus; with anteflexion of the uterus, a common position when the bladder is empty, the fundus may be located inferior to the lower corpus and cervix. The elongated apex of the triangle is the endocervical cavity, which has a jagged contour because of the cervical mucosa ( Fig. 1-14 ). The shape and size of the endometrial cavity should be assessed, and any displacement of the cavity or filling defects within it should be reported.

The fallopian (uterine) tubes insert at the cornua of the uterus. From medial to lateral, there are four named parts of each fallopian tube: interstitial (intramural), isthmus, ampullary, and infundibulum (see Fig. 1-14B ). The patency, size, and shape of the fallopian tubes should be evaluated. The examination is concluded when contrast material can be seen around loops of small bowel or if tubal occlusion is demonstrated. When there is tubal obstruction, the site of the blockage should be reported. It is also important to report if there is pooling of contrast material in the peritoneal cavity around the patent fallopian tube, which suggests adhesions.
Complications of hysterosalpingography include pain and infection. Pelvic pain, similar to menstrual cramps and typically mild or moderate, may begin during the procedure and continue for as long as several hours afterward. As many as 80% of patients may experience varying degrees of pelvic pain, which is not related to the type of contrast material administered. Fever, which complicates hysterosalpingography in 0.3% to 3% of patients, may be the harbinger of infection and requires prompt evaluation. Patients with tubal obstruction and hydrosalpinx or those with a history of pelvic inflammatory disease appear to be at higher risk for the development of infection after hysterosalpingography; such an infection is most often the result of retrograde introduction of cervical flora.
Renal and upper urinary tract sonography is a commonly performed examination because it is accurate and safe and because it does not require exposure to ionizing radiation or radiographic contrast material, nor is normal renal function necessary for image production. With modern instrumentation, bedside imaging and imaging-directed procedures can be performed on even the most seriously ill patients. Several of the more common indications for urinary tract sonography include:
Evaluation of collecting system obstruction
Evaluation of suspected or known nephrolithiasis
Evaluation of renal cystic disease
Detection of a renal, adrenal, or perinephric mass lesion
Characterization of a renal mass lesion as solid, or cystic
Guidance for aspiration or biopsy of a renal or adrenal mass.
Renal parenchyma is anatomically and functionally divided into the peripheral cortex and central medulla. Normal invaginations of cortical tissue, called columns of Bertin, separate adjacent medullary pyramids. On sonograms, normal renal cortex is hypoechoic compared with liver parenchyma. The renal pyramids, which represent that portion of the renal medulla cupped by the calyces, are hypoechoic compared with adjacent renal cortex ( Fig. 1-15 ). This sonographic corticomedullary differentiation or definition is accentuated in children aged up to 6 months, in whom the pyramids may appear echo-free. The relative size ratio of cortex to medulla is 1.6 : 1 in children, but 2.6 : 1 in adults. The arcuate arteries mark the true corticomedullary junction and can be seen as punctate, echogenic foci in approximately 25% of patients.

The renal sinus contains the calyces, infundibula, a portion of the renal pelvis, fibrous tissue, fat, vessels, and lymphatics. On sonograms, the renal sinus typically appears as a central hyperechoic area, largely because of its fat content (see Fig. 1-15 ). It contrasts with the hypoechoic renal pyramids that border the sinus. It is important to note the gradation of tissue echogenicity on an ultrasound examination. The normal order of tissue echogenicity from greatest to least in the adult is: renal sinus, pancreas, liver and spleen, renal cortex, and renal medulla.
The determination of renal size with ultrasonography is more accurate than with standard radiography because the kidney is imaged without magnification or contrast-induced osmotic diuresis. The normal superomedial inclination or tilt of the kidney may create some factitious shortening on sonograms. As a result, renal size is approximately 15% smaller than that measured on a radiograph. Renal length decreases with age, although the volume of the renal sinus increases somewhat in the elderly. For a 30- to 50-year-old individual, the upper tenth percentile of renal length is 10 cm for the right kidney and 10.3 cm for the left kidney. For people aged 60 to 69 years, the upper tenth percentile of right renal length is 9.6 cm, and for those aged 70 to 79 years this value is 9.2 cm; for the left kidney, 0.3 cm is added to the tenth percentile value of the right kidney. The range of normal renal size at sonography is 9 cm to 13 cm.
