Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Indications for evaluation of the adolescent pelvis usually are based on complaints of abdominal pain, pelvic pain, or a mass. Other clinical symptoms resulting in imaging evaluation in female patients relate to abnormalities of development of secondary sexual characteristics of puberty. These changes may be seen earlier than normal (i.e., precocious puberty), may be delayed (i.e., delayed puberty), or may fail to develop (e.g., hypogonadism and sexual infantilism) in adolescents. The other key reason for adolescent gynecologic evaluation is amenorrhea (lack of menses), whether primary or secondary. Conditions causing pubertal delay may also cause primary or secondary amenorrhea.
Puberty among girls is the stage of development between childhood and adulthood when activation of the hypothalamic-pituitary-ovarian-uterine axis produces maturation of the gonads, resulting in an increased production of sex hormones, development of secondary sex characteristics, a growth spurt, and development of reproductive capability. The earliest signs of puberty among girls are breast development (usually occurring between ages 8–13 years) and pubic hair growth (usually between ages 8–14 years). These signs are followed by a growth spurt (usually between ages 9.5–14.5 years), axillary hair development, and menarche (usually between ages 10–16 years). Puberty is typically completed within about 4 years.
Puberty among boys usually begins between 9 and 14 years of age and is completed in 3.5 to 4 years. It begins with testicular enlargement (usually occurring between ages 9–13.5 years), followed by the appearance of pubic hair (usually between ages 10–15 years), enlargement of the penis (usually between ages 11–12.5 years), and development of axillary and facial hair as well as a growth spurt (usually between ages 10.5–16 years).
Ordinarily, until the age of at least 8 years, an unknown “central restraining mechanism” prevents the pulsatile release of gonadotropin-releasing hormone (GnRH) from the arcuate nucleus of the hypothalamus. Pulsatile release of GnRH appears necessary for ovulation and development of the corpus luteum. In early puberty, the pulsatile GnRH release is maximal only at night, but with time the typical adult pattern of continuous pulsatile GnRH secretion develops.
With the earliest activation of GnRH, most girls undergo ovarian folliculogenesis without ovulation. Unopposed estrogen production leads to progressive uterine growth and endometrial proliferation. Breast budding, physiologic leukorrhea, and accelerated linear growth occur. As the hypothalamic-pituitary-ovarian-uterine axis matures over approximately a 2-year span, cycles with subnormal progesterone production and shortened intermenstrual intervals are replaced by fertile cycles and normal corpus luteum function.
The typical ovulatory cycle has a 24- to 35-day intermenstrual interval and usually a premenstrual molimina (mild group of symptoms, including breast tenderness, food craving, fatigue, headaches, sleep problems, and/or fluid retention). Longer intervals often are associated with anovulation. Improved nutrition and living conditions are thought to be at least in part responsible for the gradual decrease in mean menarchal age during the past century. In North America, the mean menarchal age currently is 12.4 years, with a range of 9 to 17 years. Menarche usually occurs 2 to 5 years after breast bud development.
Premature thelarche and adrenarche are relatively common, self-limited variants of normal pubertal development in girls. Premature thelarche refers to premature breast development without other signs of precocious sexual maturation in girls younger than 8 years of age. It usually is seen between 1 and 4 years of age; one-third of the cases resolve spontaneously. Premature adrenarche refers to the appearance of pubic and axillary hair without other signs of precocious sexual maturity. In both premature thelarche and premature adrenarche, bone age and patient height are normal to only slightly advanced/increased. The cause of premature thelarche or adrenarche is not certain. The levels of circulating sex hormones usually are normal. Increased end-organ sensitivity to normal levels of estrogen or androgen is a possible cause.
In boys, premature appearance of pubic and axillary hair without other signs or only minor signs of precocious puberty is a relatively common variant of normal development that may be due to an increase in circulating androgens from premature maturation of the adrenal glands of unknown cause. No penile enlargement is present, presumably because the levels of circulating androgens are not sufficiently elevated. The bone age and growth rate are slightly increased. The remainder of pubertal development occurs at a normal age.
Precocious puberty refers traditionally to the appearance of secondary sex characteristics before 8 years of age in girls and before 9 years of age in boys. A Pediatric Endocrine Society task force suggested that the definition ages be modified to secondary sexual characteristics noted in white girls under age 7 and African American girls under age 6. Precocious puberty is divided into two main types: (1) complete, central, gonadotropin-dependent, or true precocious puberty and (2) incomplete, peripheral, gonadotropin-independent, pseudoprecocious puberty, or precocious pseudopuberty. Whereas the complete form is characteristically isosexual, with development of secondary sex characteristics that are appropriate for the patient's gender, the incomplete form may be either isosexual or heterosexual. Incomplete heterosexual precocious puberty is manifested by signs of virilization in girls and by gynecomastia or other signs of feminization in boys.
Complete or central isosexual precocious puberty results from premature activation of the hypothalamic-pituitary-gonadal complex with increased production of gonadotropic and sex hormones and an early onset of ovulation or spermatogenesis. Complete precocious puberty may be idiopathic or secondary to organic central nervous system (CNS) lesions.
The cause of precocious puberty in girls is idiopathic in at least 80% of cases. About 20% of affected girls have a hypothalamic or pituitary lesion. Fewer than 10% of cases of true precocious puberty in boys have an idiopathic cause. A familial tendency to idiopathic early pubertal development is observed in some cases (i.e., constitutional or genetic precocious puberty).