Transabdominal and endocavitary ultrasonography can be used to produce high-resolution images of the lower urinary tract and pelvis. Particularly in female patients of childbearing age, it is the test of first choice for the evaluation of acute or chronic pelvic pain. Pelvic sonography can also be used to evaluate most pelvic masses, although large masses (i.e., those > 8 to 10 cm) are more effectively characterized with CT or MRI. Ultrasonography is also the test of choice in evaluating the patient with a suspected intrauterine or extrauterine gestation.
Transabdominal (transvesical) sonography is performed using the full urinary bladder as an acoustic window to deeper pelvic structures. In most patients, transabdominal sonography is performed with a 3.5-MHz transducer, in some patients a 5.0-MHz or even a 7.5-MHz transducer may be used to optimize imaging of the near field. In the majority of situations, endovaginal sonography supplements the transabdominal study ( Appendix F ). It is used to clarify ambiguities discovered on the transabdominal examination of the pelvis and is unnecessary if the transabdominal examination is technically satisfactory. Because its field of view is limited, endovaginal sonography is unsatisfactory for the evaluation of a large pelvic mass and can be misleading if performed without the orientation provided by a transabdominal study.
The uterine wall consists of an external serosa (perimetrium), a middle muscularis (myometrium), and an internal mucosa (endometrium). The perimetrium is not normally visible on ultrasound examination, although subserosal veins may be seen as a normal variant. By sonography, the myometrium has two, and sometimes three, distinct layers. The inner myometrium (subendometrial halo) appears as a thin hypoechoic area surrounding the echogenic endometrium ( Fig. 1-16 ). The outer myometrium is relatively hyperechoic compared with the inner myometrium and may have a bilaminar appearance.

The endometrium appears as a central echogenic zone that varies during the menstrual cycle. In the early proliferative phase, the endometrium appears as a single, echogenic line (see Fig. 1-16 ). In the midfollicular phase, three longitudinal lines can be seen in the center of the uterus; these characterize the proliferative endometrium. The outer lines represent the echo-interface between the myometrium and endometrium, and the central line is the uterine canal. The hypoechoic region between these linear borders represents the functional endometrial layer, which thickens as endometrial development progresses in the late proliferative phase (see Fig. 1-16 ). Within 48 hours of ovulation, this hypoechoic layer gradually becomes more hyperechoic, and the triple-line sign disappears, signaling the onset of the secretory endometrium. The distance between the outer lines of the endometrial echo complex can be measured (outer-to-outer border), and cycle-specific normal limits have been established for each phase: menstrual, 2 to 3 mm; early proliferative, 5 ± 1 mm; periovulatory, 10 ± 1 mm; and late secretory, up to 16 mm.
On a sagittal image through its long axis, the maximal length of the uterus is measured from the top of the uterine fundus to the external cervical os. The uterine height is measured perpendicular to the length measurement on the same sagittal image ( Fig. 1-17 ). The width of the uterus is measured in a transverse plane orthogonal to the sagittal plane in which the height was measured. For the nulliparous woman, the normal upper limits of uterine dimensions are: length, 9 cm; height, 4 cm; and width, 5 cm. Parity will increase the normal dimensions of the uterus.

The adnexa consist of the ovaries, uterine tubes, ligaments, and vessels. In nulliparous women, the ovaries are situated in the ovarian fossa. The ovarian (Waldeyer) fossa is bounded by the internal iliac artery posteriorly, the external iliac vein superiorly, and the obliterated umbilical artery anteriorly. However, in parous patients or in those with a pelvic mass, the ovary is often displaced from the ovarian fossa. The ovaries are hypoechoic ellipsoid structures and have a thin hyperechoic rim. Cohen and associates, using the formula for a prolate ellipse (Volume (ml) = length × height × width × 0.52) report a mean ovarian volume of 9.8 cm 3 (95% confidence value, 21.9 cm 3 ) in adult menstruating women ( Figs. 1-17B and C ). The mean for postmenopausal women was 5.8 cm 3 (95% confidence value, 14.1 cm 3 ). The ovaries are distinctive because of the presence of developing follicles in the cortex. Graafian follicles become visible as fluid accumulates in the antrum of the follicle. With endovaginal sonography, follicles can be seen when they reach 1 to 2 mm in diameter. Nondominant follicles are usually less than 14 mm in diameter, and dominant follicles reach a maximum diameter of approximately 20 to 25 mm ( Figs. 1-17 and 1-18 ).

The interstitial part of the fallopian tube is occasionally seen on an endovaginal sonogram as a thin echogenic line extending to the wall of the uterus from the endometrium. However, unless dilated or surrounded by pelvic ascites, the isthmus and ampulla of the fallopian tube are not seen routinely. Similarly, the suspensory ligament of the ovary and the broad ligament are usually not visualized with sonography unless there is free intraperitoneal fluid.
Some of the more common indications for ultrasonography of the bladder include:
Determination of the existence and rate of urine flow through the vesicoureteral junction in patients with dilated ureters ( Fig. 1-19 )

Determination of prevoid and postvoid bladder volume
Detection of bladder stone or bladder wall mass
Detection and quantification of bladder wall thickening
Direction for bladder aspiration or cystostomy.
The normal distended bladder is an anechoic structure that occupies the midline of the true pelvis. It is triangular in the sagittal plane and oval in the transaxial plane. Normally, the wall of the bladder is uniform and 3 to 4 mm in thickness. The capacity of the bladder can also be estimated with the formula of the prolate ellipse.
Transrectal sonography of the prostate is used as an adjunct to the digital rectal examination to quantify prostate volume, assess a palpable nodule, guide prostate biopsy, and evaluate the infertile patient ( Appendix G ). Less commonly, it is used to investigate perirectal masses or collections, particularly when biopsy is contemplated.
The normal prostate gland is a triangular ellipse that is surrounded by a thin capsule. The glandular tissue or parenchyma of the prostate is composed of the peripheral zone, central zone, and transitional zone ( Fig. 1-20 ). The parenchyma is bounded anteriorly by fibromuscular stroma. The prostatic “capsule” is seen as a bright echogenic structure posteriorly and laterally, but it is thin or absent at the apex of the gland. The normal prostate measures approximately 4 cm in length (cephalocaudal), 4 cm in transverse diameter, and 3 cm in height (anteroposterior); the normal volume ranges from 20 to 25 mL, and the normal weight is approximately 20 g.

Seminal vesicles are cephalad to the prostate gland and posterior to the bladder. The paired structures are imaged in long axis on transverse images and cross-sectional on sagittal images. The seminal vesicles are well-defined, saccular structures that are most often symmetric in size and shape. The normal seminal vesicle measures 3 ± 0.5 cm in length and 1.5 ± 0.4 cm in width. Seminal vesicles are usually hypoechoic and contain scattered fine internal echoes. The vasa deferentia are located medially to the seminal vesicles and just cephalad to the prostate gland ( Fig. 1-20B ). The dilated terminus of the vas deferens is called the ampulla. On the transaxial plane, the paired vasa deferentia appear as a pair of oval, convoluted tubular structures and are isoechoic to the seminal vesicles. The confluence of the seminal vesicle and ampullary portion of the vas deferens form the ejaculatory duct. The neurovascular bundle consists of paired cords of nerves and vessels that extend along the posterolateral margins of the prostate. The neurovascular bundles, which are frequently identified on sonograms, are important routes for extraprostatic spread of cancer. The periprostatic venous plexus is seen anteriorly and can be prominent. Vessels can be identified entering the gland laterally—most often near the apex, where they can simulate a hypoechoic tumor.
Ultrasonography of the scrotum is used to evaluate a palpable mass or scrotal enlargement because it can accurately distinguish an intratesticular lesion from one that originates from extratesticular tissues ( Appendix H ). This distinction is important because the majority of lesions that originate in an extratesticular location are benign (e.g., hydrocele, varicocele, epididymitis), unlike testicular neoplasm, the most common intratesticular mass. The integrity of the traumatized testis can also be evaluated quickly with sonography. Duplex sonography of the acute scrotum can be used to distinguish testicular torsion from epididymoorchitis. Finally, ultrasonography has been used to identify the undescended inguinal testicle but is less accurate than CT, MRI, and testicular phlebography for locating the abdominal testis. Other indications for scrotal sonography include the evaluation of male infertility, the follow-up evaluation of sexual precocity, and the evaluation of occult primary tumors in patients with metastatic disease.