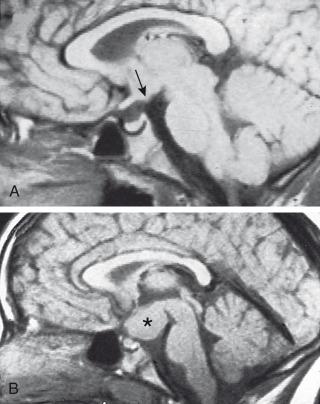
Possible causes of precocious puberty in either sex include intracranial tumors or cysts, hydrocephalus, sequelae of intracranial inflammatory processes or trauma, and other intracranial lesions that may activate the hypothalamus by pressure or invasion. Of the CNS tumors/neoplasms that may cause true precocious puberty, hamartoma of the hypothalamic tuber cinereum ( Fig. 127.1 ) is the most common. This usually small CNS tumor secretes GnRH, is more common in boys than in girls, is generally benign and nonprogressive, and usually cannot be surgically corrected. It may be pedunculated or appear as an excrescence of the tuber cinereum, below the mammillary body. The onset of puberty in patients with this lesion is usually at a younger age (~2 years) than in patients with idiopathic precocious puberty. Other CNS neoplasms that may cause true precocious puberty in either sex usually are located in or near the hypothalamus and include hypothalamic or optic gliomas, astrocytoma, ependymoma, dysgerminoma, and prolactinoma. Suprasellar dysgerminoma in boys also may cause incomplete precocious puberty through tumor secretion of human chorionic gonadotropin (hCG).
True precocious puberty may be observed in some children with long-standing untreated hypothyroidism. The increased production of gonadotropic hormones and prolactin seen in these patients may be due to a hormonal overlap in the pituitary response to thyroid deficiency. Affected patients show little, if any, development of secondary sexual characteristics, especially pubic hair. These changes regress with treatment of the hypothyroidism.
Pseudosexual (or incomplete) precocious puberty in girls usually presents before 5 years of age. Excess circulating estrogens or related substances develop independently of the hypothalamus and pituitary gland. These estrogens usually are produced by the ovaries or adrenal glands or may be from an exogenous source, such as food, parenteral or oral medications, or other substances.
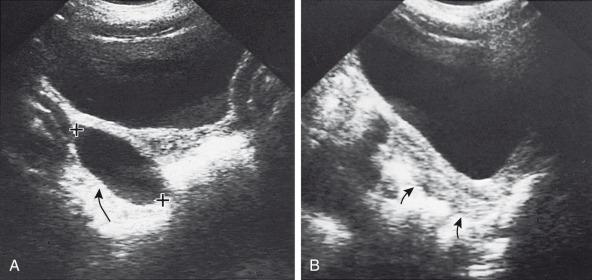
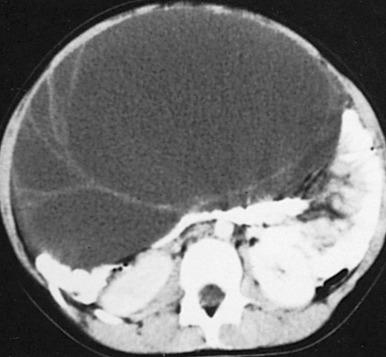
Gonadotropin levels are low, and the gonads remain immature. The most common ovarian source is an autonomous estrogen-secreting follicular (granulosa-thecal) cyst ( Fig. 127.2 ). Other causes are estrogen-producing ovarian neoplasms, particularly granulosa cell (or granulosa-theca cell) tumors ( Fig. 127.3 ) and rare estrogen-secreting adrenal neoplasms (adenomas or carcinomas).
McCune-Albright syndrome consists of polyostotic fibrous dysplasia, cutaneous café au lait spots, and precocious puberty. The syndrome is found predominantly in females. Autonomously functioning ovarian cysts are seen in some cases. In others, no anatomic cause is noted. McCune-Albright syndrome occasionally presents as complete or true precocious puberty. It has been suggested that the initially partial or incomplete precocious puberty in these patients becomes complete (gonadotropin dependent) as the result of an early maturation of the hypothalamic-pituitary-gonadal complex, which is caused by a long-standing exposure to estrogen.
Testosterone is produced in normal females by the adrenal gland (25%), by the ovary (25%), and by peripheral conversion of D4-androstenedione (50%), with only 1% typically free and biologically active. A form of pseudosexual precocity may occur in girls when androgen levels are excessive or end organs become excessively sensitive to normal amounts of circulating androgens. Either case may lead to heterosexual pseudoprecocious puberty as the girls undergo virilization and masculine secondary sexual characteristics develop. Clinical findings include increases in body and facial hair (hirsutism), acne, deepening of the voice, clitoromegaly, increased muscle mass, and temporal balding. Menstrual abnormalities are common among affected adolescents.
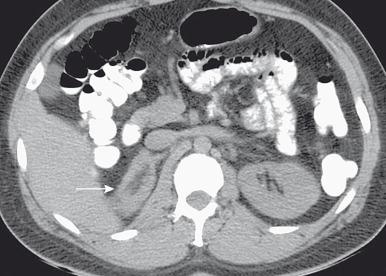
Congenital adrenal hyperplasia (CAH) is the most common form of hyperandrogenism. The increased production of androgenic substance is due in most cases to a deficiency of 21-hydroxylase and far less commonly to deficiencies in 11β-hydroxylase, 3β-hydroxysteroid dehydrogenase, or other enzymes involved in cortisol and/or aldosterone synthesis. Imaging of the adrenal gland will show enlargement ( Fig. 127.4 ) that is usually bilateral and diffuse. The normal adrenal gland shape is maintained.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here