The normal testis is an ovoid structure, measuring 3.5 cm in length and 2.5 to 3 cm in width and height (anteroposterior diameter). The echogenicity of the two testes should be similar and should consist of uniform, medium-level echoes. The mediastinum represents an invagination of the tunica albuginea along the posterior aspect of the testis and appears as an echogenic line parallel to the epididymis ( Fig. 1-21 ). Septations within the testis may be visualized as linear echogenic or hypoechoic structures and may divide the testicle into lobules.
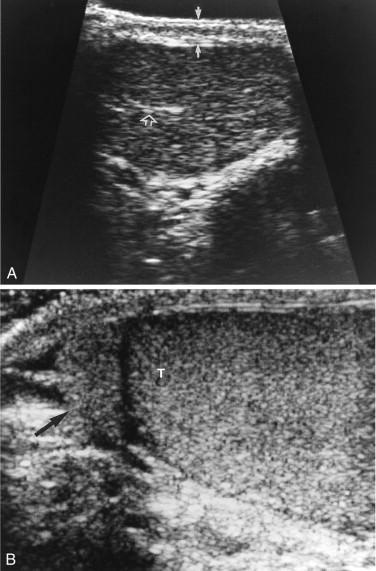
Of the normal extratesticular structures, the epididymis is most commonly identified with sonography. The epididymal head (globus major) is the largest component. It measures 5 to 15 mm in diameter and is found lateral and superior to the upper pole of the testicle. The head of the epididymis is usually slightly more echogenic than the body and tail and isoechoic to testicular parenchyma ( Fig. 1-21B ). The body and tail (globus minor) extend inferiorly along the posterior margin of the testicle and appear as a thin cord approximately 1 to 3 mm in thickness on sonograms. Color Doppler sonography shows no detectable flow in the normal epididymis. On occasion, small protuberances from the head of the epididymis and superior aspect of the testicle can be identified; these protuberances represent the appendices of epididymis and testicle, respectively.
The scrotum is composed of several layers that are derived from the skin, abdominal wall muscles, and peritoneum. Sonographically, the scrotal wall is 5 to 7-mm thick and is hyperechoic compared with the testicle ( Fig. 1-21A ). The tunica vaginalis is derived from peritoneum and applies the testicle to the posterior scrotal wall. Bursa-like, the tunica vaginalis envelops the testicle and normally contains 1 to 2 mL of fluid. The spermatic cord contains the testicular and deferential arteries and the pampiniform plexus of veins. Normal vessels are no more than 1 to 2 mm in diameter, and the presence and direction of flow can be ascertained by using color Doppler sonography.
In medical ultrasonography, images are produced by tissue reflection of a beam of sound at a frequency of 2 to 15 MHz. For a moving point reflector, such as an erythrocyte, the frequency of sound received by the transducer will differ from that of the transmitted frequency. This frequency difference, or shift, can be used to determine the direction and velocity of reflector movement. Johann C. Doppler is credited with the elucidation of this principle and the relationship between frequency shift and flow velocity.
Information about the Doppler shift frequency is displayed graphically as a series of spectra in real time. These spectra are vertical line graphs showing the relative strength of frequency shifts with respect to time, which is represented on the horizontal axis. Thus four variables are displayed on the Doppler spectra: frequency shift (velocity), distribution of frequency shifts by a population of point reflectors, direction of flow, and time. A number of different indices have been used to quantitate impedance, the total resistance to flow. The pulsatility index is equal to ( S − D )/ M , where S is the peak systolic frequency, D is the peak diastolic frequency, and M is the mean frequency shift. Other such indices include the resistive index, ( S − D ) /S , and the systolic/diastolic ratio, S/D . In duplex Doppler sonography, the pulsed Doppler system is interfaced with a real-time image so that the source of the shifted frequency can be user-selected from a gray-scale image. Color Doppler uses colors to encode directional and velocity information about flow.
Doppler sonography has been used to evaluate normal flow of urine into the urinary bladder (see Fig. 1-19 ), testicular parenchymal perfusion to exclude spermatic cord torsion, renal parenchymal perfusion to identify glomerulotubular disease (see Fig. 1-15 ), and cavernosal artery flow to investigate vasculogenic causes of impotence.
CT creates a two-dimensional image of the body from measurements of relative linear attenuation collected from multiple projections around a thin tomographic slice. This technique can demonstrate density differences between tissues of 1% or less. The reconstructed image is displayed as a matrix of pixels. The numerical value of each pixel is the relative linear attenuation coefficient of the volume of tissue represented by that pixel. The attenuation value assigned to each pixel is based on a reference scale in which − 1000 Hounsfield units (HU) are assigned to air, + 1000 HU to dense bone, and 0 HU to water. The relative linear attenuation coefficient assigned to each pixel is correlated with a shade of gray in the image. User selection of the number of shades of gray (window width) in the image and the central hue of gray (window level) permits modification of image contrast.
Spiral, helical, multidetector, or volume-acquisition CT is a technique that permits continuous acquisition of data by constant rotation of a slip-ring x-ray tube-detector system while the patient moves through the gantry. Most helical and multidetector CT scanners rotate at a rate of approximately 360 degrees/second, but the rate of patient movement through the gantry and the collimation of the beam are selected by the radiologist. Pitch is defined as the ratio of the rate of table movement (per 360 degrees of tube rotation) to beam collimation; if the scan time is 1 second, pitch is equal to the rate of table movement in millimeter/second divided by the beam width in millimeters. The tradeoff in selecting the appropriate pitch is between spatial resolution (optimized with a smaller pitch) and coverage (optimized with a larger pitch). Recent work suggests that the optimal pitch for single-row helical CT of the abdomen is 1.5 to 1.6.
There are several advantages of helical and multidetector CT over conventional CT. First, very thin collimation can be used over large areas of the body to evaluate small lesions. Also, helical CT data can be manipulated through postprocessing, which permits targeted image reconstruction to an area of interest. For instance, overlapping images could be reconstructed in narrow intervals to increase the sensitivity for smaller lesions. Second, because of shortened scan times, less contrast material is needed for some studies, and scanning can be optimized to specific phases of contrast enhancement. Finally, CT angiography is possible because three-dimensional data acquisition is combined with an imaging method that optimizes the detection of contrast enhancement in vessels.
Renal CT is used to characterize masses and can evaluate the perinephric spread of primary renal disease or the secondary involvement of the kidneys by primary intraperitoneal or retroperitoneal disease. CT is also the imaging procedure of choice in patients with suspected or known renal trauma because it accurately characterizes injuries to the parenchyma and vascular pedicle. The more common genitourinary indications for abdominal and pelvic CT include ( Appendices I , J ):
Detection of urolithiasis
Evaluation of patients with hematuria
Detection and characterization of an adrenal, renal, or pelvic soft-tissue mass
Staging of kidney, adrenal, ureter, and bladder malignancy
Staging of gynecologic malignancy (ovary and uterus)
Staging of male genital malignancy (testis and prostate)
Evaluation of known or suspected retroperitoneal disease
Evaluation of chronic pelvic pain
Evaluation of cryptorchidism
Directed aspiration, biopsy, or drainage.
The renal parenchyma is homogeneous on nonenhanced CT, and measured attenuation ranges from 30 to 60 HU. Renal sinus and perinephric fat provide intrinsic contrast for the renal parenchyma on noncontrast and contrast-enhanced CT ( Fig. 1-22 ). After administration of contrast material, there is gradual enhancement of the large vessels, renal parenchyma, and collecting system. During the vascular phase, there is opacification of the aorta and major arteries. Sequential enhancement of the renal cortex and medulla follow during the nephrogram phase. Normal corticomedullary differentiation is demonstrated during the early nephrogram phase (approximately 20 to 85 seconds after the administration of contrast material); one can miss small medullary tumors on CT during this phase ( Fig. 1-23A ). The exact timing of the early nephrogram phase depends on the dose and method of intravenous contrast administration and on the cardiac output and renal function of the patient. Scans obtained approximately 85 to 240 seconds after intravenous contrast administration demonstrate more homogeneous attenuation of the parenchyma during the nephrogram (equilibrium) phase ( Fig. 1-23B ). After contrast-material injection, the attenuation value of the normal renal parenchyma will increase to 80 to 120 HU. During and after the nephrogram phase, there is progressive opacification of the renal collecting system.


The adrenal glands are variable in shape, although the posterior limbs are usually uniform in width. The normal width of the adrenal limbs, measured perpendicular to the long axis, is less than 8 mm (see Fig. 1-23A ).
In the abdomen, the nondilated ureter may be difficult to distinguish from gonadal vessels or other retroperitoneal vessels and can be identified on the medial part of the psoas muscle ( Fig. 1-23C ). In the pelvis, the ureter descends posterolaterally, and opposite the ischial spine it turns anteromedially in front of the seminal vesicles or vaginal fornices to reach the base of the bladder ( Fig. 1-23D ). The bladder is identified in the midline of the true pelvis; on noncontrast CT, its wall is uniformly thin and is outlined by urine and perivesical fat. The presence of dense contrast material in the bladder may create artifacts and obscure the bladder wall.
Lymph nodes are routinely identified on CT; normal lymph nodes have the attenuation of soft tissue, are homogeneous, oval, and measure 4 to 10 mm in short-axis diameter. Abdominal and most pelvic lymph nodes are abnormal if they are larger than 10 mm in short axis diameter; retrocrural nodes are enlarged if more than 6 mm in diameter. The pelvic nodes are grouped around the common iliac, external iliac, and internal iliac vessels; the inguinal nodes lie medial and anterior to the femoral vessels ( Fig. 1-24 ). The surgical obturator nodes are particularly important because cancer of the prostate and bladder frequently spreads to these nodes first (sentinel nodes). These nodes actually form the medial chain of the external iliac lymph nodes and can be identified adjacent to the lateral pelvic wall at the level of the acetabulum ( Fig. 1-24C ).

The seminal vesicles are oblong, tubular structures that lie at the superior aspect of the prostate, posterior to the lower urinary bladder, and anterior to the rectum. A plane of fat separates the seminal vesicles from the bladder ( Figs. 1-22B and 1-23D ). The prostate gland is identified posterior and inferior to the bladder neck, anterior to the rectum, and posterior to the pubic symphysis. When healthy, it is homogeneous in attenuation but may contain punctate calcifications ( Fig. 1-23E ). The spermatic cord is formed at the internal inguinal ring by the confluence of the vas deferens with vessels and nerves. The spermatic cord is seen medial to the ipsilateral femoral vein and anterolateral to the symphysis pubis. The vas deferens leaves the cord at the inguinal ring and continues posteriorly and medially toward the ipsilateral seminal vesicle (see Fig. 1-23D ).
The uterus is located between the urinary bladder and the rectosigmoid colon in the true pelvis. The fundus and corpus of the uterus have a triangular shape, and the cervix has a rounded contour. The attenuation of the uterus is usually homogeneous on noncontrast CT, although the normal endometrial cavity may appear slightly hypodense if it contains secretions. The endometrium appears relatively hypodense after administration of intravenous contrast medium because of intense enhancement of the myometrium ( Fig. 1-25 ). The ovaries may be slightly hypodense, particularly if follicles are prominent. The ovaries can be identified by following the ovarian ligament from the cornua of the uterus to the ovary or by following the course of the gonadal vein inferiorly from its origin from the inferior vena cava on the right or the renal vein on the left. The broad ligaments are peritoneal folds that connect the lateral margin of the uterus to the pelvic side walls.

For many radiologic problems in genitourinary radiology, MRI is used as a supplementary examination to further investigate or characterize disease detected with other imaging modalities. For example, MRI is often utilized to evaluate renal masses or adnexal masses that remain indeterminate after sonographic or CT evaluation. MRI may also be utilized as a supplement to the clinical staging of certain malignancies, including gynecologic malignancies such as cervical and endometrial cancer, and prostate cancer. In women with uterine fibroids, MRI can play a role in determining which patients might benefit from uterine artery embolization versus surgical or hysteroscopic management. In women with clinical evidence of pelvic floor dysfunction, dynamic MRI of the pelvic floor can provide a global assessment of the various compartments of the pelvic floor and influence management decisions. MRI is, therefore, often performed as a carefully targeted study ( Appendix K ). MRI is not usually intended to be a screening study or a substitute for biopsy because, with a few notable exceptions, specific tissue characterization is not possible.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here